Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Compost and Biochar
2.2. Experimental Design
2.3. Sample Analysis
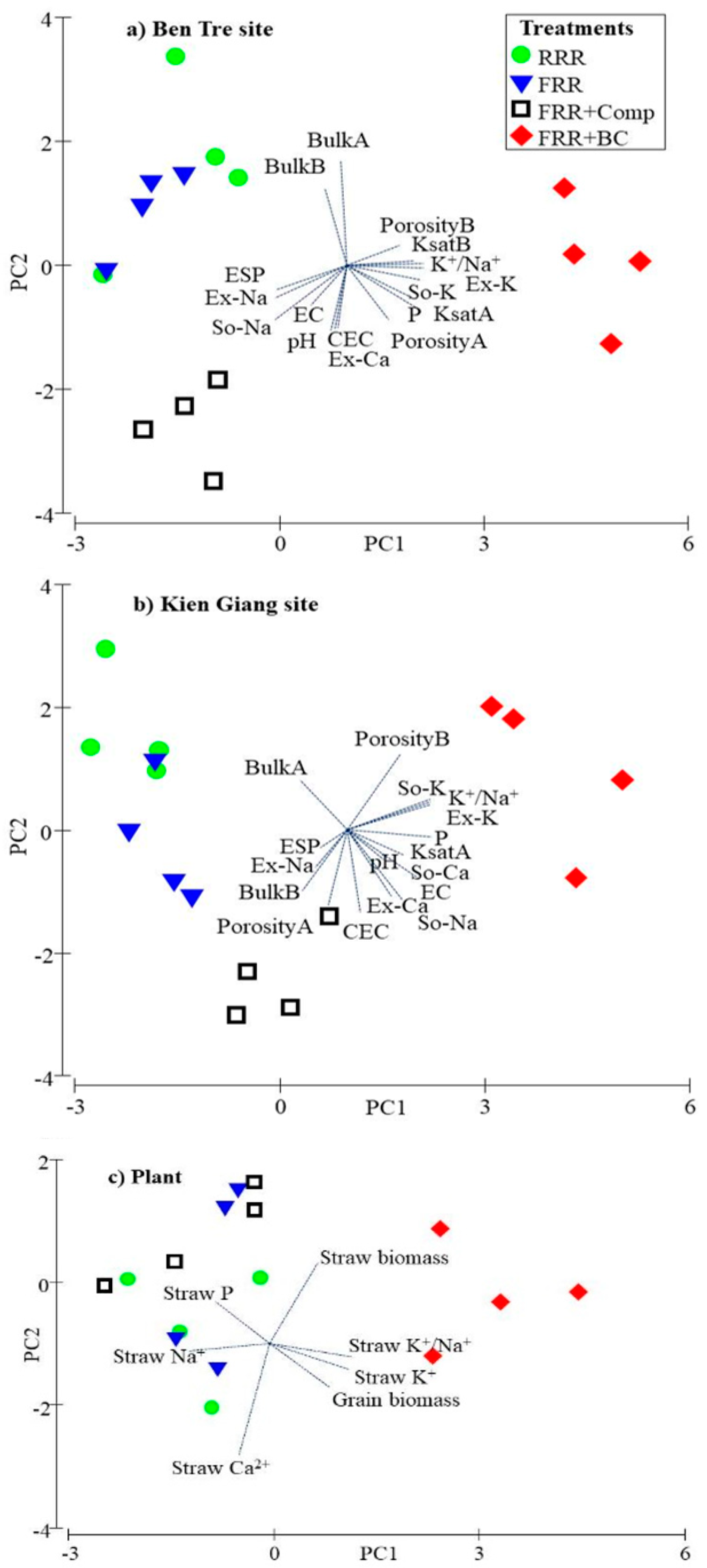
2.4. Data Analysis
3. Results
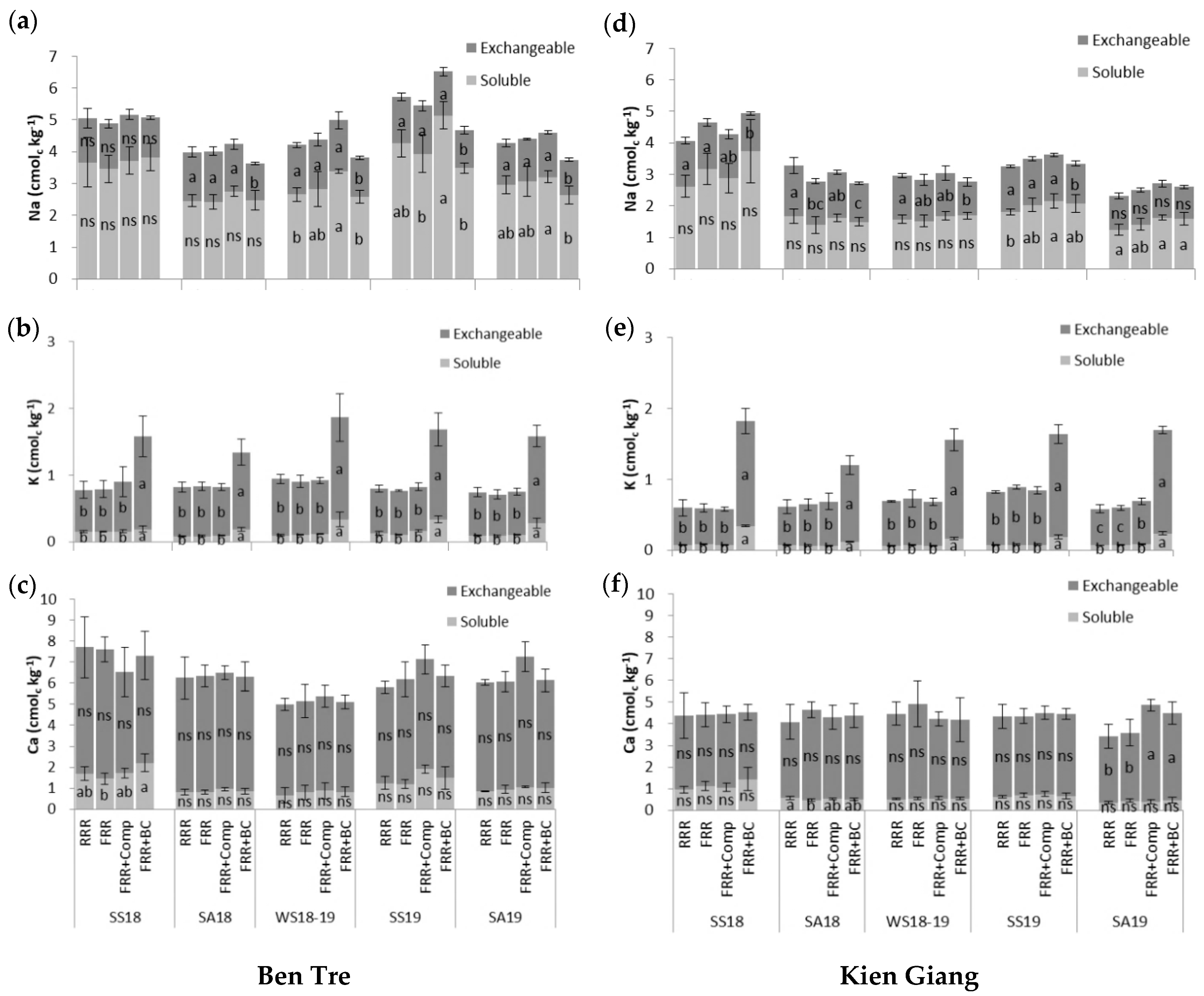
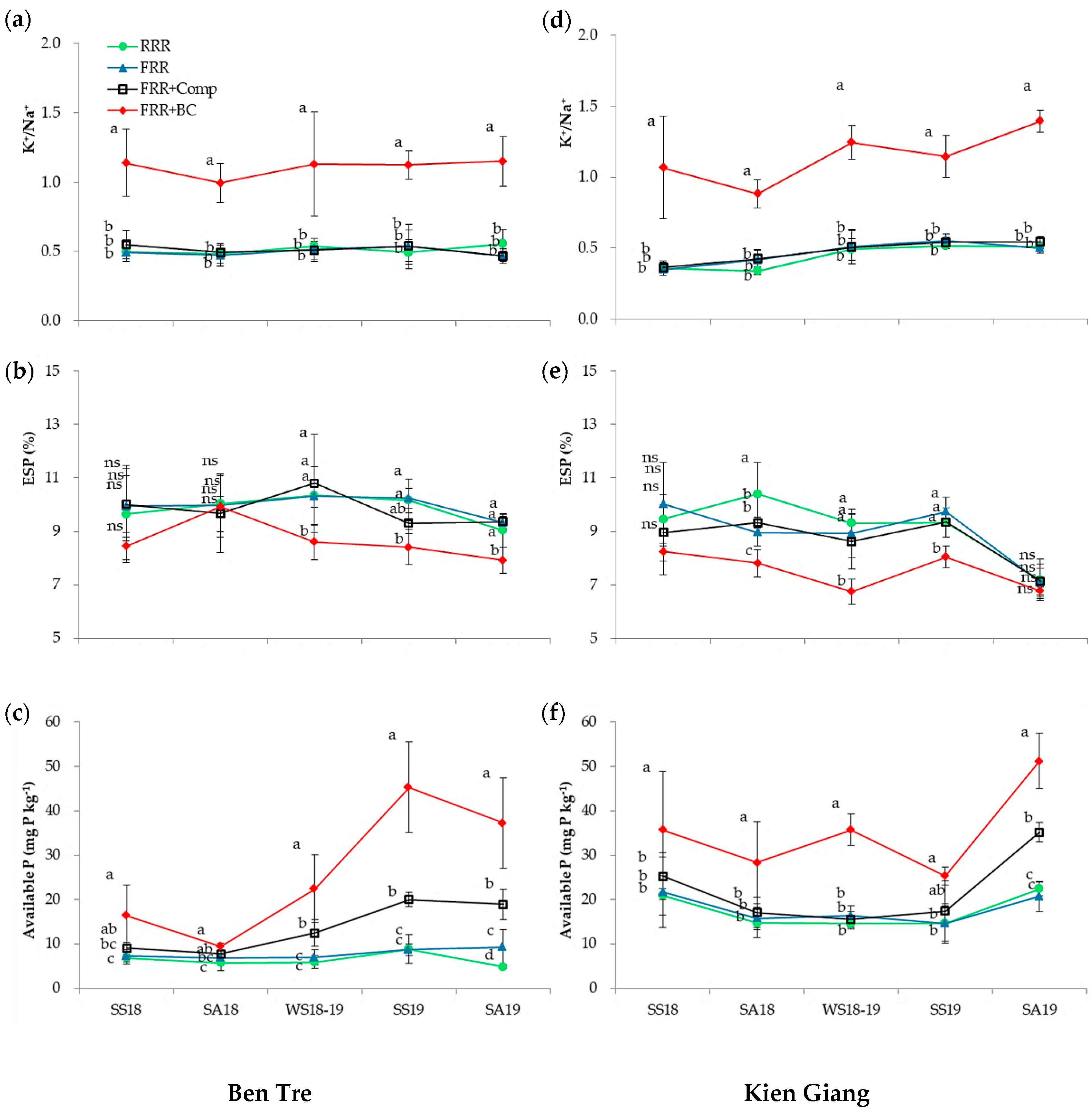
3.1. Nutritional and Chemical Properties of Experimental Soil
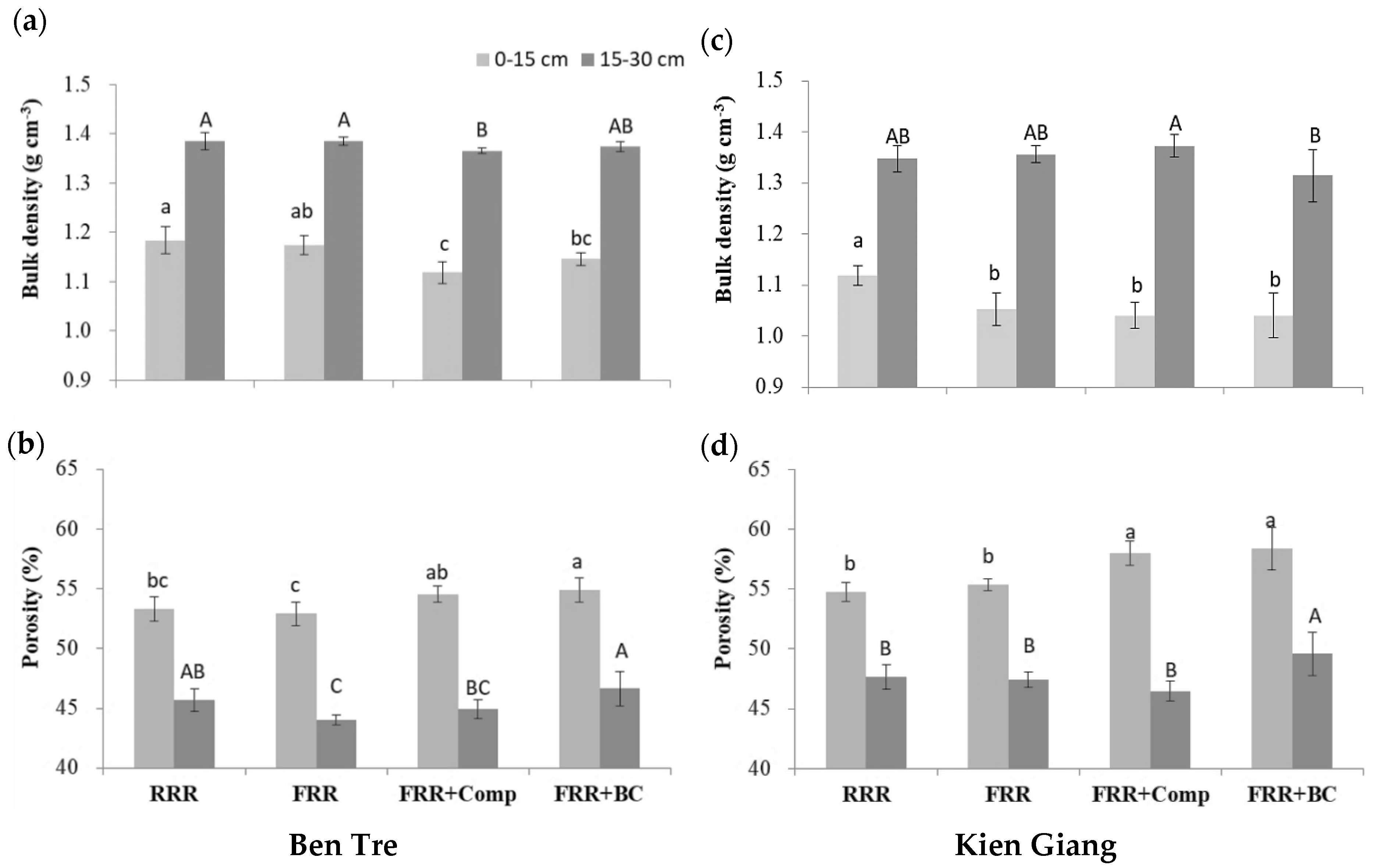
3.2. Physical Properties of Experimental Soil
3.3. Rice Growth and Plant Uptake
3.4. Relationship Between Plant Uptake and Examined Properties of the Soil
4. Discussion
4.1. Effects of Compost and Biochar on Soil Physicochemical Properties
4.2. Effects of Compost and Biochar Amendments on Plant Dry Biomass and Nutrient Concentrations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, S.K.; Goyal, M.R.; Singh, A. Engineering Practices for Management of Soil Salinity: Agricultural, Physiological, and Adaptive Approaches; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Doula, M.K.; Sarris, A. Chapter 4-Soil environment. In Environment and Development; Poulopoulos, S.G., Inglezakis, V.J., Eds.; Elsevier: Amsterdam, The Netherland, 2016; pp. 213–286. [Google Scholar]
- Abrol, I.; Yadav, J.S.P.; Massoud, F. Salt-Affected Soils and Their Management; Food & Agriculture Org.: Rome, Italy, 1988. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of salt-induced land degradation and restoration. Nat. Resour. Forum. 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- IPCC. Mitigation of Climate Change; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Raine, S.R.; Loch, R.J. What Is a Sodic Soil? Identification and Management Options for Construction Sites and Disturbed Lands; Workshop on soils in rural Queensland: Queensland, Australia, 2003. [Google Scholar]
- Sys, C.; Van Ranst, E.; Debaveye, J.; Beernaert, F. Land Evaluation Part III, Crop Requirements; G.A.D.C: Brussels, Belgium, 1993; p. 191. [Google Scholar]
- Khai, H.V.; Dang, N.H.; Yabe, M. Impact of salinity intrusion on rice productivity in the Vietnamese Mekong Delta. J. Fac. Agric. Kyushu Univ. 2018, 63, 143–148. [Google Scholar]
- Eynard, A.; Lal, R.; Wiebe, K. Crop response in salt-affected soils. J. Sustain. Agric. 2005, 27, 5–50. [Google Scholar] [CrossRef]
- Asch, F.; Wopereis, M.C.S. Responses of field-grown irrigated rice cultivars to varying levels of floodwater salinity in a semi-arid environment. Field Crops Res. 2001, 70, 127–137. [Google Scholar] [CrossRef]
- Chi, C.; Zhao, C.; Sun, X.; Wang, Z. Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma 2012, 187, 24–30. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259, 45–55. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- McConnell, D.B.; Shiralipour, A.; Smith, W.H. Compost application improves soil properties. Biocycle 1993, 34, 61–63. [Google Scholar]
- Rasool, R.; Kukal, S.S.; Hira, G.S. Soil physical fertility and crop performance as affected by long term application of FYM and inorganic fertilizers in rice–wheat system. Soil Till. Res. 2007, 96, 64–72. [Google Scholar] [CrossRef]
- Khaleel, R.; Reddy, K.R.; Overcash, M.R. Changes in soil physical properties due to organic waste applications: A review. J. Environ. Qual. 1981, 10, 133–141. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; García-Martínez, A.M.; Parrado, J. Effects of different green manures on soil biological properties and maize yield. Bioresour. Technol. 2008, 99, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Bui, E.; Krogh, L.; Lavado, R.; Nachtergaele, F.; Toth, P.; Fitzpatrick, R. Sodic soils. Distribution, properties, management and environmental consequences. In Sodic Soils, The World Scene; Oxford University Press: New York, NY, USA, 1998; pp. 19–34. [Google Scholar]
- Dabin, Z.; Zhiyuan, Y.; Jiao, C.; Pengwei, Y.; Na, Z.; Wenxiang, H.; Yangyang, L.; Suiqi, Z.; Bingnian, Z.; Zhaohui, W.; et al. Improving soil aggregation, aggregate-associated C and N, and enzyme activities by green manure crops in the Loess Plateau of China. Eur. J. Soil Sci. 2019, 70, 1267–1279. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Nikolic, M.; Peng, Y.; Chen, W.; Jiang, Y. Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol. Biochem. 2005, 37, 1185–1195. [Google Scholar] [CrossRef]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumari, S.; Chakraborty, A.; Gupta, A.; Chakrabarti, K.; Bandyapadhyay, B.K. Microbial biomass and its activities in salt-affected coastal soils. Biol. Fertility Soils 2006, 42, 273–277. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan Ltd: London, UK, 2009; p. 404. [Google Scholar]
- Chaganti, V.N.; Crohn, D.M.; Šimůnek, J. Leaching and reclamation of a biochar and compost amended saline–sodic soil with moderate SAR reclaimed water. Agric. Water Manag. 2015, 158, 255–265. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Van Sinh, N.; Chiem, N.H.; Toyota, K. Effects of rice husk biochar and calcium amendment on remediation of saline soil from rice-shrimp cropping system in vietnamese mekong delta. JEAI 2019, 39, 1–12. [Google Scholar] [CrossRef]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, Z.; Dugan, B. Biochar-induced changes in soil hydraulic conductivity and dissolved nutrient fluxes constrained by laboratory experiments. PLoS ONE 2014, 9, e108340. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, W.N.; Lin, Q.M.; Li, G.T.; Zhao, X.R. Improving salt leaching in a simulated saline soil column by three biochars derived from rice straw (Oryza sativa L.), sunflower straw (Helianthus annuus), and cow manure. J. Soil Water Conserv. 2016, 71, 467–475. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Shao, H.B. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Sappor, D.; Osei, B.; Ahmed, M. Reclaiming sodium affected soil: The potential of organic amendments. Inter. J. Plant Soil Sci. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Trinh, N.N.; Le, C.M.T.; Nguyen, T.T.; Tran, T.V.; Thai, B.V.; Le, T.V. The interactive effects of biochar and cow manure on rice growth and selected properties of salt-affected soil. Arch. Agron. Soil Sci. 2018, 64, 1744–1758. [Google Scholar] [CrossRef]
- Drake, J.A.; Cavagnaro, T.R.; Cunningham, S.C.; Jackson, W.R.; Patti, A.F. Does biochar improve establishment of tree seedlings in saline sodic soils? Land Degrad. Dev. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, K.-R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.-H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Thanh, N.K.; Tripathi, N.K.; Duan, H.D.; Gallardo, W.G. GIS–Based Planning for Sustainable Shrimp Farming in Thanh Phu, Ben Tre Province, Viet Nam; International Symposium on Geoinformatics for Spatial Infrastructure Development in Earth and Allied Sciences: Hanoi, Vietnam, 2008. [Google Scholar]
- Van Cuong, C.; Russell, M.; Brown, S.; Dart, P. Using shoreline video assessment for coastal planning and restoration in the context of climate change in Kien Giang, Vietnam. Ocean Sci. J 2015, 50, 413–432. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Sinh, N.V.; Tarao, M.; Toyota, K. Potential use of rice husk biochar and compost to improve P availability and reduce GHG emissions in acid sulfate soil. Agronomy 2020, 10, 685. [Google Scholar] [CrossRef]
- Dirksen, C. Soil Physics Measurements; Catena Verlag: Reiskirchen, Germany, 1999. [Google Scholar]
- Houba, V.J.G.; Vanderlee, J.J.; Novozamsky, I. Soil and plant analysis: A series of syllabi. In Part 5B Soil Analysis Procedures Other Procedures, 6th ed.; Department of Soil Science and Plant Nutrition, Wageningen Agricultural University: Wageningen, The Netherlands, 1995. [Google Scholar]
- Schaffers, A.P.; Sýkora, K.V. Reliability of ellenberg indicator values for moisture, nitrogen and soil reaction: A comparison with field measurements. J. Veg. Sci. 2000, 11, 225–244. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy, Agronomy Monographs: Madison, WI, USA, 1986; Volume 5, pp. 383–411. [Google Scholar]
- Grossman, R.; Reinsch, T. Bulk density and linear extensibility. In Methods of Soil Analysis. Part 4; Dane, J.H., Topp, G.C., Eds.; SSSA Book Series No. 5; Soil Science Society of America: Madison, WI, USA, 2002. [Google Scholar]
- Blake, G.; Hartge, K. Particle density. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986. [Google Scholar]
- ASTM. Standard test method for determination of iodine number of activated carbon. In ASTM Annual Book; ASTM International: West Conshohocken, PA, USA, 1999; Volume 4. [Google Scholar]
- Zhou, H.; Fang, H.; Zhang, Q.; Wang, Q.; Chen, C.; Mooney, S.J.; Peng, X.; Du, Z. Biochar enhances soil hydraulic function but not soil aggregation in a sandy loam. Eur. J. Soil Sci. 2019, 70, 291–300. [Google Scholar] [CrossRef]
- Hossain, M.; Rabbi, S.; Khanom, S.; Amin, M.; Islam, M.; Rahman, A. Effect of salinity and potassium fertilizer application on yield contributing characteristics of rice. Khulna Univ. Stud. 2010, 10, 145–152. [Google Scholar]
- Wakeel, A. Potassium–sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Hanay, A.; Büyüksönmez, F.; Kiziloglu, F.M.; Canbolat, M.Y. Reclamation of saline-sodic soils with gypsum and msw compost. Compost Sci. Util. 2004, 12, 175–179. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68. [Google Scholar] [CrossRef]
- Rostamian, R.; Heidarpour, M.; Mousavi, S.; Afyuni, M. Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J. Agric. Sci. Tech. 2015, 17, 1057–1069. [Google Scholar]
- Angın, D.; Şensöz, S. Effect of pyrolysis temperature on chemical and surface properties of biochar of rapeseed (Brassica napus L.). Int. J. Phytorem. 2014, 16, 684–693. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Miyanishi, T.; Okayama, T.; Kose, R. Pore characteristics & adsorption capacities of biochars derived from rice residues as affected by variety and pyrolysis temperature. Am. J. Innov. Res. Appl. Sci. 2016, 2, 336–346. [Google Scholar]
- Claoston, N.; Samsuri, A.; Ahmad Husni, M.; Mohd Amran, M. Effects of pyrolysis temperature on the physicochemical properties of empty fruit bunch and rice husk biochars. Waste Manag. Res. 2014, 32, 331–339. [Google Scholar] [CrossRef]
- Oladele, S.O. Changes in physicochemical properties and quality index of an alfisol after three years of rice husk biochar amendment in rainfed rice–maize cropping sequence. Geoderma 2019, 353, 359–371. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of hkt transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138. [Google Scholar] [CrossRef]
- Maathuis, F.J.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamada, S.; Masunaga, T.; Yamamoto, S.; Tsuji, W.; Murillo-Amador, B. Comparison of nutrient uptake and antioxidative response among four labiatae herb species under salt stress condition. Soil Sci. Plant Nutr. 2018, 64, 589–597. [Google Scholar] [CrossRef]
- Igartua, E.; Gracia, M.P.; Lasa, J.M. Field responses of grain sorghum to a salinity gradient. Field Crops Res. 1995, 42, 15–25. [Google Scholar] [CrossRef]
- Sharma, S.K. Effects of salinity on uptake and distribution of Na+, Cl- and K+ in two wheat cultivars. Biol. Plant. 1996, 38, 261. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, L.; Zheng, J.; Qu, J.; Zheng, J.; Zhang, X.; Pan, G. Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, F.; Li, J.; Duan, Z.; Yang, F.; Li, X.; Lu, J.; Li, F. Effects of maize straw-derived biochar application on soil temperature, water conditions and growth of winter wheat. Eur. J. Soil Sci. 2019, 70, 1280–1289. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Ventura, M.; Zhang, C.; Baldi, E.; Fornasier, F.; Sorrenti, G.; Panzacchi, P.; Tonon, G. Effect of biochar addition on soil respiration partitioning and root dynamics in an apple orchard. Eur. J. Soil Sci. 2014, 65, 186–195. [Google Scholar] [CrossRef]




| Compost * | Biochar * | Ben Tre | Kien Giang | |
|---|---|---|---|---|
| Sand (%) | 1.93 | 0.78 | ||
| Silt (%) | 56.2 | 42.0 | ||
| Clay (%) | 41.8 | 57.2 | ||
| Soil texture | Silty clay | Silty clay | ||
| pHw | 8.7 a | 7.7 a | 4.64 b | 4.65 b |
| EC (mS cm−1) | 17.1 c | 4.1 c | 1.24 d | 1.16 d |
| ECe (mS cm−1) | 4.50 | 4.10 | ||
| Total C (g kg−1) | 154 | 471 | 15.7 | 15.0 |
| Total N (g kg−1) | 26 | 4.72 | 1.55 | 1.32 |
| CEC (cmolc kg−1) | 66 | 6.5 | 14.6 | 15.4 |
| Soluble Na (cmolc kg−1) | 1.59 | 0.24 | 3.67 | 2.62 |
| Soluble K (cmolc kg−1) | 20 | 3.35 | 0.15 | 0.07 |
| Soluble Ca (cmolc kg−1) | 7.29 | 0.15 | 1.70 | 0.96 |
| Exchangeable Na (cmolc kg−1) | 0.6 | 0.24 | 1.38 | 1.21 |
| Exchangeable K (cmolc kg−1) | 15 | 12.9 | 0.63 | 0.52 |
| Exchangeable Ca (cmolc kg−1) | 61.6 | 0.16 | 6.01 | 3.44 |
| ESP (%) | 9.64 | 8.00 | ||
| Available P (mg kg−1) | 3600 | 800 | 6.91 | 20.9 |
| Total porosity (%) | 76.2 | 92.3 | 53.4 | 56.4 |
| Df | pH | EC | Avail-P | So-Na | So-K | So-Ca | Ex-Na | Ex-K | Ex-Ca | CEC | ESP | K+/Na+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ben Tre | |||||||||||||
| T | 3 | * | *** | *** | *** | *** | ** | *** | *** | ns | *** | *** | *** |
| S | 4 | *** | *** | *** | *** | *** | *** | ** | ** | *** | *** | ** | ns |
| T x S | 12 | ns | ** | *** | ** | *** | ns | ns | ns | ns | ns | ns | ns |
| Kien Giang | |||||||||||||
| T | 3 | *** | ** | *** | ** | *** | ns | *** | *** | ns | ns | *** | *** |
| S | 4 | *** | *** | *** | *** | *** | *** | *** | *** | ns | ns | *** | *** |
| T x S | 12 | * | * | ** | ** | *** | * | ns | *** | ns | ns | * | ns |
| Treatment | Straw (Oven Dry) | Grain (Oven Dry) | Total Aboveground Biomass |
|---|---|---|---|
| SS18 | |||
| RRR | NA | 0 | NA |
| FRR | - | - | - |
| FRR + Comp | - | - | - |
| FRR + BC | - | - | - |
| SA18 | |||
| RRR | NA | 2.76 ns | NA |
| FRR | NA | 2.44 ns | NA |
| FRR + Comp | NA | 3.06 ns | NA |
| FRR + BC | NA | 2.40 ns | NA |
| WS18–19 | |||
| RRR | 6.32 b | 3.15 ns | 9.47 b |
| FRR | 7.10 ab | 3.16 ns | 10.3 ab |
| FRR + Comp | 7.71 a | 3.21 ns | 11.0 a |
| FRR + BC | 7.67 a | 2.92 ns | 10.5 ab |
| SS19 | |||
| RRR | NA | 0 | NA |
| FRR | - | - | - |
| FRR + Comp | - | - | - |
| FRR + BC | - | - | - |
| SA19 | |||
| RRR | 5.59 b | 2.79 ns | 8.57 b |
| FRR | 5.80 b | 2.40 ns | 8.76 ab |
| FRR + Comp | 6.16 ab | 2.48 ns | 8.64 ab |
| FRR + BC | 6.41 a | 3.23 ns | 9.64 a |
| Treatment | WS18–19 | SA19 | ||
|---|---|---|---|---|
| Straw | Grain | Straw | Grain | |
| Total Na (mmol kg−1) | ||||
| RRR | 232 a | 4.86 ns | 448 a | NA |
| FRR | 257 a | 4.84 ns | 404 a | NA |
| FRR + Comp | 262 a | 4.72 ns | 409 a | NA |
| FRR + BC | 146 b | 5.77 ns | 268 b | NA |
| Total K (mmol kg−1) | ||||
| RRR | 436 b | 87.8 ns | 392 b | NA |
| FRR | 433 b | 88.3 ns | 394 b | NA |
| FRR + Comp | 381 c | 83.5 ns | 383 b | NA |
| FRR + BC | 618 a | 75.0 ns | 540 a | NA |
| Total Ca (mmol kg−1) | ||||
| RRR | 61.3 ns | 6.50 ns | 87.5 ns | NA |
| FRR | 63.9 ns | 6.46 ns | 84.3 ns | NA |
| FRR + Comp | 69.3 ns | 6.05 ns | 82.3 ns | NA |
| FRR + BC | 66.3 ns | 7.39 ns | 80.7 ns | NA |
| K+/Na+ | ||||
| RRR | 1.92 b | 16.6 ns | 0.91 b | NA |
| FRR | 1.79 b | 18.3 ns | 0.95 b | NA |
| FRR + Comp | 1.54 b | 15.5 ns | 0.94 b | NA |
| FRR + BC | 4.40 a | 16.0 ns | 1.99 a | NA |
| Total P (g P2O5 kg−1) | ||||
| RRR | 3.28 ab | 10.7 ns | 4.64 ns | NA |
| FRR | 2.78 b | 10.6 ns | 4.54 ns | NA |
| FRR + Comp | 3.39 a | 10.5 ns | 5.18 ns | NA |
| FRR + BC | 3.43 a | 10.6 ns | 4.94 ns | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Linh, T.B.; Minh, D.D.; Duc, T.A.; Sinh, N.V.; Linh, T.T.; Toyota, K. Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields. Agronomy 2020, 10, 1101. https://doi.org/10.3390/agronomy10081101
Phuong NTK, Khoi CM, Ritz K, Linh TB, Minh DD, Duc TA, Sinh NV, Linh TT, Toyota K. Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields. Agronomy. 2020; 10(8):1101. https://doi.org/10.3390/agronomy10081101
Chicago/Turabian StylePhuong, Nguyen Thi Kim, Chau Minh Khoi, Karl Ritz, Tran Ba Linh, Dang Duy Minh, Tran Anh Duc, Nguyen Van Sinh, Thi Tu Linh, and Koki Toyota. 2020. "Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields" Agronomy 10, no. 8: 1101. https://doi.org/10.3390/agronomy10081101
APA StylePhuong, N. T. K., Khoi, C. M., Ritz, K., Linh, T. B., Minh, D. D., Duc, T. A., Sinh, N. V., Linh, T. T., & Toyota, K. (2020). Influence of Rice Husk Biochar and Compost Amendments on Salt Contents and Hydraulic Properties of Soil and Rice Yield in Salt-Affected Fields. Agronomy, 10(8), 1101. https://doi.org/10.3390/agronomy10081101






