Abstract
The sustainable management of weeds is one of the main challenges in agriculture. Recent studies have demonstrated the potential of plant phytotoxins, such as ailanthone from Ailanthus altissima (Mill) Swingle, as bioherbicides. Since a complex extract may be more active than a single compound, we explored the phytotoxicity of A. altissima extracts obtained from the leaves, samaras, rachises, and secondary roots, and we evaluated their application potential for weed control in horticulture. The pre-emergence activity of all plant extracts was evaluated over varying concentrations on two indicator species (i.e., Lepidium sativum L. and Raphanus sativus L.) under controlled conditions. As the leaf extract was able to be generated in sufficient quantities, it was therefore further evaluated in glasshouse experiments with seven common weed species as indicators, as well as in a nursery production system for the cultivation of three horticultural crops (i.e., Salvia officinalis L., S. rosmarinus Schleid., and Dianthus caryophyllus L.). Following the application of the extract, the index of germination (IGe%), the index of biomass, and the density of weeds per pot were evaluated, along with the impact on crop growth and quality (i.e., plant growth index and leaf damage). Under controlled conditions, the extract from the secondary root was the most active in reducing the IGe%, with greater persistence across time in both indicator species. At 18 days following application, the lowest concentration of the leaf extract at 1.8 mg L−1 ailanthone reduced the IGe%by up to 15% and 45% in R. sativus and L. sativum, respectively. In R. sativus, all of the extract types affected the IGe%, but extract activity was greater in L. sativum. Under glasshouse conditions, leaf extracts containing 50 and 200 mg L−1 ailanthone showed strong inhibition (98%–99%) in the biomass of all treated indicator and weed species. Under nursery conditions, leaf extracts formulated at 100 and 200 mg L−1 ailanthone performed similarly, and no weeds were observed in any of the treated pots of S. officinalis and S. rosmarinus in the 60-day study period. Conversely, in the D. caryophyllus pots, an increase in the percentage of weed presence per pot was observed after 40 days. A reduction in the growth index and an increase in leaf phytotoxicity were observed during the cultivation experimentation, especially in S. officinalis when the extract was applied post-emergence to the crop canopy. Phytotoxicity was alleviated by the application of the extract directly to the soil or growth media. These results provide new insights into A. altissima extracts and their phytotoxicity to support their additional use as a sustainable solution for weed management in horticultural crops.
1. Introduction
Weeds reduce crop yield by competing for nutrients, light, and moisture [1]. Sustainable weed management is one of the main challenges for both conventional and organic horticulture [2]. Current weed control in horticultural production includes synthetic herbicides and physical methods [3].
Recently, bioherbicides have been developed from allelochemicals [4]. Allelochemicals, such as saponins, tannins, flavonoids, terpenoids, and lactones, are plant secondary metabolites that can be found in different plant organs, such as in the leaves, stems, roots, seeds, fruits, flowers, and pollen [5,6]. These molecules are produced by plants and are released in the surrounding environment to affect the growth, development, survival, and reproduction of neighboring plants, directly or indirectly; this phenomenon is known as allelopathy [7,8]. Their use is of interest in weed management as they are biodegradable, have structural complexity, and a wide variety of sites of action. Additionally, they are not halogenated and are generally safer for non-target organisms [9,10,11,12]. For this purpose, several studies have aimed to identify species with phytotoxic activity [13]. Rice (Oryza sativa L.) hull extracts inhibit Echinochloa crus-galli (L.) Beauv. germination, seedling growth, and weight [14], and plant extracts of Everniastrum sorocheilum (Vain.) Hale ex Sipman, Usnea roccellina Motyka, and Cladonia confusa R. Sant. inhibit the germination and root growth of Trifolium pratense L. [15]. In addition, seed extracts from Sicyos angulatus L. inhibit the germination of Lactuca sativa L. [16].
Ailanthus altissima (Mill.) Swingle is a perennial invasive species known to cause major negative impacts on human activities, especially in urban areas. Since 1959, it has been known that A. altissima produces phytotoxic compounds and that the major identified toxin is in the quassinoid ailanthone (Ail), firstly isolated by Heisey [17] from bark and foliage. Quassinoids are highly oxygenated triterpenes, which have been isolated as bitter principles from the plants of the Simaroubaceae family. Their synthesis has attracted much attention because of the wide spectrum of their biological properties. The extracts obtained from different parts of A. altissima have been reported to exhibit diverse biological activities, such as antiproliferative, central nervous depressant, antimicrobial, and antioxidant activities [18,19,20,21,22,23,24]. Ail phytotoxic activity has been observed both in the pre-emergence and the post-emergence stages of several species, showing a broad spectrum of herbicidal effects on monocots and dicots under controlled conditions [25,26]. More recently, Demasi et al. [12,27] tested Ail in a growth chamber and controlled cultivation conditions, highlighting its extremely efficient phytotoxicity on the seed germination of Lepidium sativum L. “Inglese” and Raphanus sativus L. “Tondo Rosso BIO”.
Nevertheless, as well as other natural compounds, several constraints have so far prevented the commercial development of natural Ail-based herbicides [24,28,29,30,31,32,33]. The main ailanthone drawbacks are its high extraction and purification costs and its short-term efficacy in soil, demonstrated both in greenhouses [24,34] and fields [25]. As emerges from the literature, ailanthone is not the only molecule with phytotoxic activity present in the tissues of A. altissima, but it acts better in synergy with other compounds that differ on the basis of the used tissue [35].
Thus, this study aimed to provide new insights into the phytotoxicity of A. altissima extracts obtained from the leaves, samaras, rachises, and secondary roots in pre-emergence and to assess their application potential for weed control in horticulture. Four extracts at different dilutions were evaluated by a combination of in vitro and in vivo bioassays on two indicator species (i.e., Lepidium sativum L. and Raphanus sativus L.), seven common weeds (i.e., Achillea millefolium L., Centaurea cyanus L., Matricaria chamomilla L., Portulaca oleracea L., Stellaria media (L.) Vill., Digitaria sanguinalis (L.) Scop., and Veronica persica Poir.), and during the cultivation of three horticulture crops (i.e., Salvia officinalis L., Salvia rosmarinus (L.) Schleid., and Dianthus caryophyllus L.).
2. Materials and Methods
2.1. Plant Material
Mature leaves, samaras, rachises, and secondary roots of A. altissima were collected during the period August–September 2016 from the campus of the Department of Agricultural, Forest and Food Sciences (DISAFA) of the University of Torino (Italy; 45°06′23.21″ N Lat, 7°57′82.83″ E Long; 300 m above sea level). Plant materials were oven-dried at 70 °C until constant weight and stored at room temperature in glass jars until the preparation of extracts and their use in the experiments, as summarized in Table 1 and Table 2.

Table 1.
Summary of the bioassays performed to evaluate the phytotoxic activity of Ailanthus altissima extracts.

Table 2.
List and characteristics of the plants used in the bioassays.
2.2. Extraction and Ailanthone Quantification
2.2.1. Extraction
First, 50 g of the mature leaves, samaras, rachises, or secondary roots of A. altissima was pulverized using a food chopper and then stirred for 24 h at room temperature in a 250 mL deionized water/methanol 50:50 (v/v) solution. After filtration, the insoluble fraction was recovered and added to a fresh deionized water/methanol solution, and then extracted a second time, as described above. The water/methanol solutions were then extracted three times with dichloromethane (DCM) in a separatory funnel. Subsequently, the DCM was removed in a rotatory evaporator, and a viscous oily sample adhering to the surface of the flask was obtained. This sample was added to approximately 20 mL deionized water and stirred at room temperature for a few minutes. Then, the aqueous fraction was recovered and replaced with a fresh amount of deionized water. The step described above was repeated three times. Finally, the three aqueous solutions were combined and freeze-dried. By the end, four powder extracts were obtained from the mature leaves, samaras, rachises, and secondary roots of A. altissima, which were stored at −20 °C.
2.2.2. Quantification
The content of ailanthone in each extract was determined by High Performance Liquid Chromatography (HPLC) analysis. Specifically, 0.5 mg of the extract was dissolved in 1.00 mL ultrapure water/methanol 75:25 (v/v) solution, filtered with a polytetrafluoroethylene (PTFE) 0.2 μm syringe disc filter, and then injected into a PerkingElmer HPLC apparatus, equipped with a Flexar isocratic pump, flushing at 1 mL min−1 and a Flexar UV detector set at 254 nm. The column used for the HPLC analysis was a PerkinElmer C18 (250 × 4.6 mm, 5 μm), while the composition of the mobile phase was ultrapure water/methanol 75:25 (v/v). The total analysis time was 14 min and the retention time of the ailanthone was approximately 7 min. The quantification of ailanthone was carried out using an external calibration curve (R2 = 0.9999) obtained with eight ailanthone standards (1, 2.5, 5, 7.5, 10, 25, 50, 75, and 100 μg mL−1). All standards were prepared in the mobile phase by dilution using a 1000 μg mL−1 stock solution.
2.3. Growth Chamber Assay
The phytotoxicity of the leaf, samara, rachis, and secondary root extracts was evaluated at the Dept. of Agricultural, Forest and Food Sciences (DISAFA) laboratory, through in vitro seed germination tests, as shown in Table 1 and Table 2, using the indicator plant species L. sativum “Inglese” and R. sativus “Tondo rosso” (Fratelli Ingegnoli Spa, Milano, Italy). Based on the quantification of the concentration of ailanthone in each extract, dilutions of the extracts with deionized water were performed to obtain solutions containing 1.8, 11.5, 25, and 50 mg L−1 Ail.
Ten seeds of each species were placed on two layers of filter paper (Whatman No.1) in a petri plate (9 cm diameter) and treated with 5 mL of each dosage, with ten replicates for a total of 100 seeds per dosage in each extract. The germination test took place in a growth chamber at 25 °C for 72 h in darkness. Seeds moistened with 5 mL deionized water were used as control. The persistence of the phytotoxic activity of the extracts across time was evaluated by removing seeds and/or developed seedlings every 72 h and by replacing them with new seeds without any more treatment; only deionized water was added to prevent dryness. This procedure was carried out six times for a total of 18 days (3, 6, 9, 12, 15, and 18 days after treatment (DAT)). The trial was repeated three times. Every 72 h, the Index of germination (IGe%) was calculated as follows, according to Demasi et al. [27]:
where n is the number of germinated seeds and r is the mean root length in the treated sample (s) and the control (c).
IGe% = (ns × rs/nc × rc) × 100
2.4. Greenhouse Assay
Leaf extract was diluted with deionized water to obtain dosages of 50 and 200 mg L−1 Ail. One hundred seeds from two indicator species and seven weeds, as shown in Table 2, were sown in plastic seed pot trays (five seeds per pot) of 5 cm in diameter and 5 cm in height, filled with a volume of 250 mL growth substrate (Floragard®, Germany), and treated with 1.7 mL of the solutions. Seeds moistened with deionized water were used as the control. A randomized block experiment was conducted with four replications (25 seeds each). The plastic seed pot trays were placed in a heated greenhouse of the DISAFA, where they were regularly watered, for a total of 30 days (30 DAT). At the end of the experiment, the number of germinated seeds was counted, and the aerial parts of the seedlings were cut, oven-dried at 70 °C for one week, and the dry biomass was then weighed. The trial was repeated twice. The index of biomass (IBi%) was calculated as follows:
where n is the number of germinated seeds and b is the mean dry weight biomass in the treated sample (s) and the control (c).
IBi% = (ns × bs/nc × bc) × 100
2.5. Nursery Assays
The Ailanthus altissima leaf extract was diluted with deionized water to reach dosages of 100 and 200 mg L−1 Ail, which were then used in the experiments conducted in the nursery Azienda Agricola di Paolo Damonte (Albenga, Italy).
Two-month-old clonally propagated plants of S. rosmarinus, S. officinalis, and D. caryophyllus were open cultivated in plastic pots (12 cm in diameter, 1.2 L), containing cultivation substrate (TS1 Turco Silvestro, Albenga, Italy) and Agriperlite® (80:20). Irrigation was provided when needed by a sprinkler system.
In experiment 1 (March–April 2018), the 7.5 mL pot−1 of each dosage was sprayed by a commercial sprayer (Eva Professional 2L, Di Martino s.p.a., Mussolente, VI, Italy) once (0 DAT) on 90 plants of each species. In experiment 2 (May–June 2018), the 7.5 mL pot−1 of each dosage was sprayed once (0 DAT) on 90 plants, only in the S. officinalis pots, directly on the substrate surface, covering the plant canopy.
In both experiments, untreated water for the irrigation of the plants was used as a control. The pots were arranged in a randomized block design, with three replicates for each treatment. Experiments were terminated after 60 DAT. Every 20 days (i.e., 20, 40, and 60 DAT), the percentage of weed presence per pot (calculated as the ratio between the number of pots with the presence of at least one weed and the total number of pots) and the density of weeds (calculated as the ratio between the total number of weeds and the total number of pots) were measured. Every 20 days (i.e., 20, 40, and 60 DAT), in the nursery assay, the height and diameter of each plant at the canopy level per treatment were measured to calculate the growth index (GI) as an increment in size per unit of time. The growth index (GI) was calculated as follow:
where D′ is the width, D″ is the perpendicular value of D′, and H is the height [36]. In the same experiment, the phytotoxicity was assessed (i.e., necrosis and chlorosis) using a visual rating scale of 0–4 (0 = 0%; 1 = 1%−25%; 2 = 26%−50%; 3 = 51%−75%; 4 = 76%−100%) for the leaf area, transformed to percentage (%) of leaf damage, as reported by Caser et al. [37].
GI = (Π × {[(D′+D″)/2]/2}2 × H; cm3)
2.6. Data Analysis
Arcsin transformation was performed on all percent incidence data before statistical analysis in order to improve the homogeneity of variance (using the Levene test). For all parameters, mean differences were computed using a one-way and univariate ANOVA with the Tukey post-hoc test (p ≤ 0.05), and pairwise comparisons were done using the Student’s t-test. All analyses were performed with SPSS 24.0 Inc. software (Chicago, IL, USA).
3. Results
3.1. Extraction and Ailanthone Quantification
Separate extracts were obtained from the mature leaves, samaras, rachises, and secondary roots of A. altissima using a multistep extraction procedure. To optimize the uniformity of the extraction and the extraction efficiency, all freeze-dried samples were dissolved in the same amount of deionized water. HPLC analysis revealed contents of ailanthone of approximately 44, 176, 275, and 387 μg Ml−1 in the extracts from the leaves, samaras, rachises, and secondary roots, respectively.
3.2. Herbicidal Activity of A. altissima Extracts in the Growth Chamber
Ailanthus altissima extracts showed the strong phytotoxicity and growth inhibition of R. sativus and L. sativum seeds, with R. sativus being significantly less sensitive, as shown in Table 3, Table 4 and Table 5.

Table 3.
Effect of the extract type, dosage, and days after treatment (DAT) on the index of germination (IGe%) of Raphanus sativus and Lepidium sativum seeds in the growth chamber assay.

Table 4.
Effect of the extract type, dosage, and days after treatment on the persistence across time and on the index of germination (IGe%) of Raphanus sativus seeds in the growth chamber bioassay.

Table 5.
Effect of the extract type, dosage, and days after treatment on the persistence across time and on the index of germination (IGe%) of Lepidium sativum seeds in the growth chamber bioassay.
Differences in phytotoxicity were highlighted among the extract types. Secondary root extracts were the most inhibitory, as assessed by IGe% (2.9%), with greater inhibition observed over time in both species. The nearly complete inhibition of seedling growth, as assessed by the radicle length, was obtained at 1.8 mg L−1 of ailanthone (IGe% = 1.85%–14.84% and 2.56%–44.70% in R. sativus and L. sativum, respectively). Phytotoxicity was stable throughout the experimental period (i.e., 18 DAT) in R. sativus, shown in Table 4 and for up to 15 DAT in L. sativum, shown in Table 5.
A similar pattern was also observed by applying the samara and rachis extracts, although with lower efficacy. Both extracts reduced IGe%, already at the lowest concentration, to 15 DAT in R. sativus (IGe = 30.19% with the samara extract and IGe = 42.01% with the rachis extract), as shown in Table 4, and up to 6 DAT in L. sativum (39.06% and 24.56%, respectively), as shown in Table 5. The most effective and persistent concentration for the samara and rachis extracts was 11.5 mg L−1 Ail, with IGe% values ranging between 0% and 2.43% and 0% and 1.43% in R. sativus, and between 0% and 10.71% and 0% and 5.07% in L. sativum, respectively. The leaf extract was the least effective in reducing the studied parameters, as shown in Table 3. At lower concentrations, it was effective in reducing IGe% up to 9 DAT in R. sativus (IGe% values ranging between 15.94% and 37.91%) and up to 6 DAT in L. sativum (IGe% values ranging between 31.78% and 35.51%). Differences were also observed among DATs with IGe% gradually increasing from 2.5% at 3 DAT to 29.8% at 18 DAT.
3.3. Effects of Leaf Extract Under Greenhouse Condition
The leaf extract at the concentrations of 50 and 200 mg L−1 Ail eq. showed a clear inhibitory effect on the index of biomass (IBi%) of all the treated model and weed species, as shown in Figure 1.
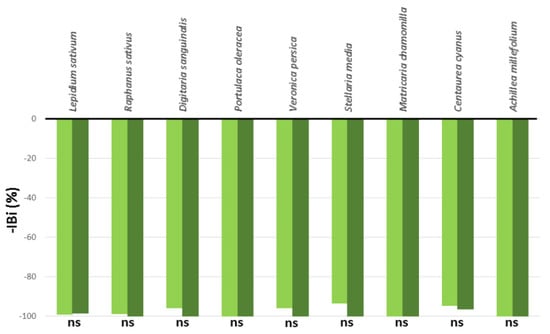
Figure 1.
Phytotoxic effects of Ailanthus altissima leaf extracts at 50 (light green bars) and 200 (dark green bars) mg L−1 Ail equivalent on the reduction in the index of biomass (–IBi%) of Lepidium sativum, Raphanus sativus, Digitaria sanguinalis, Portulaca oleracea, Veronica persica, Stellaria media, Matricaria chamomilla, Centaurea cyanus, and Achillea millefolium seeds, cultivated under greenhouse conditions. Pairwise comparisons were done using a Student’s t-test (p < 0.05; ns = not significant). The black line at 0% indicates the IBi% of the control plants.
On average, the IBi% values were reduced to circa −97.47% and −99.41% by applying 50 and 200 mg L−1 Ail, respectively, in comparison to the untreated seeds. No differences between concentrations were observed in any species.
3.4. Effect of the Leaf Extract on Weeds, Salvia officinalis, Salvia rosmarinus, and Dianthus caryophyllus under Pot Cultivation in the Nursery
3.4.1. Experiment 1
Considering the onset of the weeds, the main species detected during both of the nursery trials were Sonchus oleraceus L., Galium mollugo L., Sagina procumbens L., Erigeron annuus (L.) Pers., and Capsella bursa-pastoris (L.) Medik.
In S. officinalis and S. rosmarinus cultivation, the leaf extracts at 100 and 200 mg L−1 Ail performed similarly and no weeds were observed in any of the treated pots during the 60-day experimental period, as shown in Table 6. Conversely, weeds appeared at 60 DAT in the control pots of both species, with 13% of the infested pots belonging to S. officinalis and 1.5% to S. rosmarinus with weed densities of 1.7 and 1.5, respectively. In the D. caryophyllus pots, weeds had already appeared at 40 DAT, with differences among the treatments and the control up to the end of the experiment (i.e., 60 DAT).

Table 6.
Phytotoxic effects and persistence across time (0, 20, 40, and 60 DAT) of Ailanthus altissima leaf extracts (100 and 200 mg L−1 Ail) on the percentage of weed presence per pot (%), weed density (n pot−1), leaf damage (%), and growth index (cm3) of Salvia officinalis, Salvia rosmarinus, and Dianthus caryophyllus in pot cultivation during experiment 1 in nursery cultivation.
The percentage of weed presence per pot was highest in the untreated pots (43.3% at 40 DAT and 60.8% at 60 DAT) and lowest in pots treated with 100 mg L−1 Ail (10.3% at 40 DAT and 20.0% at 60 DAT). Similar results were observed in weed density, equal to 3.1, in the untreated pots and 1.8 and 1.2 in the pots treated with 100 and 200 mg L−1 Ail, respectively.
The side effects of the applied leaf extracts on the crops were mainly related to the reduction in the growth index and the increase in leaf damage during cultivation, as shown in Table 6. The species most affected by the treatments was S. officinalis. At 20 DAT, S. officinalis plants, treated with both concentrations of leaf extract, showed leaf damage that exceeded 97% and reached complete plant senescence at 40 DAT. At the end of the experiment, lower leaf damage was observed only in plants treated with 100 mg L−1 Ail (93.7%), with the subsequent slight recovery of vegetative growth (165 cm3). This species also showed leaf damage in the control plants (50.5% after 20 DAT). This damage was then reduced during the experiment, reaching a value of circa 20% at 60 DAT. However, the plants continued to grow, reaching 1673 cm3 at 60 DAT. In S. rosmarinus, at 20 DAT, the treatment at 100 mg L−1 Ail caused leaf damage of 69.3%, while the treatment at 200 mg L−1 Ail caused damage of 96.1%. At 40 DAT, the percentages rose to 86.1% and 100%, respectively. At the end of the experiment, a reduction in leaf damage and a recovery of vegetative growth were observed (GI = 185 cm3), with the exception of the plants treated with the highest concentration. Among the three treated species, D. caryophyllus suffered less damages. On the whole, this species showed leaf damage only at 20 DAT (2.5% and 6.6% at 100 and 200 mg L−1 Ail, respectively). During the entire experiment, no significant differences in leaf damage and the growth index were observed.
3.4.2. Experiment 2
The effects on the emergence of weeds of A. altissima leaf extracts applied directly on the substrate surface in S. officinalis cultivation are shown in Table 7.

Table 7.
Phytotoxic effects and persistence across time (0, 20, 40, and 60 DAT) of Ailanthus altissima leaf extracts (100 and 200 mg L−1 Ail) on the percentage of weed presence per pot (%), weed density (n pot−1), and growth index (cm3) of Salvia officinalis in pot cultivation during experiment 2 in nursery cultivation.
At 20 DAT, the percentage of weed presence per pot ranged between 36.1% and 43.1%, regardless of the treatment. At 40 DAT, the treated pots showed less weeds (16.7% and 18.1% weed presence per pot for 100 and 200 mg L−1 Ail eq., respectively) than the control (44.0% weed presence per pot). At 60 DAT, no differences between the control (88.9%) and the extract at 100 mg L−1 Ail eq. (76.4%) were observed. Conversely, a significant reduction was imposed by the extract at 200 mg L−1 Ail, with 48.6% weed presence per pot. Concerning the weed density, the leaf extract treatments induced a reduction in the number of weeds per pot by circa 50% (in 100 mg L−1 Ail) and 70% (in 200 mg L−1 Ail) compared to the control. No side effects on the leaves or growth were observed; however, at the end of the experiment, S. officinalis plants grown in the substrate treated with the leaf extract at 200 mg L−1 Ail showed a higher growth index (+77.5%).
4. Discussion
Ailanthus altissima water extracts were used to simulate the natural release of water-soluble phytotoxins into the environment. The measurement of the physiochemical properties of aqueous extracts, such as pH or conductivity, is commonly explored, since they can cause changes in cellular processes that could be mistaken as possible phytotoxic effects [38]. Taking into account that the used extracts present pH values around 4.5 and electrical conductivity (EC) values below 0.02 dS m−1 (data not shown), the inhibitory effects observed in the bioassays could be attributed a priori to the phytotoxins present in the extracts.
In the in vitro phytotoxicity tests, among the measured macroscopic traits, seed germination and root growth are considered as the most sensitive criteria to detect the toxicity of allelopathic compounds [39,40]. In turn, the seed index of germination (IGe%) is directly affected by root growth and germination and may thus contribute to the unraveling of the herbicidal effects of the studied extracts. In the present study, all the applied A. altissima extracts showed a marked inhibition in the seed germination and root growth of the indicator plants L. sativum and R. sativus during the growth chamber assay. Thus, this indicates that they could be a promising natural herbicide. The inhibition of IGe% was observed in the seeds of all the treated species at different concentrations and during the entire experiment, with some limitations at 1.8 mg L−1 Ail, particularly at the end of the trial. However, some differences among extract types were observed. Among them, the secondary root-derived extract presents the most promising efficacy in reducing seed germination and root growth under in vitro germination conditions. In addition, the leaf, samara, and rachis extracts presented results of interest, albeit to a lesser extent. The concentration of 25 mg L−1 Ail was found to be the most promising, both in terms of its efficacy and persistence for all extracts. As shown in Figure 2, by comparing the effects of the extracts used in this study with the results obtained by Demasi et al. [12,27] with pure Ail, there are significant differences between the treatments at the concentration of 25 mg L−1 Ail.
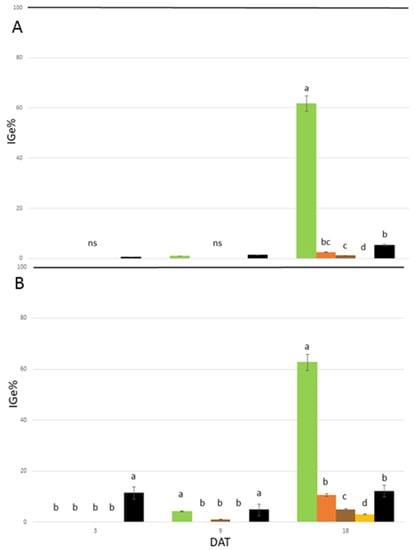
Figure 2.
Phytotoxic effects of Ailanthus altissima extracts from the leaf (green bars), samara (orange bars), rachis (brown bars), and secondary root (yellow bars) at 25 mg L−1 Ail, as well as of ailanthone pure ([12] black bars) at 25 mg L−1, on the index of germination (IGe%) of Raphanus sativus (A) and Lepidium sativum (B) after 3, 9, and 18 DAT under the growth chamber conditions. Mean values showing the same letter are not statistically different at p ≤ 0.05, according to the Tukey post-hoc test (ns = not significant). Black line at 100% indicates the IGe% of the control plants.
In R. sativus, the studied extracts affected IGe% as well as pure Ail (IGe% values ranged between −98.60% and −99.75%) without significant differences up to 9 DAT. At 18 DAT, the leaf extract was the least effective, while the rachis and secondary root extracts significantly reduced IGe% in comparison to pure Ail. In L. sativum, the tested extracts were significantly more effective than the pure Ail up to 9 DAT, with the exception of the leaf extract (9 DAT). At the end of the experiment, a pattern similar to R. sativus was observed.
Although the leaf extract was found to be less effective and less persistent than the other extracts tested in the growth chamber assay, it was nevertheless used for the greenhouse and nursery assays, because leaf biomass is more available and easy to collect and process. This result implies that plant extracts obtained by the applied multistep solvent extraction method can be successfully used without incurring huge purification costs, as for quassinoids such as Ail. These findings are in agreement with the known pronounced phytotoxic activity of the outer tissues of A. altissima, such as the trunk bark of the rachis and, in particular, of the roots [25,34]. Only few and ambiguous reports on the phytotoxic activity of A. altissima extracts under in vitro conditions are present in the literature. Tsao et al. [41] highlighted that the secondary metabolite extracts from the leaves of A. altissima have inhibitory effects on the seed germination and plant growth of Medicago sativa L. El Ayeb-Zakhama et al. [42] evaluated the phytotoxicity of A. altissima essential oils obtained from different parts of the plant. In agreement with our results, these authors observed a dose-dependent inhibitory effect on L. sativa seed germination and growth (range between −50% and −85%), with the essential oil obtained from the roots as the most effective. In contrast, Bagheri and Cici [20] showed no statistical differences among the leaf, stem, root, bark, and fruit extracts on L. sativum seed germination, with only limited inhibition (circa −5%). However, the same authors indicated that a marked reduction in radicle length was observed, with the highest activity from the bark and root (circa −65%) extracts under greenhouse conditions. De Feo et al. [35] indicated that water extracts from the roots and leaves resulted in the inhibition of circa 80%, 70%, and 50% of the germination of L. sativum, R. sativus, and P. oleracea, respectively, markedly less than our studied extracts. Our extracts were more effective than that, reported in the abovementioned studies. The effectiveness of a phytoextract depends on the extraction procedure used. Specifically, at the moment there are no studies in the literature that have used the method proposed in this work. The advantage in utilizing water extracts rather than a single purified compound is suggested by many authors, demonstrating that a mixture of compounds can act synergistically and could be more phytotoxic than their respective individual compounds [2]. Furthermore, the applied water extracts could help in reducing the dose of the application of synthetic herbicides, if applied in combination, serving as an effective management strategy in controlling weeds without causing much harm to the environment quality [43]. Jabran et al. [44] reported a reduction in weed population by using a mixture of water extracts of Helianthus annuus L. and Oryza sativa L. when applied in combination, with a significant reduction in the dose of pendimethalin. Accurate scientific studies must, however, be conducted to evaluate the environmental impact of A. altissima water extracts, for any other pesticides or biologically active compounds used in agriculture.
Based on these promising results, further in vivo experiments were conducted, this time both under greenhouse and nursery conditions by testing more seed species belonging to the weed and horticulture sector. Although the leaf extract was found to be less effective and less persistent than the other extracts tested, it was nevertheless selected for this purpose. A higher amount of this extract can be obtained because leaf biomass is more abundant than the other organs and is easier to collect and process, leading to lower production costs. Moreover, considering that an organic substrate is buffered due to its physico-chemical properties, higher concentrations (50, 100, and 200 mg L−1 Ail) were evaluated compared to the growth chamber assay, where filter paper was used.
In the greenhouse experiment, all of the treated weed species were strongly inhibited by the used leaf extract, without differences among extract concentrations, suggesting the possibility to further reduce the concentration of this leaf extract. In the literature, it is reported that the influence of phytotoxic compounds on seed germination also depends on the size and permeability of the seed integument [45,46], and that seeds of weeds tend to be less sensitive to the action of phytochemicals because the phytotoxic effects can be ameliorated due to lower absorption and translocation or faster degradation of phytotoxins [47].
In the present assay, as also observed in the laboratory trial, only a residual number of roots emerged from the seeds, but their development was inhibited early, resulting in senescence and rotting. This can be explained by the fact that the secondary root surfaces are more permeable to our extracts in comparison to hypocotyl surfaces due to the presence of a less pronounced protective cuticle layer, which can result in a greater penetration and concentration of these compounds in root tissues [48]. However, in this regard, little is known about the mode of action of A. altissima natural compounds in inhibiting seed germination. Dayan et al. [49] and Duke et al. [29] suggested that ailanthone, similar to other quassinoids, might act as a mitosis inhibitor. The strong inhibitory activity of quassinoids, particularly on seed germination and early root growth, corroborates previous studies that investigated the inhibitory effects of other terpenoids obtained from Ageratina adenophora (Spreng.) R.M. King & H. Rob. and Drimys brasiliensis Miers on the early growth of O. sativa, Barbarea verna (Mill.) Asch., Echinochola crus-galli (L.) P. Beauv., and Ipomea grandiflora (Dammer) O’Donnell [50,51]. This has already been interpreted as a result of the loss of mitotic activity, capable of reducing both the germination and seedling growth of several seed species, such as Amaranthus retroflexus L. and Setaria viridis (L.) P. Beauv., treated with different plant extracts and essential oils [52]. Very little information about A. altissima extracts in pot cultivation under greenhouse conditions has been reported. Bagheri and Cici [20] affirmed that the root extract had no effect on E. crus-galli growth, while it significantly affected the dry biomass of different weed species, such as A. retroflexus, Abutilion theophrasti Medik., and Carthamus tinctorius L. However, the indicated reduction was lower than in this experiment, settling in a range between −34% and −80%.
Ailanthone stability in a sterile environment has already been demonstrated by previous tests performed by Heisey [24], in which biological activity was detected on sterile substrates for up to 21 days, versus 3–5 days on non-sterile substrates. More recently, Demasi et al. [12,27] reported that Ail in paper was extremely active at low doses (i.e., 7.5 mg L−1) up to 30 DAT on radish and 20 DAT on garden cress, while on cultivation substrate, it lasted 30 DAT on radish when using a concentration of 60 mg L−1. These authors also indicated that Ail remained highly toxic up to 21 DAT if the soil was sterilized. Here, the assays in the growth chamber and the greenhouse showed that the studied extracts are able to maintain their phytotoxic activity for a period ranging between 18 and 30 DAT, both in sterile and, as is particularly evident, in non-sterile substrates.
Further details highlighting the biological effects and the persistence of the leaf extract were provided by the two experiments performed in the nursery, which, to the best of our knowledge, is the first time such effects have been noted. In this context, the leaf extract showed a potential herbicidal effect in pre-emergence for the whole duration of both experiments, particularly in the first. In this experiment, the substrate contained only a few weed seeds (1.3–3.1 weeds per pot germinated); therefore, it was not possible to evaluate the susceptibility of different weed species to the studied extracts, as was done in the greenhouse assay. For the same reason, the parameter “weed presence per pot (%)” was used instead of the commonly used “weed density.” The lower phytotoxic activity highlighted in the second experiment could be related to the higher temperatures of late spring (mean temperature ranging between 11.2 and 17.2 °C during experiment 1, and between 20.5 and 24.1 °C during experiment 2), which more likely increased the number and the growth of weeds.
Bioherbicides are a future target for development in weed management, although research in this area has been somewhat slow. As reported by Seiber et al. [53], the toxicity of non-target species is one of the main problems that limit the use of natural compounds as herbicides. Here, during the first experiment in the nursery, the leaf extract negatively affected the plant growth and health status of the cultivated crops, particularly in S. officinalis and slightly less in S. rosmarinus. The senescence and the presence of wilted leaves in the treated crops might indicate a reduction in chlorophyll content and the inhibition of the photosynthesis [54]. Such observations could reflect different modes of action, depending on the site of phytotoxin entry, with the growth and morphological parameters being affected early by spraying on the canopy. The phytotoxic effect on D. caryophyllus was markedly less intense. This behavior could be due to the selectivity of the phytocomplex contained within the extract. However, at present, it is not yet possible to accurately explain the selectivity of these complexes. Meanwhile, the indirect effects on the growth and quality of the untreated S. officinalis and S. rosmarinus plants can be supposed as the effect of the volatilization of some herbicide-carrying molecules during or after treatment, although there is currently no bibliographical evidence. Mastelic and Jerkovic [55] characterized the volatiles produced by A. altissima plants and listed several oxygenated aliphatic compounds and terpenes that are commonly known for their phytotoxicity. On the other hand, during the second experiment, the highest leaf extract concentration promoted the growth of plants. Evidence of the growth stimulation of crops by plant-released compounds is available in the literature [56,57]. However, this phenomenon, called hormesis [58], is usually related to lower doses of phytotoxins, not as high as in this study, with the leaf extract at 200 mg L−1 Ai eq. Regarding this topic, Hussain et al. [59] espoused that a foliar spray containing 3% aqueous extract of Moringa oleifera Lam., Sorghum vulgare Pers., and Brassica rapa L. increases maize grain yield by circa 50%. Unfortunately, the literature is lacking data on these aspects, and further studies are needed to elucidate them.
5. Conclusions
Ailanthus altissima water extracts showed significant phytotoxic activity on various weed seedlings at a low dosage in in the growth chamber and greenhouse assays. The extracts were generally more effective and, particularly rachis and secondary root extracts, provided more persistent activity (up to 18 days) than pure ailanthone, as previously tested. These findings support the higher efficacy of complex non-purified extracts that likely contain other related phytotoxins, biosynthesized by Ailanthus residues and tissues. The extract derived from the secondary roots was the most bioactive. Nevertheless, the difficulties in obtaining a large quantity of secondary roots makes the leaves preferable as a source of extract for the development of a bioherbicide for the organic and sustainable management of weeds in horticulture. At this time, the phytotoxins that contribute the greatest activity have not yet been identified.
Currently, pure Ail is marketed by various manufacturing companies at a price ranging between EUR 2000 and EUR 3000 per gram. In general, plant material could be available at a low cost, as the pruning waste of A. altissima allows for the cost-effective use of A. altissima extracts as herbicides, being more convenient that other bioherbicides. However, it is necessary to upscale the process for evaluating commercial production costs at an industrial level. Attention should be paid to the application method, as the experimental leaf extract caused side effects on crops when applied post-emergence to the crop canopy, but such effects did not occur when applied to pre-emergence.
Author Contributions
Conceptualization, M.C., S.D., and V.S.; data curation, M.C. and S.D.; formal analysis, M.C., S.D., F.C., and N.K.D.; resources, V.S.; supervision, F.T. and V.S.; validation, F.T. and V.S.; writing—original draft, M.C.; writing—review and editing, S.D. and V.S.; project administration, V.S.; funding acquisition, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project titled “Natural plant protection products for weed control in urban environments and nurseries”—Bando per finanziamento di progetti di ricerca di Ateneo, anno 2014: Call 01 Excellent Young PI—Torino_call2014_L1_141 and by the program Interreg V-A Francia Italia Alcotra “Attività Innovative per lo sviluppo della filiera transfrontaliera del fiore edule - ANTEA” n. 1139.
Acknowledgments
Walter Gaino and Giancarlo Padovan are thanked for their helpful support in the set-up of the bioassays.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and basic aspects for agroecosystem control. Sustain. Agric. Rev. 2018, 28, 47–101. [Google Scholar]
- Puig, C.G.; Reigosa, M.J.; Valentão, P.; Andrade, P.B.; Pedrol, N. Unravelling the bioherbicide potential of Eucalyptus globulus Labill: Biochemistry and effects of its aqueous extract. PLoS ONE 2018, 13, e0192872. [Google Scholar]
- Cai, X.; Gu, M. Bioherbicides in organic horticulture. Horticulture 2016, 2, 3. [Google Scholar] [CrossRef]
- Bachheti, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Pandey, D.P. Plant allelochemicals and their various applications. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 441–465. ISBN 978-3-319-96397-6. [Google Scholar]
- Rice, E.L. Allelopathy; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Bertin, C.; Paul, R.N.; Duke, S.O.; Weston, L.A. Laboratory assessment of the allelopathic effects of fine leaf fescues. J. Chem. Ecol. 2003, 29, 1919–1937. [Google Scholar] [CrossRef]
- Scognamiglio, M.; Esposito, A.; D’Abrosca, B.; Pacifico, S.; Fiumano, V.; Tsafantakis, N.; Monaco, P.; Fiorentino, A. Isolation, distribution and allelopathic effect of caffeic acid derivatives from Bellis perennis L. Biochem. Syst. Ecol. 2012, 43, 108–113. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Putnam, A.R.; Duke, W.B. Allelopathy in agroecosystems. Ann. Rev. Phytopathol. 1978, 16, 431–451. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef]
- Anese, S.; Grisi, P.U.; de Jatobá, L.J.; de Pereira, V.C.; Gualtieri, S.C.J. (Phytotoxic activity of differents plant parts of Drimys brasiliensis miers on germination and seedling development. Biosci. J. 2015, 31, 923–933. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Vanara, F.; Fogliatto, S.; Vidotto, F.; Negre, M.; Trotta, F.; Scariot, V. Ailanthone from Ailanthus altissima (Mill.) Swingle as potential natural herbicide. Sci. Hortic. 2019, 257, 108702. [Google Scholar] [CrossRef]
- Mendes, I.D.S.; Rezende, M.O.O. Assessment of the allelopathic effect of leaf and seed extracts of Canavalia ensiformis as postemergent bioherbicides: A green alternative for sustainable agriculture. J. Environ. Sci. Health Part B 2014, 49, 374–380. [Google Scholar] [CrossRef]
- Ahn, J.K.; Chung, I.M. Allelopathic potential of rice hulls on germination and seedling growth of barnyardgrass. Agric. J. 2000, 92, 1162–1167. [Google Scholar] [CrossRef]
- Nieves, J.A.; Acevedo, L.J.; Valencia-Islas, N.A.; Rojas, J.L.; Dávila, R. Fitotoxicidad de extractos metanólicos de los líquenes Everniastrum sorocheilum, Usnea roccellinay and Cladonia confusa. Glalia 2011, 4, 96. [Google Scholar]
- Lee, C.W.; Kim, D.; Lee, H. The riparian vegetation disturbed by two invasive alien plants, Sicyos angulatus and Paspalum distichum var. indutum in South Korea. Ecol. Resil. Infrastr. 2015, 2, 255–263. [Google Scholar]
- Heisey, R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 1996, 83, 192–200. [Google Scholar] [CrossRef]
- Zhao, C.C.; Shao, J.H.S.; Li, X.; Xu, J.; Zhang, P. Antimicrobial constituents from fruits of Ailanthus altissima Swingle. Arch. Pharm. Res. 2005, 28, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Fang, C.; Yang, G.; Xie, Y.; Nong, X.; Zhu, J.; Wang, S.; Peng, X.; Yan, Q. Acaricidal properties of an Ailanthus altissima bark extract against Psoroptes cuniculi and Sarcoptes scabiei var. cuniculi in vitro. Exp. Appl. Acarol. 2014, 62, 225–232. [Google Scholar] [CrossRef]
- Popa, C.V.; Lungu, L.; Cristache, L.F.; Ciuculescu, C.; Danet, A.F.; Farcasanu, I.C. Heat shock, visible light or high calcium augment the cytotoxic effects of Ailanthus altissima (Swingle) leaf extracts against Saccharomyces cerevisiae cells. Nat. Prod. Res. 2015, 29, 1744–1747. [Google Scholar] [CrossRef]
- Bagheri, F.; Cici, S.Z.H. Study on inhibitory effects of Ailanthus altissima on the growth of weeds and agricultural plants. Biol. Forum 2015, 7, 506–511. [Google Scholar]
- Khan, S.; Hussain, A.; Mehmood, A.; Mehmood, R.; Perveen, S.; Imran, M. Ailanthus altissima (Miller) Swingle fruit-new acyl β-sitosteryl glucoside and in vitro pharmacological evaluation. Nat. Prod. Res. 2016, 30, 2629–2636. [Google Scholar] [CrossRef]
- Ni, J.C.; Shi, J.T.; Tan, Q.W.; Chen, Q.J. Phenylpropionamides, piperidine, and phenolic derivatives from the fruit of Ailanthus altissima. Molecules 2017, 22, 2107. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Heisey, R.M.; Heisey, T.K. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 2003, 256, 85–99. [Google Scholar] [CrossRef]
- Pedersini, C.; Bergamin, M.; Aroulmoji, V.; Baldini, S.; Picchio, R.; Pesce, P.G.; Ballarin, L.; Murano, E. Herbicide Activity of Extracts from Ailanthus altissima (Simaroubaceae). Nat. Prod. Commun. 2011, 6, 593–596. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Fogliatto, S.; Vidotto, F.; Trotta, F.; Scariot, V. Ailanthone inhibition data on seed germination and seedling growth of Lepidium sativum L. and Raphanus sativus L. Data Brief 2019, 26, 104550. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Inderjit Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003, 22, 661–671. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicides: Current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Heisey, R.M. Development of an Allelopathic Compound from Tree-of-Heaven (Ailanthus altissima) as a Natural Product Herbicide; Biologically Active Natural Products: Agrochemicals; Cutler, J.S., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–68. [Google Scholar]
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowska, A. Allelochemicals as bioherbicides—Present and perspectives. In Herbicides-Current Research and Case Studies in Use; InTech: London, UK, 2013; pp. 517–542. [Google Scholar] [CrossRef]
- Heisey, R.M. Alleopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- De Feo, V.; De Martino, L.; Quaranta, E.; Pizza, C. Isolation of phytotoxic compounds from Tree-of-heaven (Ailanthus altissima Swingle). J. Agric. Food Chem. 2003, 51, 1170–1180. [Google Scholar] [CrossRef]
- Caser, M.; Lovisolo, C.; Scariot, V. The influence of water stress on growth, ecophysiology and ornamental quality of potted Primula vulgaris ‘Heidy’plants. New insights to increase water use efficiency in plant production. Plant Growth Regul. 2017, 83, 361–373. [Google Scholar] [CrossRef]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant Growth Regul. 2018, 84, 383–394. [Google Scholar] [CrossRef]
- Macias, F.A.; Chinchilla, N.; Arrojo, E.; Molinillo, J.M.G.; Marin, D.; Varela, R.M. Combined strategy for phytotoxicity enhancement of benzoxazinones. J. Agric. Food Chem. 2010, 58, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Aragão, F.B.; Queiroz, V.T.; Ferreira, A.; Costa, A.V.; Pinheiro, P.F.; Carrijo, T.T.; Andrade-Vieira, L.F. Phytotoxicity and cytotoxicity of Lepidaploa rufogrisea (Asteraceae) extracts in the plant model Lactuca sativa (Asteraceae). Rev. Biol. Trop. 2017, 65, 435–443. [Google Scholar]
- Sousa Carvalho, M.S.; Andrade-Vieira, L.F.; dos Santos, F.E.; Correa, F.F.; das Cardoso, M.G.; Vilela, L.R. Allelopathic potential and phytochemical screening of ethanolic extracts from five species of Amaranthus spp. in the plant model Lactuca sativa. Sci. Hortic. 2019, 245, 90–98. [Google Scholar] [CrossRef]
- Tsao, R.; Romanchuk, F.E.; Peterson, C.J.; Coats, J.R. Plant growth regulatory effect and insecticidal activity of the extracts of the Tree of Heaven (Ailanthus altissima L.). BMC Ecol. 2002, 2, 1. [Google Scholar]
- El Ayeb-Zakhama, A.; Ben Salem, S.; Sakka-Rouis, L.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and phytotoxic effects of essential oils obtained from Ailanthus altissima (Mill.) Swingle cultivated in Tunisia. Biochem. Biod. 2014, 11, 1216–1227. [Google Scholar]
- Tharayil, N.; Bhowmik, P.C.; Xing, B. Bioavailability of allelochemicals as affected by companion compounds in soil matrices. J. Agric. Food Chem. 2008, 56, 3706–3713. [Google Scholar] [CrossRef]
- Jabran, K.; Cheema, Z.A.; Farooq, M.; Hussain, M. Lower doses of pendimethalin mixed with allelopathic crop water extracts for weed management in canola (Brassica napus). Int. J. Agric. Biol. 2010, 12, 335–340. [Google Scholar]
- Hanley, M.E.; Whiting, M.D. Insecticides and arable weeds: Effects on germination and seedling growth. Ecotoxicology 2005, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Statti, G.A.; Abenavoli, M.R. Phytotoxic activity of foliar volatiles and essential oils of Calamintha nepeta (L.) Savi. Nat. Prod. Res. 2013, 27, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Duke, S.O. Clues in the search for new herbicides. In Allelopathy; Reigosa, M., Pedrol, N., González, L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 63–83. [Google Scholar]
- Bessire, M.; Chassot, C.; Jacquat, A.C.; Humphry, M.; Borel, S.; Petétot, J.M.C.; Métrauz, J.P.; Nawrath, C. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 2007, 26, 2158–2168. [Google Scholar] [CrossRef]
- Dayan, F.E.; Watson, S.B.; Galindo, J.C.G.; Hernández, A.; Dou, J.; McChesney, J.D.; Duke, S.O. Phytotoxicity of quassinoids: Physiological responses and structural requirements. Pestic. Biochem. Physiol. 1999, 65, 15–24. [Google Scholar] [CrossRef]
- Yang, Q.; Wan, F.; Guo, J.Y.; Liu, W.X. Cellular and ultrastructural changes in the seedling roots of upland rice (Oryza sativa) under the stress of two allelochemicals from Ageratina adenophora. Weed Biol. Manag. 2011, 11, 152–159. [Google Scholar] [CrossRef]
- Anese, S.; Grisi, P.U.; Cassia Pereira, V.; Gualtieri, S.C.J. Fitotoxicidade de extratos etanólicos de frutos e folhas de Banisteriopsis oxyclada (A. Juss.) B. Gates sobre o crescimento de plantas daninhas. Capa 2016, 29, 1. [Google Scholar] [CrossRef]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Pest management with biopesticides. Front. Agric. Sci. Eng. 2018, 5, 205–230. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Durga Devi, D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium–an antioxidative protectant in soybean during senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Mastelic, J.; Jerkovic, I. Volatile constituents from the leaves of young and old Ailanthus altissima (Mili.) Swingle tree. Croat. Chem. Acta 2002, 75, 189–197. [Google Scholar]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Chauhan, B.S. Can hormesis of plant-released phytotoxins be used to boost and sustain crop production? Crop Prot. 2017, 93, 69–76. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxic. Appl. Pharm. 2005, 202, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Farooq, M.; Basra, S.M.A.; Lee, D.J. Application of Moringa allelopathy in crop sciences. In Allelopathy; Cheema, Z., Farooq, M., Wahid, A., Eds.; Springer: Berlin, Heidelberg, 2013; pp. 469–483. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).