Abstract
Weed management is one of the main challenges of conservation agriculture. Although all three components of conservation agriculture (minimal tillage, permanent soil cover and crop diversification) can reduce weed populations, these effects may only become apparent in the medium to long term. This study evaluated weed biomass, density and diversity with and without herbicide control in a long-term trial initiated in 1991 in the Mexican Highlands to evaluate all three components of conservation agriculture. Data were collected in 2004, 2005, 2013, 2014 and 2015. Weed density and biomass were generally lower in conservation agriculture than with conventional tillage. The three components of conservation agriculture significantly reduced weed biomass, which was lower when all three components were applied together. When herbicides were applied, weed biomass in conservation agriculture was 91% lower in maize and 81% lower in wheat than in conventional tillage. Different treatments favored different weed species, but no trend toward increased perennial weeds was observed in conservation agriculture. These data supported claims stating that if adequate weed control is achieved in the initial years, weed populations in conservation agriculture systems are lower than in conventional tillage systems.
1. Introduction
Conservation agriculture (CA), a production system based on minimum tillage, permanent soil cover and crop diversification, increases yield and yield stability of maize and wheat under rainfed conditions [1,2]. Despite the potential benefits, uptake by smallholder farmers in the tropics and subtropics is limited. Competing interests using residue for fodder make it challenging for farmers to maintain adequate soil cover, and small farmers’ lack of market access restricts the availability of profitable alternative crops for crop diversification [3,4]. However, when zero tillage (ZT) is applied without adequate soil cover and crop rotation, this can result in soil degradation, problems with weeds, pests and diseases and, consequently, lower yields [1,5,6]. Furthermore, farmers often lack access to knowledge regarding the management of CA-based production systems, which strongly differ from conventional practices [3]. Weeds are another major constraint for the widespread adoption of CA [7,8,9]. The main weed control methods in smallholder agriculture include tillage, hand weeding, mechanical control and cheap herbicides. In CA, tillage is not generally seen as an option for weed control. Instead, herbicides, mulching and cultural practices or integrated weed management are the primary means of weed management [10]. Weeds were reported to be problematic particularly in the initial years after changing from conventional agriculture to CA [10]. Therefore, the success of CA depends on optimum crop establishment and effective weed management.
With adequate weed control in the first years of CA adoption, weed problems could be reduced in the long term in CA compared to conventional production systems [10,11]. All three components of CA have the potential to lower weed pressure if applied correctly [11]. In conventional production systems, tillage controls weeds but also generates optimum conditions for weed germination and brings weed seeds from the seedbank to the soil surface [12,13]. In CA, weeds are not killed by tillage, but conditions favoring weed germination are not generated either [11]. Weed seeds are not buried but remain on the soil surface, where they are more prone to predation and germination [14]; therefore, if weeds are prevented from producing more seeds and weed seed predation is augmented, weed seedbanks can be depleted in the long run in ZT [15]. Soil cover or mulching reduce the number of weed seeds that germinate, and cause delays in weed germination that give crops an early competitive advantage over weeds [11] and favor increased seed predation [16,17]. Some crop residues produce allelochemicals that may inhibit seed germination of many weeds [18]. The weed-suppressing effect of mulch depends on the amount of soil cover and thus on the productivity of the system [19]. Crop rotation reduces weed pressure by allowing for contrasting weed management practices in varying crops due to crops being associated with different weeds [17]. Although these components have beneficial effects on weed management, no studies exist regarding the effect of all three components of CA and their interactions, with most studies reporting only on one or two of these individual components.
Weed community composition and diversity depend on the growing conditions and crop association, which may differ between CA and conventional tillage (CT) and cause a shift in weed flora when changing from CT to CA [20]. It is possible that CA benefits perennial weeds, since these are not necessarily controlled by tillage [21,22]. For example, in a 14-year trial of tillage methods in corn and soybean in Iowa, perennial weeds increased more over time in the reduced tillage treatments than in the moldboard plow system [21], while no increase in perennials was observed in a similar trial in Argentina [20]. Mechanical weed control and hand-weeding are more difficult in CA due to the mulch cover, which increases dependence on herbicides for weed control [7]. The stronger reliance on herbicides in CA favors certain weeds and even generates problems regarding resistant weeds if herbicide management is not appropriate [23]. Ideally, integrated weed management promotes a diverse weed community to prevent one species from becoming problematic [24].
Weed control in Mexico relies mainly on the use of herbicides in commercial agriculture and/or on manual or animal-drawn mechanical control in traditional agriculture. Yield penalties due to inadequate weed control can range from 26% to 48% in CT and 58% to 92% in ZT [25]. Little research exists regarding the effect of CA components and their interactions with weed populations under Mexican highland conditions. Furthermore, very little long-term data about the effect of implementing all three CA components together on weed populations is available in the literature from other regions. The goal of this study was therefore to evaluate the long-term effects of all three CA components (ZT, crop residue and rotation) on weed biomass and density in wheat (Triticum aestivum L.) and maize (Zea mays L.). Weed biomass and density were evaluated in a long-term experiment with treatments unchanged for over 20 years. The research hypothesis behind this study was that CA would reduce weed biomass and weed density in the long term. The following specific questions were asked: (1) Were weed biomass and density higher in CA or CT, and what were the effects of the components of CA (ZT, crop residue and rotation) on weed biomass and density? (2) Was there a difference in weed diversity between the treatments with varying crop management? and (3) Did CA favor perennial weeds in maize and wheat systems?
2. Materials and Methods
2.1. The Long-Term Experiment
Weeds were studied in the “D5” long-term experiment to evaluate the effects of the three components of CA (rotation, tillage and soil cover) on maize and wheat yield. The experiment began in 1991 and was located in the experiment station of the International Maize and Wheat Improvement Center (CIMMYT) at El Batan, Texcoco, in the state of Mexico in Mexico. This experiment station was located in the semi-arid, subtropical highlands of Central Mexico (2240 masl; 19.318 N, 98.508 W), with a mean annual temperature of 14 °C and 616 mm average annual rainfall between 1995 and 2015, of which 567 mm fell on average during the growing season (May–October). The soil of the experimental plot is classified as a Haplic Phaeozem (clayic) in the World Reference Base system. The soil has 1.97% organic matter, 54.5 kg ha−1 phosphorus (P2O5) and 210 kg ha−1 potassium (K2O).
The experimental design was a randomized complete block design with two replications. Individual plots measured 7.5 m by 22 m (165 m2). The 32 treatments combined different wheat-maize rotations, tillage/planting methods and residue management practices. This study only included the first 16 treatments of the experiment, which consisted of the full factorial combination between the factors of 1) crop rotation, 2) tillage and 3) soil cover (Table 1). Crop rotation included continuous wheat, continuous maize (Mon) and wheat and maize rotations (Rot), with both phases of the double crop rotation included each year. Tillage was evaluated as CT or ZT with direct seeding. Conventional tillage consisted of one pass with a chisel plow to a depth of 30 cm depth, followed by two passes with a disk harrow to a depth of 20 cm and two passes with a spring tooth harrow to 10 cm. To prepare the seed bed before sowing, the tillage operations in December were repeated but including only one pass with the spring teeth harrow. No tillage operations were performed in ZT. The crop was planted by a custom-built, multicrop, multiuse, zero-till sowing machine. Soil cover was evaluated by keeping all residue on the field (K) or removing all residue for fodder (R) after grain harvest. Retained residues were incorporated in CT or left on the surface in ZT.

Table 1.
The 16 treatments (Trt.) of the long-term trial in Texcoco, Mexico, used in this study. A total of 32 treatments were used throughout the trial.
The experimental design contained only two replicated plots, as the experiment was designed in 1991 to study the effect of CA practices on crop yields to provide agronomic recommendations for the Mexican highlands. Field experiments in agricultural research often use designs with two replicates to reduce costs while still allowing for testing of significant differences between treatments [26,27]; although the variation is larger when two replicates are used in a statistical analysis and “renders any test less powerful” [28], thus making it less likely to detect significant differences, it is still possible to determine the effect of an agricultural treatment in a two-replicate design (e.g., [29,30,31]).
Maize and wheat yields and yield stability of the trial were reported in [5,31,32]. Apart from the treatment factors, crop management for each crop was the same in all treatments. Maize was planted at 75,000 plants ha−1 in rows 75 cm apart, and wheat in at 110 kg seed ha−1 in rows 20 cm apart. The experimental crops were planted around the beginning of the rainy season, usually in the last week of May or the first week of June. In 2004 and 2005, 120 kg N ha−1 applied as urea was used as fertilization at the 4–5 leaf stage in maize and at the first node stage in wheat. In 2013, fertilization consisted of 150 kg N ha−1 as urea and was incorporated during preplanting in wheat and sowing in maize. In 2014 and 2015, fertilization was the same as in 2013, with the addition of 40 kg ha−1 P2O5 as triple superphosphate, which was applied before sowing. The experiment was rainfed, although in some years when precipitation was too low to allow germination in all treatments, one sprinkler irrigation of 10–20 mm was applied to save the experiment. The crops were generally harvested during late September for wheat and late November for maize; wheat was harvested with a combine harvester and maize was harvested manually.
2.2. Weed Management in the Long-Term Experiment
Herbicides were used for weed control during the growing season; during the winter fallow season, the spring tooth harrow was used when needed for weed control (typically twice) in CT, while weeds were controlled with glyphosate in ZT when necessary. Herbicide application was similar on all plots with the same crop and tillage for a given year, regardless of the level of weed infestation. In zero-tillage treatments, glyphosate was applied before sowing during the fallow season, depending on the presence of weeds following rainfall events. Glyphosate was also applied after harvesting when weeds and volunteers proliferated, which occurred when the end of the growing season was wet. Once the experimental crop was established, weeds were controlled mainly by applying atrazine or fluroxypyr in maize and bromoxynil and halosulfuron or bromoxynil, fluroxypyr and clodinafop in wheat in all treatments (Table 2). The amounts of active ingredients applied for each herbicide were as follows: glyphosate: 907.5 g ha−1; atrazine: 1850 g ha−1; bromoxynil: 460.5 g ha−1; halosulfuron: 75 g ha−1; clodinafop: 62.25 g ha−1; fluroxypyr: 249.75 g ha−1 or 182 g ha−1, depending on the product used; topramezone: 33.6 g ha−1; fenoxaprop: 115 g ha−1; and S-metolachlor: 1450 g ha−1. One sowing machine width, which was 1.6 m in wheat (8 rows at 20 cm) and 1.5 m in maize (2 rows at 75 cm) on the border of the plot, was left without weed control in 2005, 2013, 2014 and 2015 after the presowing glyphosate application. In these untreated strips, biomass samples and weed counts without herbicide treatment were taken. There were no long-term plots without herbicide application. The herbicides used in the trial were the herbicides locally available at that time.

Table 2.
Applications per crop, management and year during the years when weeds where studied in the trial.
2.3. Data Collection
Weed density and dry biomass content were recorded for each of the experimental units in the study. Weed data were collected after treatments were in place for more than ten years to allow the study of long-term effects and when resources or resident students were available for data collection. Weed density was determined in 2004, 2013, 2014 and 2015. Weed biomass was determined in 2005, 2013, 2014 and 2015. In 2014, observations of weed density and biomass were only made in wheat. The type of data collected differed between years because the data were collected by several resident students as part of their research projects. Weed observations were recorded from anthesis to milk stage in wheat and from V10 to tasseling in maize (Table 3). In maize, weeds were counted and collected between 2 rows with a width of 0.75 m (0.75 m × 1 m = 0.75 m2), and in wheat, weeds were counted and collected between 5 rows with a width of 0.20 m between 2 rows (4 × 0.20 m × 1 m = 0.80 m2) using a quadrate. Aboveground weed biomass was harvested in the three sampling areas per plot, then pooled and sundried. Samples were dried in a hot air oven at 70 °C for at least 24 h, after which the dry biomass was determined. When density and biomass were determined in the same year, weeds were counted in the collection area while being harvested for biomass.

Table 3.
Overview of the timing in days after sowing of measurements taken in the trial.
Weeds were identified using the keys provided by National Commission for knowledge and use of Biodiversity, Mexico [33].
2.4. Data Analysis
Indices were calculated as follows:
The Shannon–Weaver diversity index (H′) [34] was calculated using the following equation:
where pi is the relative density of the i-th species.
Species evenness was calculated using the following equation:
Weed control efficiency was calculated as:
The complete dataset is available from Dataverse [35].
Statistical analysis of the effect of the experimental factors on weed biomass and weed density was performed using R 3.1.1 [36]. Generalized linear models and analysis of variance (ANOVA) were used to assess the effects of the components of CA on weed biomass, weed density, H’ and J’, using the “glm” and “aov” functions from the “stats” package. Treatment effects were analyzed per crop and with and without weed control using the following model:
where R is the factor “Residue” (keeping all residues in the field or removing all residues), C is the factor “Crop rotation” (monocropping or wheat–maize rotation) and T is the factor “Tillage” (conventional tillage or zero tillage). The year of sampling and repetition were considered to be random effects. In the case of significant interactions between the factors, the datasets were split according to the respective factors and analyzed per factor level. The p level of significance for all effects mentioned in the text was 0.05. Post-hoc analysis was performed using the “HSD” (Honestly Significant Differences) function from the “agricolae” package, which makes multiple comparisons of treatments by means of Tukey.
Y = α + βR + γC + δT + εR × C + ζR × T + ηC × T + θR × C × T + Residual
3. Results
Over the years, 40 different weed species were observed in the trial (Table S1). Most species were annual dicots or broadleafs. Of all the identified species, 31 were annuals and 9 were perennials, with 30 dicots and 10 monocots. Only 10 weed species (Portulaca oleracea, Amaranthus hybridus, Sonchus oleraceus, Verbena bipinnatifida, Euphorbia stictospora, Galinsoga parviflora, Malva parviflora, Oxalis corniculata, Eragrostis mexicana and Bromus carinatus) were observed every year; of these, O. corniculata and E. mexicana were the most dominant. O. corniculata was mainly associated with maize, while E. mexicana was mainly associated with wheat.
Herbicides were generally effective in weed suppression. The subplots with herbicides showed significantly lower weed biomass (31 ± 5 g m−2) than the subplots without herbicide application (188 ± 16 g m−2). Lower weed biomass was recorded in maize treated with herbicides than in wheat treated with herbicides (17 ± 5 g m−2 and 34 ± 8 g m−2, respectively, excluding observations from 2014, when data were only collected from wheat). There was a significant relationship between herbicide application and the tillage system on weed biomass, so data with and without herbicide were analyzed separately. Weed control efficiency was 93% in maize on average, but lower in wheat at 68%, mainly due to presence of E. mexicana; the minimum occurred in 2014, when it was only 33%. Tillage, residues and rotation did not influence weed control efficiency of herbicide applications. The treatment with the lowest weed control efficiency was W-Mon-ZT-K with an average of 52%, which demonstrated significantly lower weed control efficiency than all maize rotation treatments. Weed density was significantly higher without herbicide application than with herbicide application, resulting in 208 ± 29 plants and 134 ± 23 plants m−2, respectively.
3.1. Effects of Conservation Agriculture and Its Components on Weed Biomass and Density
In maize with herbicide application, a significant effect of crop rotation was shown only on weed biomass, while the effects of tillage practice, residue management and interaction effects were not significant. Crop rotation in maize significantly reduced weed biomass by 87%, resulting in 31 ± 10 g m−2 in monoculture and 4 ± 1 g m−2 in rotation on average (Figure 1, Table 4). ZT and CT in maize resulted in similar weed biomasses on average (18 ± 6 g m−2 in CT and 18 ± 9 g m−2 in ZT). Treatment effects were generally consistent over the measured seasons, although weed pressure magnitude differed widely throughout the years (Figure S1).
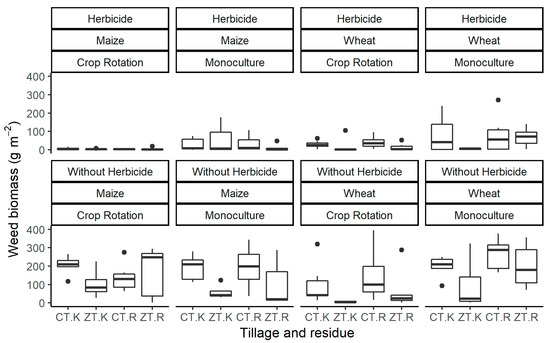
Figure 1.
Boxplots of weed biomass observed in 2005, 2013, 2014 and 2015 with and without herbicide application in terms of crop, crop rotation, tillage and residue management. CT: Conventional tillage; ZT: Zero tillage; R: Remove all residues; K: Keep all residues. n = 6 (maize) and 8 (wheat).

Table 4.
Analysis of variance (ANOVA) of weed biomass per crop (wheat or maize) and per weed management (with or without herbicide application). * indicates significant effects at p < 0.05.
In herbicide-applied wheat, a significant effect of rotation was observed, with a weed biomass of 58 ± 13 g m−2 in the monoculture treatments and 24 ± 5 g m−2 in the treatments with rotation. Weed biomass was also significantly higher in wheat with CT than with ZT. There was no overall significant effect of residue in wheat, although weeds were significantly reduced in W-Mon-ZT-K compared with W-Mon-ZT-R (treatment abbreviations in Table 1). Weed biomass was higher in the treatments with wheat monocropping, except in W-Mon-ZT-K, mainly because of an infestation with E. mexicana which was not controlled by the clodinafop or fenoxaprop herbicides used.
In maize subplots without herbicide application (Figure 1), a significant interaction was observed among all factors (tillage, rotation and residue), making interpretation of the overall effects complicated. Treatments with CT resulted in higher weed biomass than ZT treatments (220 ± 27 compared to 178 ± 43 g m−2), with treatments with rotation having lower weed biomass on average than monocultures (176 ± 29 compared to 222 ± 41 g m−2), but no apparent difference was observed due to residue retention (200 ± 35 and 198 ± 37 g m−2 with K and R, respectively). Effects of the treatments varied over the years; in 2005, the effects of all factors were significant, but this was not the case for 2013 and 2015 (Figure S1).
In wheat without herbicide application, weed biomass was significantly higher in the CT treatments compared to the ZT treatments throughout all four years, but there were no significant effects due to rotation or tillage. Treatment effects varied over the years; in 2005 and 2014, there was only an effect due to crop rotation, but 2005 demonstrated higher weed dry biomass with crop rotation, probably due to a combination of initial drought and higher soil fertility with crop rotation, whereas lower weed biomass with crop rotation was observed in 2014. In 2013, all factors produced significant effects on weed biomass in wheat, and in 2015, rotation and ZT resulted in significantly reduced weed biomass (Figure S1).
In maize and wheat, weed biomass was markedly lower when crops were planted in CA (a combination of ZT with retained residue and crop rotation) compared with treatments representing the conventional production system of the Mexican highlands, i.e., CT with residue removal and monoculture of maize or wheat. When herbicide was applied, the weed biomass was 34 ± 18 g m−2 in M-Mon-CT-R, with M-Rot-ZT-K showing a biomass of 3 ± 1 g m−2, 91% lower; similarly in wheat, weed biomass was 77 ± 33 g m−2 in W-Mon-CT-R, while W-Rot-ZT-K showed a result 81% lower at 15 ± 13 g m−2. Differences were similar without herbicide application; weed biomass was 299 ± 79 g m−2 in M-Mon-CT-R, while in M-Rot-ZT-K this was 66% lower at 102 ± 29 g m−2. Similarly, in wheat, weed biomass was 288 ± 37 g m−2 in W-Mon-CT-R, while this was 59% lower in W-Rot-ZT-K at 118 ± 75 g m−2.
Regardless of herbicide application, no significant differences were observed in weed density between factors or between treatments throughout the whole time period (Table 5) due to the large variation in weed density between the years (Figure 2, Figure S2). Without herbicide application, significantly fewer weeds were produced in ZT compared to CT in 2013, but no significant effects of the factors were observed in the other years. With herbicide application, significantly fewer weeds were observed in maize than in wheat in 2015, with 63 ± 21 and 136 ± 49 plants m−2, respectively. In maize, treatments with rotation showed similar weed densities, while maize monoculture produced a significantly lower weed density in CT and with residue retention. In wheat, there were significantly more weeds after monoculture treatments than with rotation. Treatments with monoculture resulted in significantly more weeds in ZT than in CT, while rotation treatments with tillage did not affect weed density. In 2013, significantly fewer weeds were observed in maize with crop rotation than with monoculture, 14 ± 4 and 122 ± 30 plants m−2, respectively, while rotation did not affect weed density in wheat. In contrast, in 2004 and 2014, no significant effects on weed density were observed as a result of any of the factors.

Table 5.
Analysis of variance (ANOVA) of weed density per crop (wheat or maize) and per weed management (with or without herbicide application).
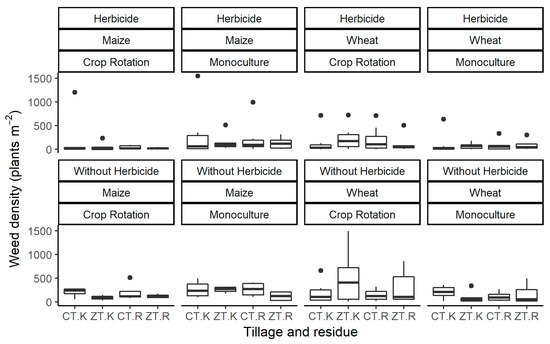
Figure 2.
Boxplots of weed density with herbicide application observed in 2004, 2013, 2014 and 2015 and without herbicide application in 2013, 2014 and 2015 in terms of crop, crop rotation, tillage and residue management. CT: Conventional tillage; ZT: Zero tillage; R: Remove all residues; K: Keep all residues. n = 6 (maize) and 8 (wheat).
3.2. Effects of Conservation Agriculture and Its Components on Weed Diversity
Overall, in ZT, perennial weeds were of minor importance in the weed community counts. Of the perennial weed species, Oxalis spp. was the most common, followed by Alternanthera caracasana and B. carinatus. With herbicide application, only the number of perennial weeds was significantly influenced by crop rotation and were shown to be significantly more abundant in treatments with maize monoculture compared to the other treatments. This was mainly due to the high incidence of O. corniculata in the maize monoculture treatments. Without herbicide application, there was a significant effect from the rotation × tillage interaction on the density of perennial weeds, with perennial weeds more abundant in maize monoculture with CT, while in the maize–wheat rotation there were no differences in perennial weed density.
In maize, an average of 45% of counted weeds were Oxalis spp., with 13% E. mexicana. In wheat, 30% of the weeds were E. mexicana and 24% were Oxalis spp. Other common weeds included Euphorbia stictospora, with 5% of observations in maize and 7% in wheat. In maize, Portulaca oleracea was another common weed, making up 5% of the observations, and in wheat Acalypha mexicana was common, with 7% of the observations. None of the other weeds observed formed more than 5% of the counted weed population in any of the years of observation. Because of the dominance of Oxalis spp. in maize and E. Mexicana in wheat, monocots were more dominant in the weed community in wheat, while dicots were more dominant in maize. Without herbicide application, significantly more monocots were observed in ZT, while there were no differences in monocot density due to tillage when herbicides were applied. Sedges were not commonly observed in the trial.
The weed community diversity per treatment was rather low in the trial, with the number of species observed per plot ranging between 0 and 12 in any given year. When herbicides were applied, the number of species was significantly impacted by management factors (Table 6). In wheat, the number of species was significantly higher in treatments with rotation compared to monoculture and with CT compared to ZT. In maize, the number of species was higher in ZT than in CT. There was also a significant rotation × residue interaction observed, with the number of species higher with residues than without in maize monoculture treatments, while rotation treatments demonstrated no effect of residue management. Without herbicides, species diversity and evenness only differed significantly with rotation, with the effect of residue management on species diversity appearing marginally significant (p = 0.06). There was higher species diversity and evenness in treatments with rotation compared to monoculture, and higher species diversity and evenness in treatments with residue retention compared to residue removal.

Table 6.
Species diversity, species evenness and number of species determined in plots without herbicide application. Averages for 2013, 2014 (only wheat plots) and 2015. Abbreviations: H’: Species diversity; J’: evenness; S: number of species. Weeds were not sampled in maize in 2014.
4. Discussion
This study demonstrated that, at least in the long term, weeds are not necessarily problematic in CA, and CA can lead to lower weed pressure compared to CT if adequate weed management is performed, as proposed in literature reviews regarding this subject [8,10]; this is true both with herbicide control and without. All three CA components showed potential to decrease weed populations. Even when the herbicides could not control weed populations adequately (e.g., the treatments with wheat monocropping showed that herbicides did not control all weed species), the combination of ZT and residue retention resulted in a significantly lower weed biomass, thereby demonstrating the usefulness of the individual CA components in weed suppression. The treatments with all three CA components generally resulted in the lowest weed biomass over the years, which may have been a result of the weed-reducing effect of all three components over the 25-year course of the experiment, leading to reduced weed seedbanks and lower weed populations in the treatments with adequate weed control through the CA components, while weed problems accumulated in treatments without adequate control, particularly in wheat.
The effect of residue retention on weeds was dependent on tillage; in CT, crop residues were incorporated by tillage, therefore, they did not form a mulch layer that suppressed weeds. Treatments with ZT resulted in low weed biomass, regardless of residue retention or removal. Weed germination, growth and development were lower in ZT, which may have been dependent on the residue management. In treatments with residue retention and ZT, soil fertility and soil water content were considerably higher compared to treatments with residue removal [5], leading to better crop growth and an improved ability of the crop to compete with weeds. The mulch cover, especially after maize, prevents weeds from germinating and may facilitate higher seed predation due to favorable conditions for soil fauna, such as ants and beetles [8,37]. In treatments with ZT but without residue retention, the soil was degraded and showed poor fertility and lower infiltration [5], which created an unfavorable environment for weeds to germinate and grow. Wheat was shown to leave more stubble when harvested than maize, and previous studies showed that ZT-treated soil with wheat monoculture or maize–wheat rotation without residue was less degraded than treatments with maize monoculture [38,39]. Therefore, in ZT treatments with residue removal, weed biomass was the lowest in M-Mon-ZT-R treatments, higher in M-Rot-ZT-R and W-Rot-ZT-R treatments, and highest in the W-Mon-ZT-R treatment, proving that weed management must be considered when designing CA systems, since the crops available for rotation and the residue that they produce affect weed control and growth.
The dominant weed species were different in maize and wheat, and their presence was related to management factors. In wheat, E. mexicana was the dominant weed, with mulching with residues helping to suppress this problem. In the W-Mon-ZT-R treatment, weed biomass was high due to the infestation with E. mexicana, while in W-Mon-ZT-K, it was significantly lower due to the presence of residues. This may have been due to the residues impeding germination, the small seeds having insufficient reserves to push through the residue cover, higher seed predation with residues present, or because of the residues’ allelopathic effects. In wheat, the same herbicides (e.g., fenoxaprop or clodinafop) were used since the beginning of the experiment for weed control, but they did not control E. mexicana, leading to an increase in the presence of this weed. Alternative active ingredients available on the local market were tested in separate experiments, but none were able to control E. mexicana. In maize, E. mexicana was not a problem due to S-metolachlor effectively controlling the weed, combined with the higher competitiveness of maize with E. mexicana. Similar results were obtained in an experiment with winter wheat in Alberta, where populations of Bromus tectorum increased over the course of six years in wheat monoculture treatments, but not in a wheat–canola rotation, where it could be controlled with herbicides in canola [40]. In maize, Oxalis spp. weeds were the most common. As Oxalis spp. do not compete strongly with maize, especially later in the growing season, applying additional herbicides to control Oxalis spp. in maize was not considered to be necessary, leading to an increase in Oxalis spp. propagules in maize monocultures. In maize, treatments with monoculture had higher weed density on average due to presence of Oxalis spp., especially in CT. Crop rotation helped suppress the presence of this weed in maize; Oxalis spp. was not common in wheat.
Treatments with rotation resulted in larger weed diversity, which may have helped the crops avoid weed problems. The more diverse the weed community in a field, the less likely one dominant weed is to cause serious problems [41]. The diversity of weeds in a field depends on the local ecology and on station conditions. Changes appeared to occur in the weed species present in the trial since its initiation in 1992. While P. oleracea, A. hybridus, S. oleraceus, V. bipinnatifida, E. stictospora, G. parviflora, M. parviflora, O. corniculata, E. mexicana and B. carinatus were the most common weeds observed in this study, Elusine, Eragrostis, Setaria, Cyperus, Oxalis, Alcalypha, Galinsoga, Taraxacum, Portulaca and Sonchus were reported to be the most common weed genera in the 1992–1995 period [42]. Taraxacum was only observed in 8 out of 256 plots sampled over the years, while Setaria was not observed during our study. These shifts in populations were similar to those reported by [43,44], who reported rapid changes in dominant weed species during a five-year trial with tillage and herbicide treatments.
No indication of a higher incidence of perennial weeds in CA was found, which was similar to the findings of other studies; for example, a study on tillage and rotation in Argentina did not find a consistent relationship between tillage reduction and the presence of perennial weeds [20]. This is, of course, not a general conclusion, as it is highly dependent on the weed species present, the climate and the field management. The most common perennial weed species at the site, O. corniculata, was more abundant in CT, especially in maize. These observations were considered to be long-term effects of CA, as the treatment was in place for more than 25 years and occurred in a research station in which weeds were controlled for over 50 years. In Mexico, agroecological conditions vary widely across the country. Therefore, to gain more conclusive results, it is necessary to study the effects of CA in on-farm trials, different agroecologies and on different time scales.
5. Conclusions
Weed density and biomass were lower in long-term conservation agriculture than in conventional tillage, confirming the hypothesis described previously. The three components of conservation agriculture (zero tillage, residue retention and maize-wheat rotation) reduced weed biomass, which was lower when all three components were applied together. The exception was the treatment with maize monoculture, zero tillage and residue removal, where low weed densities were observed due to severe soil degradation. Different treatments favored different weed species, but no trend toward increased perennial weeds was observed in conservation agriculture. The data supported claims stating that if adequate weed control is achieved in the initial years, weed populations in conservation agriculture systems would be lower than in conventional tillage systems. Given the weed-controlling effects of conservation agriculture, this practice will likely lead to lower herbicide use in the long run.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/7/962/s1. Table S1: Weed species observed and identified in the trial (some minor weed species could not be identified due to lack of flowers). Figure S1: Weed biomass per year with herbicide application and without herbicide application in function of crop, crop rotation, tillage and residue management. CT: Conventional tillage; ZT: Zero tillage; R: Remove all residues; K: Keep all residues. Figure S2: Weed density per year with herbicide application and without herbicide application in function of crop, crop rotation, tillage and residue management. CT: Conventional tillage; ZT: Zero tillage; R: Remove all residues; K: Keep all residues.
Author Contributions
Conceptualization, B.G., N.V. and S.F.; methodology, B.G. and N.V.; formal analysis, S.F., N.V. and R.G.S.; investigation, B.G. and N.V.; data curation, S.F. and N.V.; writing—original draft preparation, S.F.; writing—review and editing, N.V., R.G.S. and B.G.; visualization, S.F. All authors read and agreed to the published version of the manuscript.
Funding
This work was implemented by CIMMYT as part of the project “MasAgro Productor”, made possible by the generous support of the Mexican Government through SADER. The project was part of the CGIAR Research Program on Maize (MAIZE), which was generously supported by W1 and W2 donors, including the Governments of Australia, Belgium, Canada, China, France, India, Japan, Korea, Mexico, Netherlands, New Zealand, Norway, Sweden, Switzerland, U.K., U.S., and the World Bank. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the donors mentioned previously.
Acknowledgments
We thank several students from UGent and KULeuven for their help with data collection. We thank Fabian Enyanche, Rachael Cox, Francisco Javier Vargas, Humberto González, José de Jesús Miranda, Daniel Terrazas and Rodolfo Gómez for their help in sampling and Carolina Jaramillo Escalante for data curation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Govaerts, B.; François, I.; Verhulst, N. Conservation agriculture (CA) for sustainable intensification of maize and other cereal systems: The case of Latin America. In Conservation Agriculture for Sustainable Intensification of Maize and Other Cereal Systems; Burleigh Dodds Science Publishing: London, UK, 2017; pp. 81–105. [Google Scholar]
- Govaerts, B.; Sayre, K.D.; Goudeseune, B.; De Corte, P.; Lichter, K.; Dendooven, L.; Deckers, J. Conservation agriculture as a sustainable option for the central Mexican highlands. Soil Tillage Res. 2009, 103, 222–230. [Google Scholar] [CrossRef]
- Thierfelder, C.; Rusinamhodzi, L.; Ngwira, A.R.; Mupangwa, W.; Nyagumbo, I.; Kassie, G.T.; Cairns, J.E. Conservation agriculture in Southern Africa: Advances in knowledge. Renew. Agric. Food Syst. 2015, 30, 328–348. [Google Scholar] [CrossRef]
- Giller, K.E.; Andersson, J.A.; Corbeels, M.; Kirkegaard, J.; Mortensen, D.; Erenstein, O.; Vanlauwe, B. Beyond conservation agriculture. Front. Plant Sci. 2015, 6, 870. [Google Scholar] [CrossRef]
- Verhulst, N.; Nelissen, V.; Jespers, N.; Haven, H.; Sayre, K.D.; Raes, D.; Deckers, J.; Govaerts, B. Soil water content, maize yield and its stability as affected by tillage and crop residue management in rainfed semi-arid highlands. Plant Soil 2011, 344, 73–85. [Google Scholar] [CrossRef]
- Fuentes, M.; Govaerts, B.; De León, F.; Hidalgo, C.; Dendooven, L.; Sayre, K.D.; Etchevers, J. Fourteen years of applying zero and conventional tillage, crop rotation and residue management systems and its effect on physical and chemical soil quality. Eur. J. Agron. 2009, 30, 228–237. [Google Scholar] [CrossRef]
- Lee, N.; Thierfelder, C. Weed control under conservation agriculture in dryland smallholder farming systems of southern Africa. A review. Agron. Sustain. Dev. 2017, 37, 48. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Mahajan, G. Role of integrated weed management strategies in sustaining conservation agriculture systems. Curr. Sci. 2012, 103, 135–136. [Google Scholar]
- Wall, P.C. Tailoring Conservation Agriculture to the Needs of Small Farmers in Developing Countries: An Analysis of Issues. J. Crop Improv. 2007, 19, 137–155. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crop. Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Buhler, D.D.; Hartzler, R.G.; Forcella, F. Implications of weed seedbank dynamics to weed management. Weed Sci. 1997, 45, 329–336. [Google Scholar] [CrossRef]
- Travlos, I.S.; Cheimona, N.; Roussis, I.; Bilalis, D.J. Weed-Species Abundance and Diversity Indices in Relation to Tillage Systems and Fertilization. Front. Environ. Sci. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Liebman, M.; Gallandt, E.R. Many Little Hammers: Ecological Management of Crop-Weed Interactions. In Ecology in Agriculture; Jackson, L.E., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 291–343. [Google Scholar]
- Clements, D.R.; Benoit, D.L.; Murphy, S.D.; Swanton, C.J. Tillage Effects on Weed Seed Return and Seedbank Composition. Weed Sci. 1996, 44, 314–322. [Google Scholar] [CrossRef]
- Cromar, H.E.; Murphy, S.D.; Swanton, C.J. Influence of tillage and crop residue on postdispersal predation of weed seeds. Weed Sci. 1999, 47, 184–194. [Google Scholar] [CrossRef]
- Anderson, R.L. A multi-tactic approach to manage weed population dynamics in crop rotations. Agron. J. 2005, 97, 1579–1583. [Google Scholar] [CrossRef]
- El Keblawy, A. Impact of crop residues on seed germination of native desert plants grown as weeds. Afr. J. Biotechnol. 2012, 11, 7836–7842. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mohler, C.L. The quantitative relationship between weed emergence and the physical properties of mulches. Weed Sci. 2006, 48, 385–392. [Google Scholar] [CrossRef]
- Tuesca, D.; Puricelli, E.; Papa, J.C. A long-term study of weed flora shifts in different tillage systems. Weed Res. 2001, 41, 369–382. [Google Scholar] [CrossRef]
- Buhler, D.D.; Stoltenberg, D.E.; Becker, R.L.; Gunsolus, J.L. Perennial Weed Populations After 14 Years of Variable Tillage and Cropping Practices. Weed Sci. 1994, 42, 205–209. [Google Scholar] [CrossRef]
- Trichard, A.; Alignier, A.; Chauvel, B.; Petit, S. Identification of weed community traits response to conservation agriculture. Agric. Ecosyst. Environ. 2013, 179, 179–186. [Google Scholar] [CrossRef]
- Farooq, M.; Flower, K.C.C.; Jabran, K.; Wahid, A.; Siddique, K.H.M. Crop yield and weed management in rainfed conservation agriculture. Soil Tillage Res. 2011, 117, 172–183. [Google Scholar] [CrossRef]
- Clements, D.R.; Weise, S.F.; Swanton, C.J. Integrated weed management and weed species diversity. Phytoprotection 1994, 75, 1–18. [Google Scholar] [CrossRef]
- Fonteyne, S.; Peñaloza, O.N.; Alcalá, L.O.; Rodríguez, C.S.; Villalcantara, J. Control de malezas en maíz en tres regiones de Oaxaca: Primeros resultados de diferentes manejos de malezas en labranza convencional, mínima y cero. In Red de Plataformas de Investigación MasAgro-Resultados PV2016 y OI 2016–17; Fonteyne, S., Verhulst, N., Eds.; CIMMYT: Texcoco, Mexico, 2017; pp. 146–151. [Google Scholar]
- Federer, W.T. Experimental Designs: Theory and Applications; Oxford and IBH Publishing Company: New Delhi, India, 1977; p. 591. [Google Scholar]
- Federer, W.T.; José Crossa, J. Screening experimental designs for quantitative trait loci, association mapping, genotype by environment interaction, and other investigations. Front. Physiol. 2012, 3, 156. [Google Scholar] [CrossRef] [PubMed]
- Fay, D.S.; Gerow, K. A Biologist’s Guide to Statistical Thinking and Analysis. WormBook: The Online Review of C. Elegans Biology. 2013. Available online: http://www.wormbook.org/chapters/www_statisticalanalysis/statisticalanalysis.html (accessed on 29 May 2020).
- Fonteyne, S.; Gamiño, M.M.; Tejeda, A.S.; Verhulst, N. Conservation Agriculture Improves Long-term Yield and Soil Quality in Irrigated Maize-oats Rotation. Agronomy 2019, 9, 845. [Google Scholar] [CrossRef]
- Grahmann, K.; Verhulst, N.; Palomino, L.M.; Bischoff, W.A.; Govaerts, B.; Buerkert, A. Ion exchange resin samplers to estimate nitrate leaching from a furrow irrigated wheat-maize cropping system under different tillage-straw systems. Soil Tillage Res. 2018, 175, 91–100. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Deckers, J. Stable high yields with zero tillage and permanent bed planting? Field. Crop. Res. 2005, 94, 33–42. [Google Scholar] [CrossRef]
- Fonteyne, S.; Verhulst, N. Celebrando más de 25 años del experimento a largo plazo ‘D5’ o la plataforma Texcoco, I., Estado de México. In Red de Plataformas de Investigación MasAgro-Resultados PV2016 y OI 2016–17; Fonteyne, S., Verhulst, N., Eds.; CIMMYT: Texcoco, Mexico, 2017; pp. 188–189. [Google Scholar]
- Malezas de México. Available online: http://www.conabio.gob.mx/malezasdemexico/2inicio/home-malezas-mexico.htm (accessed on 10 February 2020).
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 3–24. [Google Scholar]
- Verhulst, N.; Fonteyne, S.; Govaerts, B. Weed Biomass and Density in a Long-term Conservation Agriculture Experiment in Mexico. hdl:11529/10548424, CIMMYT Research Data & Software Repository Network. 2020. Available online: https://data.cimmyt.org/dataset.xhtml?persistentId=hdl:11529/10548424 (accessed on 11 May 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 29 May 2020).
- Trichard, A.; Ricci, B.; Ducourtieux, C.; Petit, S. The spatio-temporal distribution of weed seed predation differs between conservation agriculture and conventional tillage. Agric. Ecosyst. Environ. 2014, 188, 40–47. [Google Scholar] [CrossRef]
- Verhulst, N.; Govaerts, B.; Nelissen, V.; Sayre, K.D.; Crossa, J.; Raes, D.; Deckers, J. The effect of tillage, crop rotation and residue management on maize and wheat growth and development evaluated with an optical sensor. Field. Crop. Res. 2011, 120, 58–67. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Deckers, J. A minimum data set for soil quality assessment of wheat and maize cropping in the highlands of Mexico. Soil Tillage Res. 2006, 87, 163–174. [Google Scholar] [CrossRef]
- Blackshaw, R.E. Rotation Affects Downy Brome (Bromus tectorum) in Winter Wheat (Triticum aestivum). Weed Technol. 1994, 8, 728–732. [Google Scholar] [CrossRef]
- Storkey, J.; Neve, P. What good is weed diversity? Weed Res. 2018, 58, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Santiveri, F.; Vidal, I. Crop rotation, tillage and crop residue management for wheat and maize in the sub-humid tropical highlands. Field Crop. Res. 2002, 79, 123–137. [Google Scholar] [CrossRef]
- Norsworthy, J.K. Effect of tillage intensity and herbicide programs on changes in weed species density and composition in the southeastern coastal plains of the United States. Crop Prot. 2008, 27, 151–160. [Google Scholar] [CrossRef]
- Murphy, S.D.; Clements, D.R.; Belaoussoff, S.; Kevan, P.G.; Swanton, C.J. Promotion of weed species diversity and reduction of weed seedbanks with conservation tillage and crop rotation. Weed Sci. 2006, 54, 69–77. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).