Modeling Climate Warming Impacts on Grain and Forage Sorghum Yields in Argentina
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. ALMANAC Model Description
2.3. ALMANAC Input Datasets for Model Calibration
2.3.1. Forage and Grain Sorghum Parameters
2.3.2. Soil Parameters and Crop Management
2.3.3. Weather Data
2.4. Model Validation
2.5. Statistical Analysis
3. Results
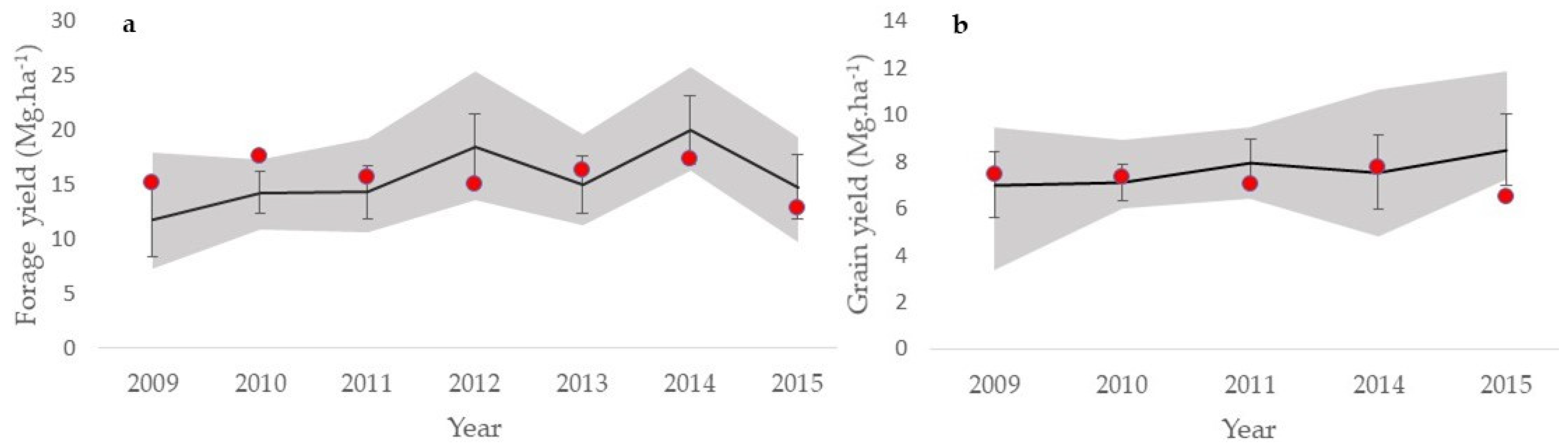
3.1. Model Validation
3.2. Forage and Grain Sorghum Yields under Different Warming Scenarios
3.3. Correlation Analysis between Yields and Days of Water and Nitrogen Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Statistics Data Base (FAOSTAT) for 2018. 2019. Available online: Http://Faostat.Fao.Org (accessed on 10 February 2020).
- Alguacil, M.D.M.; Torres, M.; Torrecillas, E.; Diaz, G.; Roldan, A. Plant type differently promote the arbuscular mycorrhizal fungi biodiversity in the rhizosphere after revegetation of a degraded, semiarid land. Soil Biol. Biochem. 2011, 43, 167–173. [Google Scholar] [CrossRef]
- Hariprasanna, K.; Rakshit, S. Economic Importance of Sorghum; Springer: Cham, Switzerland, 2016; pp. 1–25. [Google Scholar]
- Sasaki, T.; Antonio, B.A. Sorghum in sequence. Nature 2009, 457, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas, M.G.; Bertoia, L.M.; Jorge Rey, L. Nitrogen Nutrition and Hybrid Type and Their Influence on Attributes That Determine Bioenergetic Aptitude of Sorghum. AgriScientia 2018, 35, 1–9. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Tack, J.; Lingenfelser, J.; Jagadish, S.V.K. Disaggregating sorghum yield reductions under warming scenarios exposes narrow genetic diversity in US breeding programs. Proc. Natl. Acad. Sci. USA 2017, 114, 9296–9301. [Google Scholar] [CrossRef]
- Mundia, C.; Secchi, S.; Akamani, K.; Wang, G. A Regional Comparison of Factors Affecting Global Sorghum Production: The Case of North America, Asia and Africa’s Sahel. Sustainability 2019, 11, 2135. [Google Scholar] [CrossRef]
- Unger, P.W. Grain and Forage Sorghum Production with No-Tillage on Dryland. Agron. J. 1988, 80, 193–197. [Google Scholar] [CrossRef]
- Pavli, O.I.; Ghikas, D.V.; Katsiotis, A.; Skaracis, G.N. Differential Expression of Heat Shock Protein Genes in Sor-Ghum (Sorghum bicolor L.) Genotypes under Heat Stress. Aust. J. Crop Sci. 2011, 5, 511–515. [Google Scholar]
- Rogelj, J.; Elzen, M.D.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639. [Google Scholar] [CrossRef]
- IPCC. Special Report on Global Warming of 1.5 °C (SR1). 2018. Available online: http://www.ipcc.ch/report/sr15/ (accessed on 6 March 2020).
- Arnell, N.; Livermore, M.; Kovats, S.; Levy, P.; Nicholls, R.J.; Parry, M.; Gaffin, S. Climate and socio-economic scenarios for global-scale climate change impacts assessments: Characterising the SRES storylines. Glob. Environ. Chang. 2004, 14, 3–20. [Google Scholar] [CrossRef]
- Meehl, G.A.; Washington, W.M.; Collins, W.; Arblaster, J.M.; Hu, A.; Buja, L.E.; Strand, G.; Teng, H. How Much More Global Warming and Sea Level Rise? Science 2005, 307, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Magrin, G.; Travasso, M.; Diaz, R.; Rodríguez, R. Vulnerability of the agricultural systems of Argentina to climate change. Clim. Res. 1997, 9, 31–36. [Google Scholar] [CrossRef]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-Term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; IPCC, Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. [Google Scholar]
- Weng, E.; Luo, Y. Soil hydrological properties regulate grassland ecosystem responses to multifactor global change: A modeling analysis. J. Geophys. Res. Space Phys. 2008, 113. [Google Scholar] [CrossRef]
- Summers, D.M.; Bryan, B.A.; Crossman, N.D.; Meyer, W.S. Species Vulnerability to Climate Change: Impacts on Spatial Conservation Priorities and Species Representation. Glob. Chang. Biol. 2012, 18, 2335–2348. [Google Scholar] [CrossRef]
- Choudhary, S.; Guha, A.; Kholova, J.; Pandravada, A.; Messina, C.D.; Cooper, M.; Vadez, V.; Anand, P. Maize, sorghum, and pearl millet have highly contrasting species strategies to adapt to water stress and climate change-like conditions. Plant Sci. 2020, 295, 110297. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.S.; Vlek, P.L.G. Impact of Climate Change on Sorghum Production under Different Nutrient and Crop Residue Management in Semi-Arid Region of Ghana: A Modeling Perspective. Afr. Crop Sci. J. 2012, 20, 243–259. [Google Scholar]
- Ramirez-Villegas, J.; Jarvis, A.; Läderach, P. Empirical approaches for assessing impacts of climate change on agriculture: The EcoCrop model and a case study with grain sorghum. Agric. For. Meteorol. 2013, 170, 67–78. [Google Scholar] [CrossRef]
- Pembleton, K.; Cullen, B.; Rawnsley, R.P.; Harrison, M.T.; Ramilan, T. Modelling the resilience of forage crop production to future climate change in the dairy regions of Southeastern Australia using APSIM. J. Agric. Sci. 2016, 154, 1131–1152. [Google Scholar] [CrossRef]
- Sandeep, V.M.; Rao, V.U.M.; Bapuji Rao, B.; Bharathi, G.; Pramod, P.; Santhibhushan Chowdary, P.; Patel, N.R.; Mukesh, P.; Vijaya Kumar, P. Impact of Climate Change on Sorghum Productivity in India and Its Adaptation Strategies. J. Agrometeorol. 2018, 20, 89–96. [Google Scholar]
- Amouzou, K.A.; Lamers, J.P.; Naab, J.B.; Borgemeister, C.; Vlek, P.L.; Becker, M. Climate change impact on water- and nitrogen-use efficiencies and yields of maize and sorghum in the northern Benin dry savanna, West Africa. Field Crops Res. 2019, 235, 104–117. [Google Scholar] [CrossRef]
- Eggen, M.; Ozdogan, M.; Zaitchik, B.F.; Ademe, D.; Foltz, J.; Simane, B. Vulnerability of sorghum production to extreme, sub-seasonal weather under climate change. Environ. Res. Lett. 2019, 14, 045005. [Google Scholar] [CrossRef]
- Huntington, T.; Cui, X.; Mishra, U.; Scown, C.D. Machine learning to predict biomass sorghum yields under future climate scenarios. Biofuels Bioprod. Biorefining 2020, 14, 566–577. [Google Scholar] [CrossRef]
- Pena, G.; Cavaglieri, L.R.; Chulze, S.N. Fusarium species and moniliformin occurrence in sorghum grains used as ingredient for animal feed in Argentina. J. Sci. Food Agric. 2018, 99, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Barros, V.R.; Boninsegna, J.A.; Camilloni, I.A.; Chidiak, M.; Magrín, G.O.; Rusticucci, M. Climate change in Argentina: Trends, projections, impacts and adaptation. Wiley Interdiscip. Rev. Clim. Chang. 2014, 6, 151–169. [Google Scholar] [CrossRef]
- Nistor, M.M.; Cheval, S.; Gualtieri, A.F.; Dumitrescu, A.; Boţan, V.E.; Berni, A.; Hognogi, G.; Irimuş, I.A.; Porumb-Ghiurco, C.G. Crop Evapotranspiration Assessment under Climate Change in the Pannonian Basin during 1991–2050. Meteorol. Appl. 2017, 24, 84–91. [Google Scholar] [CrossRef]
- Leirós, M.; Trasar-Cepeda, C.; Seoane, S.; Gil-Sotres, F. Dependence of mineralization of soil organic matter on temperature and moisture. Soil Biol. Biochem. 1999, 31, 327–335. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J. A meta-analysis of the response of soil espiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Srivastava, A.; Naresh, K.S.; Aggarwal, P. Assessment on vulnerability of sorghum to climate change in India. Agric. Ecosyst. Environ. 2010, 138, 160–169. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Sanchez, H.; Greenwade, J.; Seidensticker, E.; Bell, J.R.; Pringle, F.; Peacock, G.; Rives, J. Simulating Grass Productivity on Diverse Range Sites in Texas. J. Soil Water Conserv. 2002, 57, 144–150. [Google Scholar]
- Meki, M.N.; Kiniry, J.R.; Worqlul, A.W.; Kim, S.; Williams, A.S.; Osorio, J.M.; Reilley, J. Field and simulation-based assessment of vetivergrass bioenergy feedstock production potential in Texas. Agron. J. 2020, 1–16. [Google Scholar] [CrossRef]
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part i: model development. J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Hammer, G. Crop modelling: current status and opportunities to advance. Acta Hortic. 1998, 456, 27–36. [Google Scholar] [CrossRef]
- Rosenthal, W.D.; Gerik, T.J.; Pendleton, G.B.; Teetes, G.; Makila, M.; Stone, N. Sorghum Midge Control Using a Plant/Insect Model; ASAE Technical Paper 894043; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1989. [Google Scholar]
- Gerik, T.; Wade, L.; Rosenthal, W.; Vanderlip, R. Optimising cultural practices for grain sorghum in relation to climatic risk at three locations in the United States, using the Sorkam model. Math. Comput. Simul. 1992, 33, 415–419. [Google Scholar] [CrossRef]
- Fritz, J.O.; Vanderlip, R.L.; Heiniger, R.W.; Abelhalim, A.Z. Simulating Forage Sorghum Yields with SORKAM. Agron. J. 1997, 89, 64–68. [Google Scholar] [CrossRef]
- Xie, Y.; Kiniry, J.R.; Nedbalek, V.; Rosenthal, W.D. Maize and Sorghum Simulations with CERES-Maize, SORKAM, and ALMANAC under Water-Limiting Conditions. Agron. J. 2001, 93, 1148–1155. [Google Scholar] [CrossRef]
- Behrman, K.D.; Kiniry, J.R.; Winchell, M.; Juenger, T.E.; Keitt, T.H. Spatial forecasting of switchgrass productivity under current and future climate change scenarios. Ecol. Appl. 2013, 23, 73–85. [Google Scholar] [CrossRef]
- Rong, L.; Zhang, C.; Zhang, X.; Wu, S.; Wang, Z. Wheat Production Simulation Based on the ALMANAC Model of North China Region. Sustain. Agric. Res. 2013, 2. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Williams, A.; Kiniry, J.R.; Hawkes, C.V. Simulating diverse native C4 perennial grasses with varying rainfall. J. Arid. Environ. 2016, 134, 97–103. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; Agriculture Handbook No. 436; US Government Printing Office: Washington, DC, USA, 1975.
- Kiniry, J.R.; Johnson, M.V.V.; Venuto, B.C.; Burson, B.L. Novel Application of ALMANAC: Modelling a Functional Group, Exotic Warm-season Perennial Grasses. Am. J. Exp. Agric. 2013, 3, 631–650. [Google Scholar] [CrossRef][Green Version]
- Monsi, M.; Saeki, T. Uber Den Lichtfaktor in Den Pflanzen-Gesellschaften Und Seine Bedeutung Fur Die Stoffproduktion. Jpn. J. Bot. 1953, 14, 22–52. [Google Scholar]
- Quinby, J.R.; Kramer, N.W.; Stephens, J.C.; Lahr, K.A.; Karper, R.E. Grain sorghum production in Texas. Texas Agric. Exp. Stn. Bull. 1958, 912, 78–218. [Google Scholar]
- Ferraris, R.; Charles-Edwards, D. A comparative analysis of the growth of sweet and forage sorghum crops. II. Accumulation of soluble carbohydrates and nitrogen. Aust. J. Agric. Res. 1986, 37, 513–522. [Google Scholar] [CrossRef]
- Hammer, G.L.; Carberry, P.S.; Muchow, R.C. Modeling genotypic and environmental control of leaf area dynamics in grain sorghum. Whole plant level. Field Crops Res. 1993, 33, 293–310. [Google Scholar] [CrossRef]
- Prasad, P.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Tischler, C.; Van Esbroeck, G. Radiation use efficiency and leaf CO2 exchange for diverse C4 grasses. Biomass Bioenergy 1999, 17, 95–112. [Google Scholar] [CrossRef]
- Williams, J.R.; Jones, C.A.; Kiniry, J.; Spanel, D.A. The EPIC Crop Growth Model. Trans. ASAE 1989, 32, 497–511. [Google Scholar] [CrossRef]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006, 17, 197–209. [Google Scholar] [CrossRef]
- Monteith, J.L. Evaporation and Environment. In Symposium of the Society Experimental Biology; Cambridge University Press: Cambridge, UK, 1965; pp. 205–234. [Google Scholar]
- Giorgi, F.; Mearns, L.O. Approaches to the simulation of regional climate change: A review. Rev. Geophys. 1991, 29, 191–216. [Google Scholar] [CrossRef]
- Fox, D.G. Judging Air Quality Model Performance. A Summary of the AMS Workshop on Dispersion Model Performance, Woods Hole, Mass., 8–11 September 1980. Bull. Am. Meteorol. Soc. 1981, 62, 599–609. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat: Statistical Software; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2013; p. 334. [Google Scholar]
- Kiniry, J.R.; Cassida, K.A.; Hussey, M.A.; Muir, J.P.; Ocumpaugh, W.R.; Read, J.C.; Reed, R.L.; Sanderson, M.A.; Venuto, B.C.; Williams, J.R. Switchgrass simulation by the ALMANAC model at diverse sites in the southern US. Biomass Bionerg. 2005, 29, 419–425. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Bockholt, A.J. Maize and Sorghum Simulation in Diverse Texas Environments. Agron. J. 1998, 90, 682–687. [Google Scholar] [CrossRef]
- Boomiraj, K.; Marimuthu, S.; Wani, S.P.; RaviKumar, S.M.; Tani, S. Vulnerability assessment of kharif rainfed sorghum to climate change in SAT regions of India. J. Agrometeorol. 2012, 14, 2–8. [Google Scholar]
- Sunoj, V.S.J.; Somayanda, I.M.; Chiluwal, A.; Perumal, R.; Prasad, P.V.V.; Jagadish, S.V.K. Resilience of Pollen and Post-Flowering Response in Diverse Sorghum Genotypes Exposed to Heat Stress under Field Conditions. Crop Sci. 2017, 57, 1658–1669. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 1–13. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Mantineo, M.; Testa, G. Water and nitrogen balance of sweet sorghum (Sorghum bicolor moench (L.)) cv. Keller under semi-arid conditions. Ind. Crops Prod. 2012, 36, 329–342. [Google Scholar] [CrossRef]
- Alexander, L.; Arblaster, J.M. Assessing trends in observed and modelled climate extremes over Australia in relation to future projections. Int. J. Clim. 2009, 29, 417–435. [Google Scholar] [CrossRef]
- Levy, P.E.; Cannell, M.; Friend, A. Modelling the impact of future changes in climate, CO2 concentration and land use on natural ecosystems and the terrestrial carbon sink. Glob. Environ. Chang. 2004, 14, 21–30. [Google Scholar] [CrossRef]
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Band, L.E.; Baron, J.S.; Davidson, E.A.; Tague, C.L.; et al. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
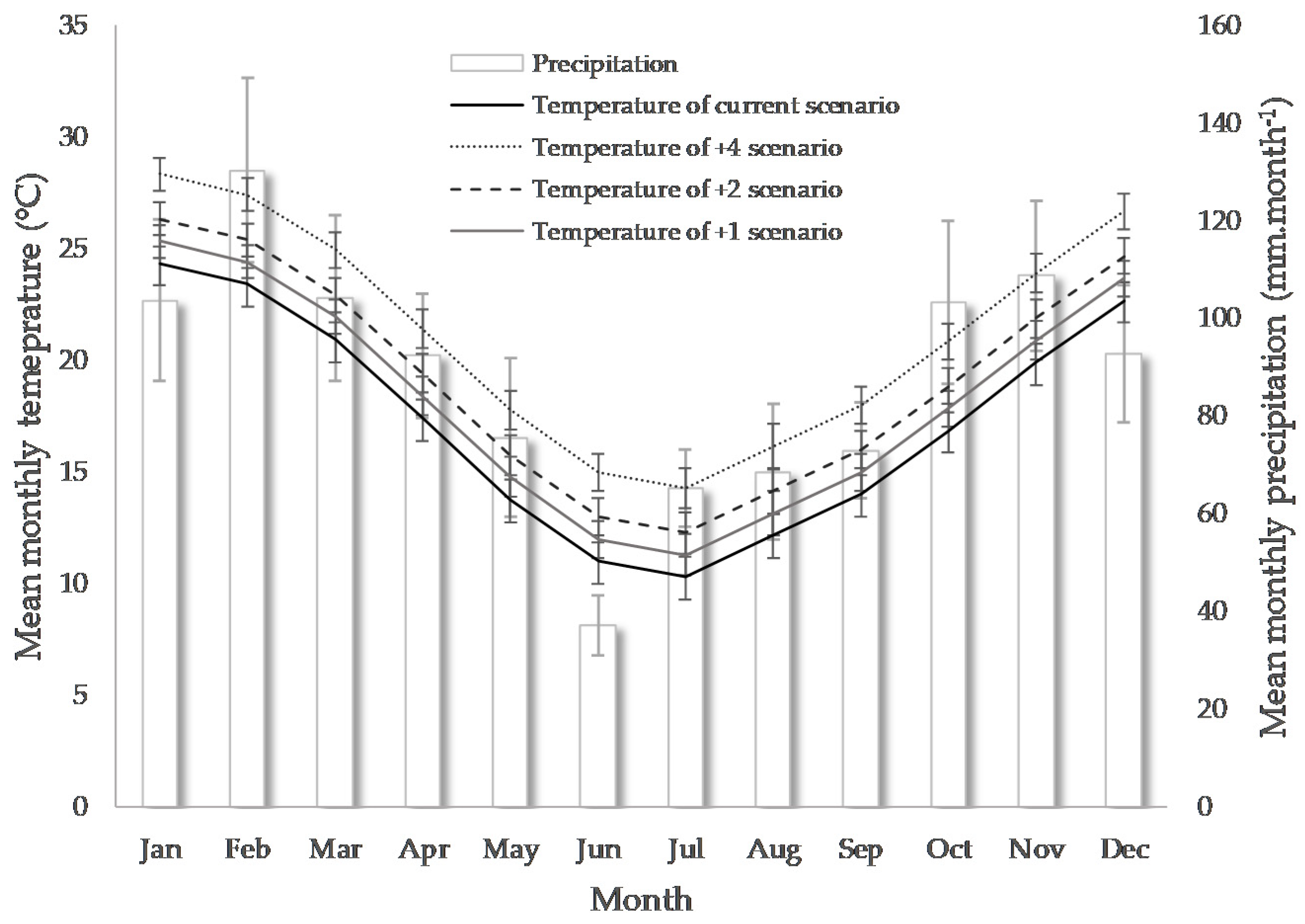


| Growing Season (GS) | Precipitation (mm/GS) | Average Minimum Temperature (°C) | Average Maximum Temperature (°C) | Sorghum Type | Nº of Hybrids Evaluated |
|---|---|---|---|---|---|
| 2008/2009 | 438 | 16.2 | 28.5 | Forage | 23 |
| Grain | 32 | ||||
| 2009/2010 | 932 | 15.7 | 26.5 | Forage | 20 |
| Grain | 32 | ||||
| 2010/2011 | 348 | 14.8 | 27.6 | Forage | 19 |
| Grain | 33 | ||||
| 2011/2012 | 430 | 15.8 | 28.2 | Forage | 16 |
| 2012/2013 | 744 | 15.4 | 27.2 | Forage | 24 |
| 2013/2014 | 843 | 15.7 | 27.3 | Forage | 14 |
| Grain | 44 | ||||
| 2014/2015 | 581 | 15.8 | 27.8 | Forage | 15 |
| Grain | 31 |
| Soil Properties | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|---|
| Organic C (%) | 1.89 | 1.71 | 2.15 | 1.86 | 1.75 | 1.82 | 2.46 |
| NO3 (g·t−1) | 91.2 | 91.2 | 81.2 | 51.7 | 39.4 | 160.1 | 72.4 |
| Growing Season (GS) | Planting Date | Planting Density * (pl·m−2) | Fertilization Date | Fertilization Rate (Kg·ha−1) | Intermediate Harvest Dates | Final Harvest Date |
|---|---|---|---|---|---|---|
| 2008/2009 | Dec 5 | 50 | 1st: Dec 12 2nd: Feb 24 | 1st: N 25, P 9; 2nd: N 9, P 10 | Jan 23, Feb 23 | Mar 31 |
| 2009/2010 | Dec 3 | 40 | Dec 5 | N 25, P 9 | Jan 15, Feb 18 | Apr 23 |
| 2010/2011 | Nov 19 | 50 | Nov 25 | N 21, P 7.5 | Jan 23 | Mar 22 |
| 2011/2012 | Nov 22 | 40 | Nov 22 | N 21.6, P 24 | Feb 13 | Apr 20 |
| 2012/2013 | Nov 15 | 45 | Nov 15 | N 18, P 20 | Jan 20 | Apr 30 |
| 2013/2014 | Dec 1 | 40 | Dec 1 | N 18, P 20 | Jan 30 | Mar 25 |
| 2014/2015 | Nov 28 | 60 | Nov 28 | N 18, P 20 | Jan 22 | Apr 22 |
| Growing Season (GS) | Planting Date | Planting Density * (pl·m−2) | Fertilization Date | Fertilization Rate (Kg·ha−1) | Harvest Date |
|---|---|---|---|---|---|
| 2008/2009 | Dec 3 | 30 | Dec 17 | N 25, P 9 | Jun 7 |
| 2009/2010 | Dec 4 | 36 | Dec 7 | N 72, P 21 | May 10 |
| 2010/2011 | Nov 25 | 42 | Nov 25 | N 21, P 7.5 | Jun 2 |
| 2013/2014 | Dec 8 | 45 | Dec 8 | N 45, P 20 | May 20 |
| 2014/2015 | Nov 28 | 27 | Nov 28 | N 68, P 20 | Jun 3 |
| Statistic | Forage Sorghum | Grain Sorghum | ||
|---|---|---|---|---|
| Measured | Simulated | Measured | Simulated | |
| Mean yield (Mg ha−1) | 15.47 | 15.69 | 7.61 | 7.22 |
| Coefficient of variation (%) | 18.1 | 10.1 | 8.1 | 6.6 |
| RMSE (Mg ha−1) | 2.6 | 1.0 | ||
| MBE (Mg ha−1) | 0.21 | −0.39 | ||
| MAE (Mg ha−1) | 2.4 | 0.7 | ||
| Variable 1 | Variable 2 | Forage Sorghum | Grain Sorghum | ||
|---|---|---|---|---|---|
| Pearson | p-Value | Pearson | p-Value | ||
| Yield | N stress | −0.94 | 0.0594 | 0.98 | 0.0161 |
| Yield | Water stress | 0.97 | 0.0313 | −0.99 | 0.0030 |
| N stress | Water stress | −0.99 | 0.0046 | −0.97 | 0.0323 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Druille, M.; Williams, A.S.; Torrecillas, M.; Kim, S.; Meki, N.; Kiniry, J.R. Modeling Climate Warming Impacts on Grain and Forage Sorghum Yields in Argentina. Agronomy 2020, 10, 964. https://doi.org/10.3390/agronomy10070964
Druille M, Williams AS, Torrecillas M, Kim S, Meki N, Kiniry JR. Modeling Climate Warming Impacts on Grain and Forage Sorghum Yields in Argentina. Agronomy. 2020; 10(7):964. https://doi.org/10.3390/agronomy10070964
Chicago/Turabian StyleDruille, Magdalena, Amber S. Williams, Marcelo Torrecillas, Sumin Kim, Norman Meki, and James R. Kiniry. 2020. "Modeling Climate Warming Impacts on Grain and Forage Sorghum Yields in Argentina" Agronomy 10, no. 7: 964. https://doi.org/10.3390/agronomy10070964
APA StyleDruille, M., Williams, A. S., Torrecillas, M., Kim, S., Meki, N., & Kiniry, J. R. (2020). Modeling Climate Warming Impacts on Grain and Forage Sorghum Yields in Argentina. Agronomy, 10(7), 964. https://doi.org/10.3390/agronomy10070964






