Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation
Abstract
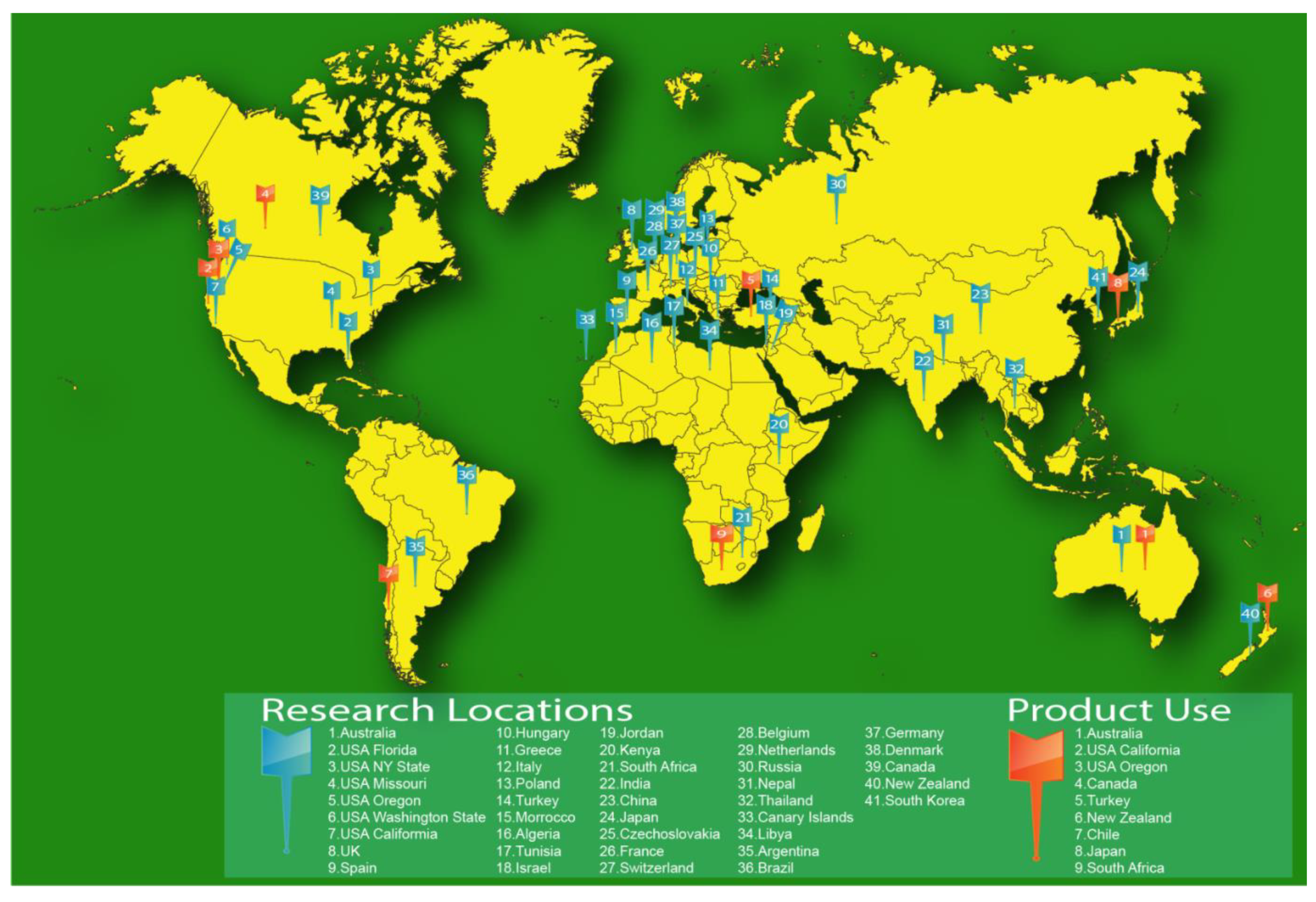
1. Introduction
2. Biocontrol
3. Challenges of Biopesticide Commercialisation—Case Study of K84 and K1026
3.1. Government Registrations
- Identification and characterisation assessments;
- A description of gene deletions and lineage to K84 (K1026);
- Human health risk assessments (pathogenicity, toxicity, allergenicity, sensitisation and mutagenicity);
- Target organism effects (mode of action);
- Non-target organism effects;
- Environmental risk assessment.
- A statement of formula;
- A certificate of analysis on samples from several production batches;
- Physical and chemical properties;
- Storage stability;
- The efficacy of the biological control product;
- Label wording and claims;
- Risk management.
- The effects of agrocin 84 on a range of non-target organisms;
- The amount of agrocin 84 in the end-product formulation;
- The amount of the agrocin 84 antibiotic in batch samples and methods of analysis;
- The effects of exposure to agrocin 84 on operators;
- The development of agrocin 84 resistance in wild type agrobacteria;
- The levels of K1026 in the air, water and in soils against background levels of agrobacteria;
- The effects from use of K1026 on soil microorganisms levels;
- Supporting information on risks to wildlife;
- Supporting information on crop safety.
3.2. Marketing and Distribution
“OPEC considered that the use of products with genes deleted as a result of a scientific intervention designed specifically to remove a gene would be inappropriate in organic farming and believed that their use could undermine consumer confidence in the certification of organic food exports. It, therefore, concluded that the definition of GMOs should extend to organisms of this type.”
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chilton, M.-D.; Drummond, M.H.; Merlo, D.J.; Sciaky, D.; Montoya, A.L.; Gordon, M.P.; Nester, E.W. Stable incorporation of plasmid DNA into higher plant cells. Cell 1977, 11, 263–271. [Google Scholar] [CrossRef]
- Lacroix, B.; Citovsky, V. Pathways of DNA transfer to plants from Agrobacterium tumefaciens and related bacterial species. Ann. Rev. Phytopath. 2019, 57, 231–251. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A. Biological control of crown gall: Seed inoculation. J. Appl. Bacteriol. 1972, 35, 493–497. [Google Scholar] [CrossRef]
- Kerr, A. Biological control of crown gall. Australas. Plant. Pathol. 2015, 45, 15–18. [Google Scholar] [CrossRef]
- Shim, J.-S.; Farrand, S.K.; Kerr, A. Biological control of crown gall: Construction and testing of new biocontrol agents. Phytopathology 1987, 77, 463–466. [Google Scholar] [CrossRef]
- Jones, D.A.; Ryder, M.H.; Clare, B.G.; Farrand, S.K.; Kerr, A. Construction of a Tra- deletion mutant of pAgK84 to safeguard the biological control of crown gall. Mol. Gen. Genet. 1988, 212, 207–214. [Google Scholar] [CrossRef]
- Kerr, A.; Htay, K. Biological control of crown gall through bacteriocin production. Physiol. Plant. Pathol. 1974, 4, 37–44. [Google Scholar] [CrossRef]
- Murphy, P.J.; Tate, M.E.; Kerr, A. Substituents at N6 and C-5′ control selective uptake and toxicity of the adenine nucleotide bacteriocin, agrocin 84 in Agrobacterium. Eur. J. Biochem. 1981, 115, 539–543. [Google Scholar] [CrossRef]
- Reader, J.S.; Ordoukhanian, P.T.; Kim, J.G.; de Crecy-Lagard, V.; Hwang, I.; Farrand, S.; Schimmel, P. Major biocontrol of plant tumors targets tRNA synthetase. Science 2005, 309, 1533. [Google Scholar] [CrossRef]
- Chopra, S.; Palencia, A.; Virus, C.; Tripathy, A.; Temple, B.R.; Valezquez-Campoy, A.; Cusack, S.; Reader, J.S. Plant tumour biocontrol agent employs a tRNA-dependent mechanism to inhibit leucyl-tRNA synthetase. Nat. Commun. 2013, 4, 1417. [Google Scholar] [CrossRef]
- Ellis, J.G.; Kerr, A.; van Montagu, M.; Schell, J. Agrobacterium: Genetic studies on agrocin 84 production and the biological control of crown gall. Physiol. Plant. Pathol. 1979, 15, 311–319. [Google Scholar] [CrossRef]
- Farrand, S.K.; Slota, J.E.; Shim, J.-S.; Kerr, A. Tn5 insertions in the agrocin 84 plasmid: The conjugal nature of pAgK84 and the locations of determinants for transfer and agrocin 84 production. Plasmid 1985, 13, 106–117. [Google Scholar] [CrossRef]
- Ryder, M.H.; Slota, J.E.; Scarim, A.; Farrand, S.K. Genetic analysis of agrocin 84 production and immunity in Agrobacterium spp. J. Bacteriol. 1987, 169, 4184–4189. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Palencia, A.; Virus, C.; Schulwitz, S.; Temple, B.R.; Cusack, S.; Reader, J.S. Structural characterization of antibiotic self-immunity tRNA synthetase in plant tumour biocontrol agent. Nat. Commun. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petit, A.; Delhaye, S.; Tempe, J.; Morel, G. Recherches sur les guanidines des tissus de Crown Gall. Mise en evidence d’une relation biochemique specifique entre les souches d’Agrobacterium tumefaciens et les tumeurs qu’elles induisent. Physiol. Veg. 1970, 8, 205–213. [Google Scholar]
- Ellis, J.G.; Murphy, P.J. Four new opines from crown gall tumours-their detection and properties. Mol. Gen. Genet. 1981, 181, 36–43. [Google Scholar] [CrossRef]
- Ryder, M.H.; Tate, M.E.; Jones, G.P. Agrocinopine A, a tumor-inducing plasmid-coded enzyme product, is a phosphodiester of sucrose and L-arabinose. J. Biol. Chem. 1984, 259, 9704–9710. [Google Scholar]
- Kim, H.; Farrand, S.K. Characterization of the acc operon from the nopaline-type plasmid pTiC58, which encodes utilization of agrocinopines A and B and susceptibility to agrocin 84. J. Bacteriol. 1997, 179, 7559–7572. [Google Scholar] [CrossRef]
- El Sahili, A.; Li, S.-Z.; Lang, J.; Virus, C.; Planamente, S.; Ahmar, M.; Guimaraes, B.; Aumont-Nicaise, M.; Vigouroux, A.; Soulère, L.; et al. A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in Agrobacterium tumefaciens. PLoS Pathog 2015, 11, e1005071. [Google Scholar] [CrossRef]
- Donner, S.C.; Jones, D.A.; McClure, N.C.; Rosewarne, G.M.; Tate, M.E.; Kerr, A.; Fajardo, N.N.; Clare, B.G. Agrocin 434, a new plasmid encoded agrocin from the biocontrol Agrobacterium strains K84 and K1026, which inhibits biovar 2 agrobacteria. Physiol. Mol. Plant. Path. 1993, 42, 185–194. [Google Scholar] [CrossRef]
- Burr, T.J.; Reid, C. Biological control of grape crown gall with Agrobacteriumn vitis strain F2/5. Am. J. Enol. Vitic. 1994, 45, 213–219. [Google Scholar]
- Kawaguchi, A.; Inoeu, K.; Tamina, K.; Nita, M. Control of grapevine grown gall using nonpathogenic Rhizobium vitis strain ARK-1. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2017, 93, 547–560. [Google Scholar] [CrossRef]
- Ellis, J.G. Can plant microbiome studies lead to effective biocontrol of plant diseases? Mol. Plant. Microbe Interact. 2017, 30, 190–193. [Google Scholar] [CrossRef]
- Dewan, V.; Reader, J.; Forsyth, K.M. Role of aminoacyl tRNA synthetases in infectious diseases and targets for therapeutic development. Top. Curr. Chem. 2014, 344, 293–329. [Google Scholar] [PubMed]
- Fravel, D.R. Commercialisation and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C. Trends for Commercialization of Biocontrol Agent (Biopesticide) Products. Plant Def. Biol. Control. 2011, 139–160. [Google Scholar]
- Stewart, A. Commercial biocontrol: Reality or fantasy? Australas. Plant Pathol. 2001, 30, 127–131. [Google Scholar] [CrossRef]
- Kerr, A.; Clare, B.G.; Ryder, M.H.; Farrand, S. Agrobacterium radiobacter K84 Carrying Transfer-Deficient pAgK84 Plasmid. U.S. Patent 5,354,684, 11 October 1994. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- OECD. Available online: https://www.oecd.org/env/ehs/pesticides-biocides/data-for-biopesticide-registration.htm (accessed on 1 August 2020).
- Kabuluk, J.T.; Svircev, A.M.; Goettel, M.S.; Woo, S.G. Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide. 2010, p. 99. Available online: https://www.researchgate.net/profile/Nguya_Maniania/publication/297758802_Microbial_Regulation-Goettel_et_al_2010-Todd-Goettel/links/56e2e9da08ae387a2483a704/Microbial-Regulation-Goettel-et-al-2010-Todd-Goettel.pdf (accessed on 1 August 2020).
- Rhizobium rhizogenes. In Proceedings of the Invasive Species Compendium, Wallingford, UK, 17 March 2020; Available online: www.cabi.org/isc/datasheet/3743 (accessed on 2 December 2019).
- Gene Technology Act. Available online: http://www.legistlation.gov.au/Details/C2016C00792 (accessed on 1 August 2020).
- Gene Technology Regulations. Available online: http://www.legislation.gov.au/Details/F2019C00781 (accessed on 1 August 2020).
- Cook, R.J.; Bruckart, W.L.; Coulson, J.R.; Goettel, M.S.; Humber, R.A.; Lumsden, R.D.; Maddox, J.V.; McManus, M.L.; Moore, L.; Meyer, S.F.; et al. Safety of Microorganisms Intended for Pest and Plant Disease Control: A Framework for Scientific Evaluation. Biol. Control. 1996, 7, 333–351. [Google Scholar] [CrossRef]
- USA EPA. Available online: https://www.epa.gov/pesticide-registration/biopesticide-registration (accessed on 1 August 2020).
- USA EPA. Agrobacterium radiobacter strains K84/Kerr-84 and K1026 Registration Review Case 4101. Available online: https://www.regulations.gov (accessed on 29 March 2010).
- Raio, A.; Peluso, R.; Puopolo, G.; Zoina, A. Evidence of pAgK84 transfer from Agrobacterium rhizogenes K84 to natural pathogenic Agrobacterium spp. in an Italian peach nursery. Plant. Pathol. 2009, 58, 745–753. [Google Scholar] [CrossRef]
- Frederiks, C.; Wesseler, J. A comparison of the EU and US regulatory frameworks for the active substance registration of microbial biological control agents Pest. In Management Science; John & Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Lindstrom, K.; Young, J.P.W. Subcommittee on the taxonomy of Agrobacterium and Rhizobium. Int. J. Syst. Evolut. Microbiol. 2011, 61, 3089–3093. [Google Scholar]
- Andreoletti, O. European Food Safety Authority Scientific Committee Scientific opinion: The maintenance of the list of QPS microorganisms intentionally added to food or feed. Scientific Opinion of the Panel on biological Hazards (Question No EFSA-Q-2008-006). EFSA J. 2008, 923, 1–48. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerr, A.; Bullard, G. Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation. Agronomy 2020, 10, 1126. https://doi.org/10.3390/agronomy10081126
Kerr A, Bullard G. Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation. Agronomy. 2020; 10(8):1126. https://doi.org/10.3390/agronomy10081126
Chicago/Turabian StyleKerr, Allen, and Gary Bullard. 2020. "Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation" Agronomy 10, no. 8: 1126. https://doi.org/10.3390/agronomy10081126
APA StyleKerr, A., & Bullard, G. (2020). Biocontrol of Crown Gall by Rhizobium rhizogenes: Challenges in Biopesticide Commercialisation. Agronomy, 10(8), 1126. https://doi.org/10.3390/agronomy10081126



