Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design
2.3. Gas Chromatography–Electron Impact–Mass Spectrometry (GC/EI/MS) Metabolomics Analysis of Cichorium spinosum L.
2.3.1. Chemicals and Reagents
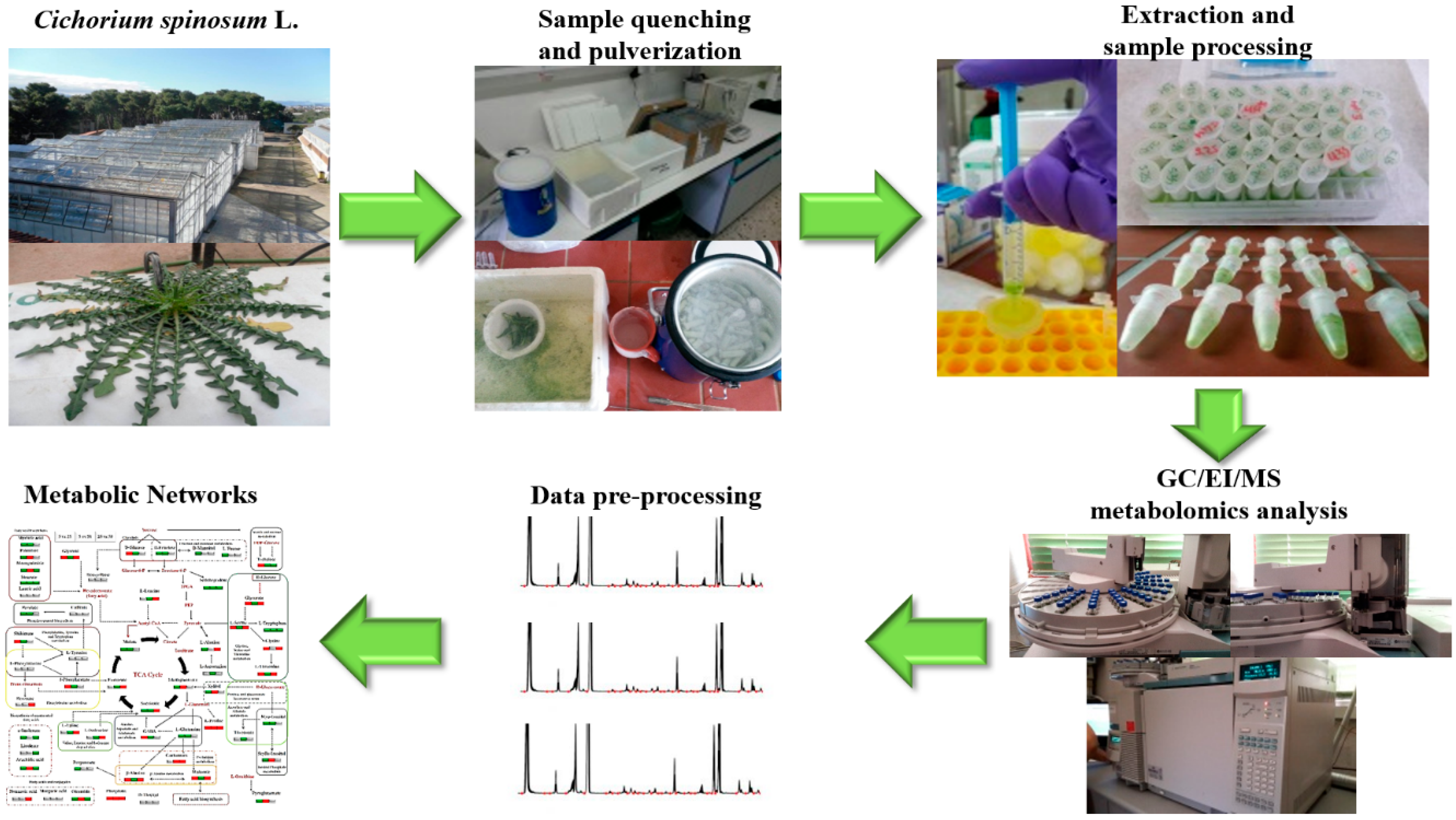
2.3.2. Sampling and Extraction of Cichorium spinosum L. Leaves and Preparation for Metabolomics Analyses
2.3.3. Gas Chromatography–Electron Impact–Mass Spectrometry (GC/EI/MS) Analyses and Bioinformatics Analysis
3. Results
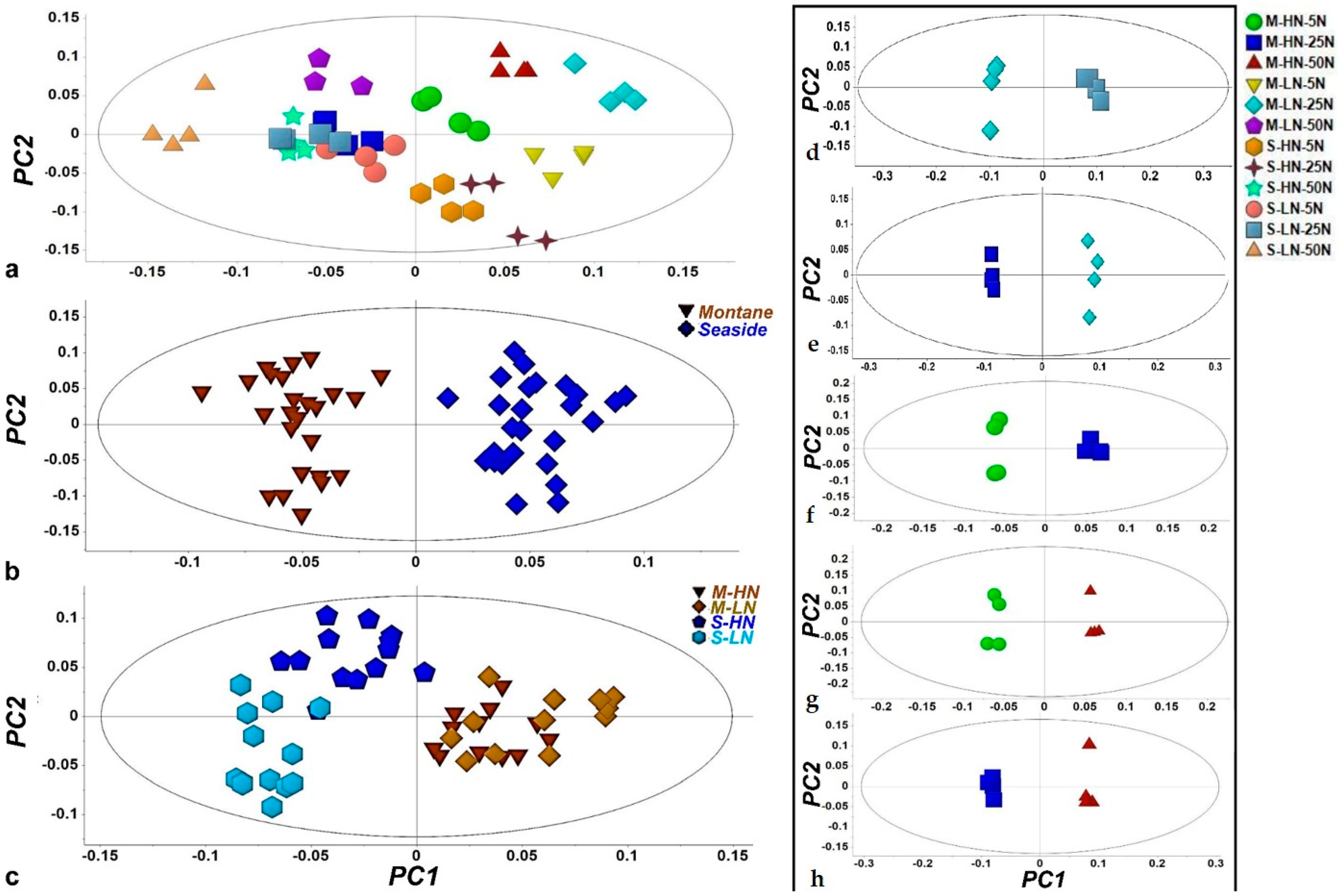
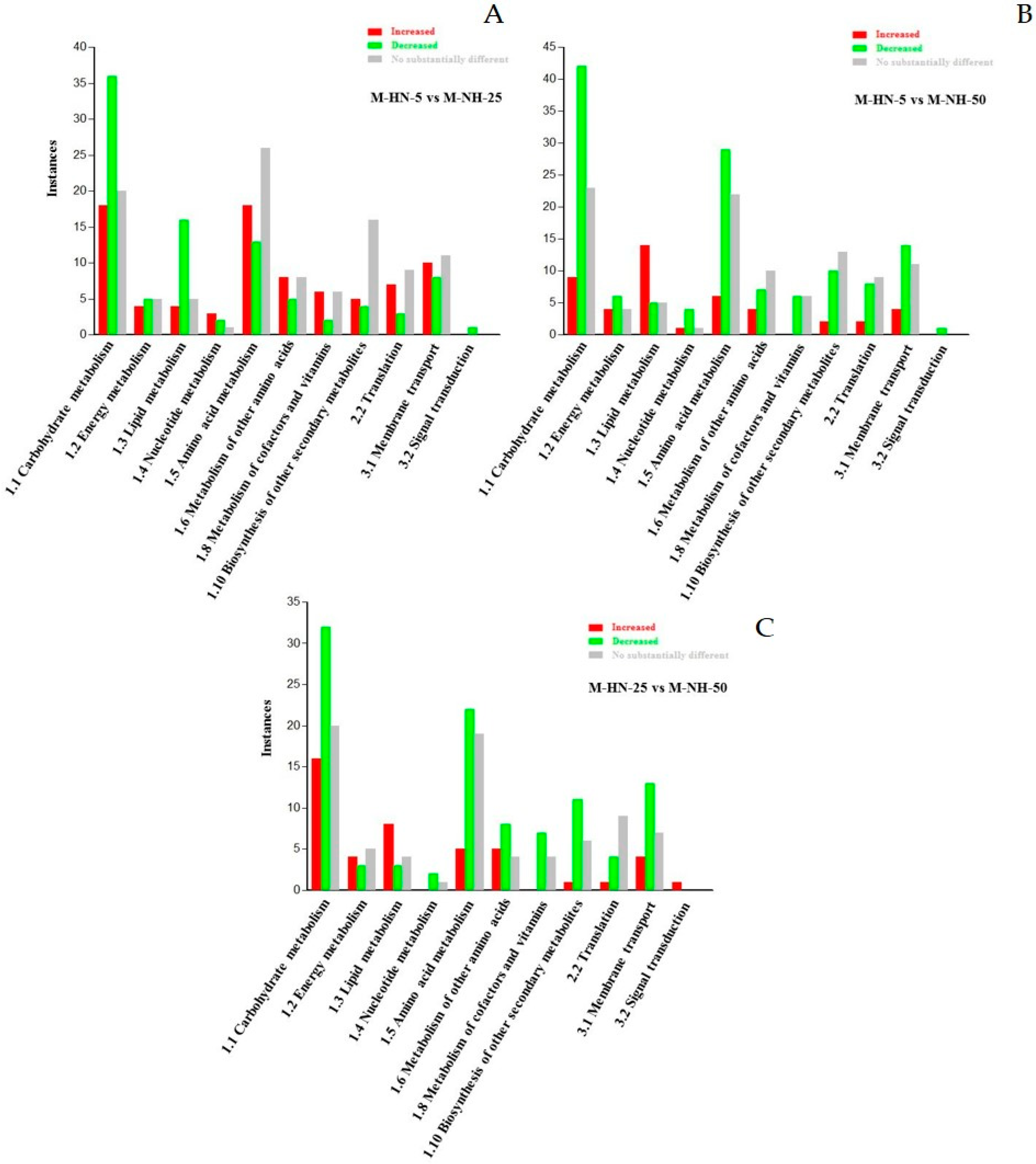
3.1. Overview of the GC/EI/MS Metabolomics Analysis
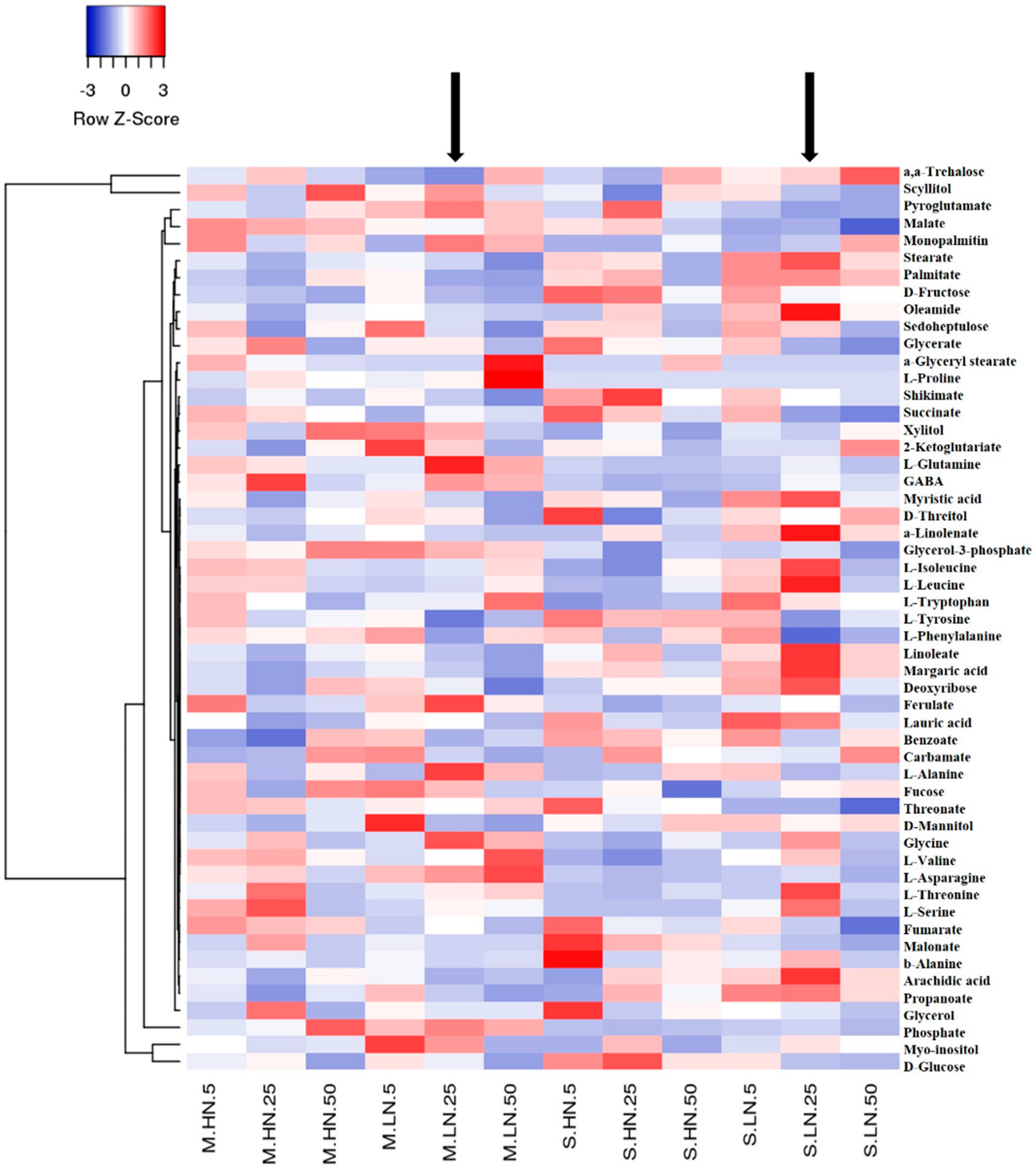
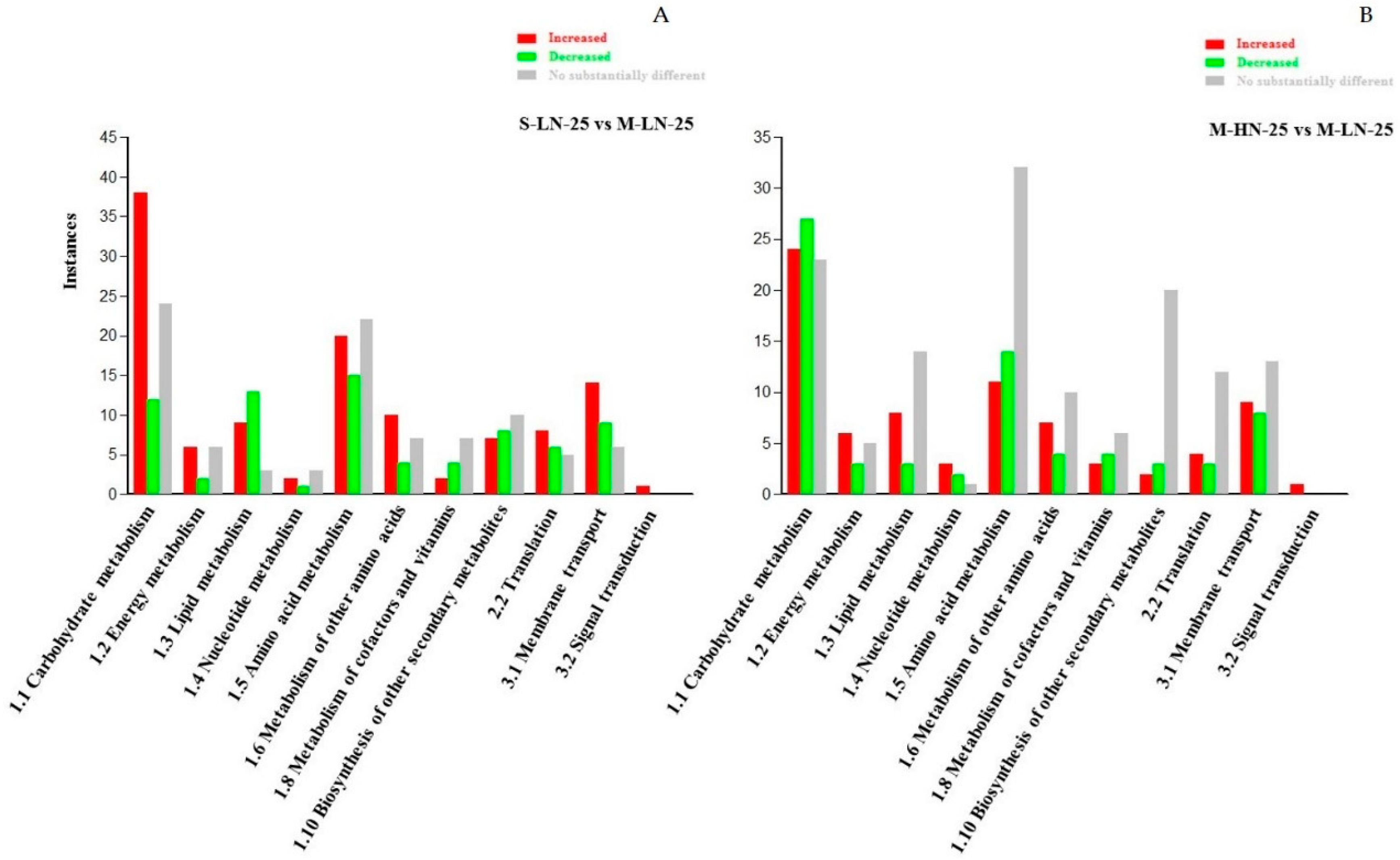
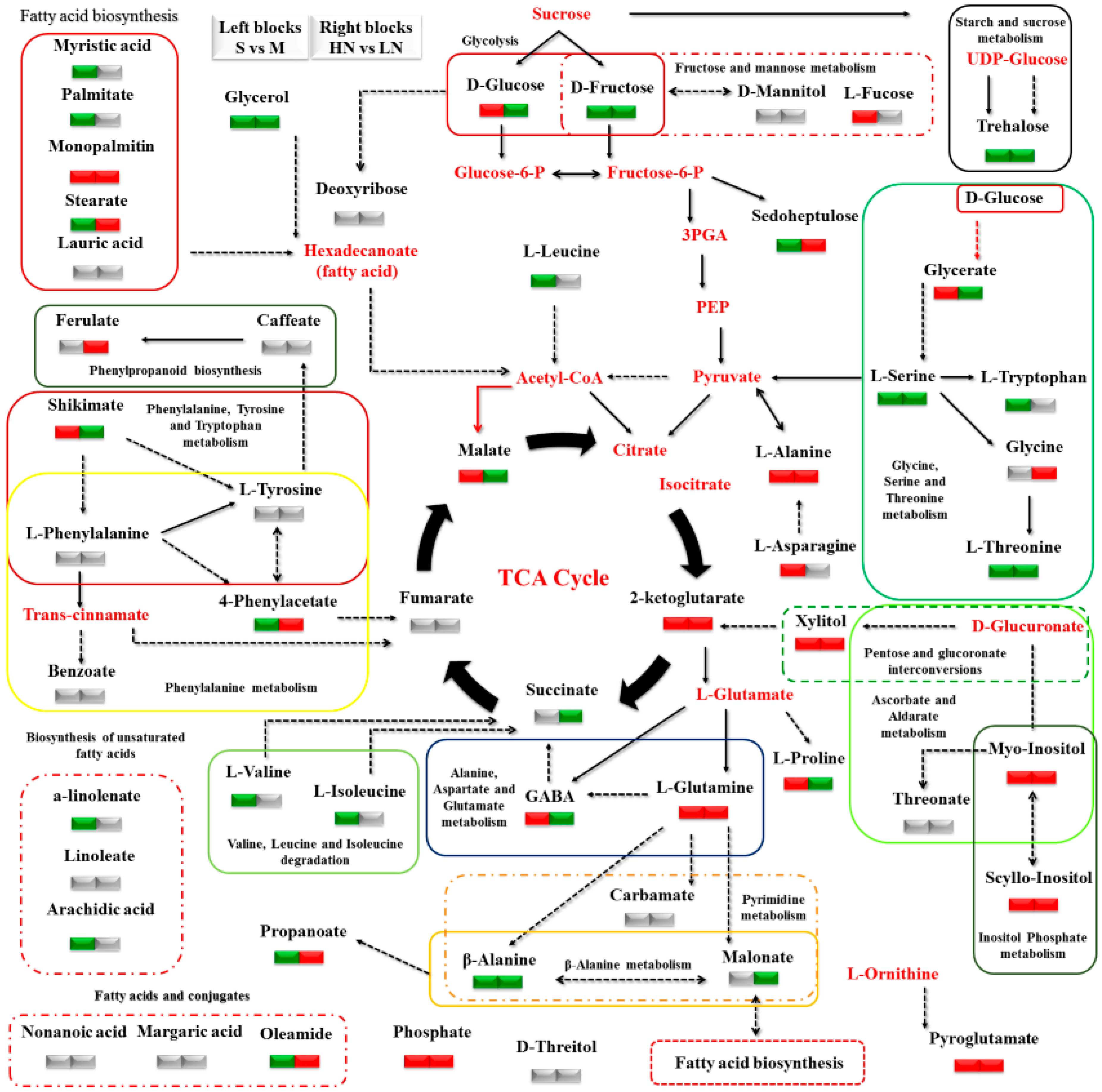
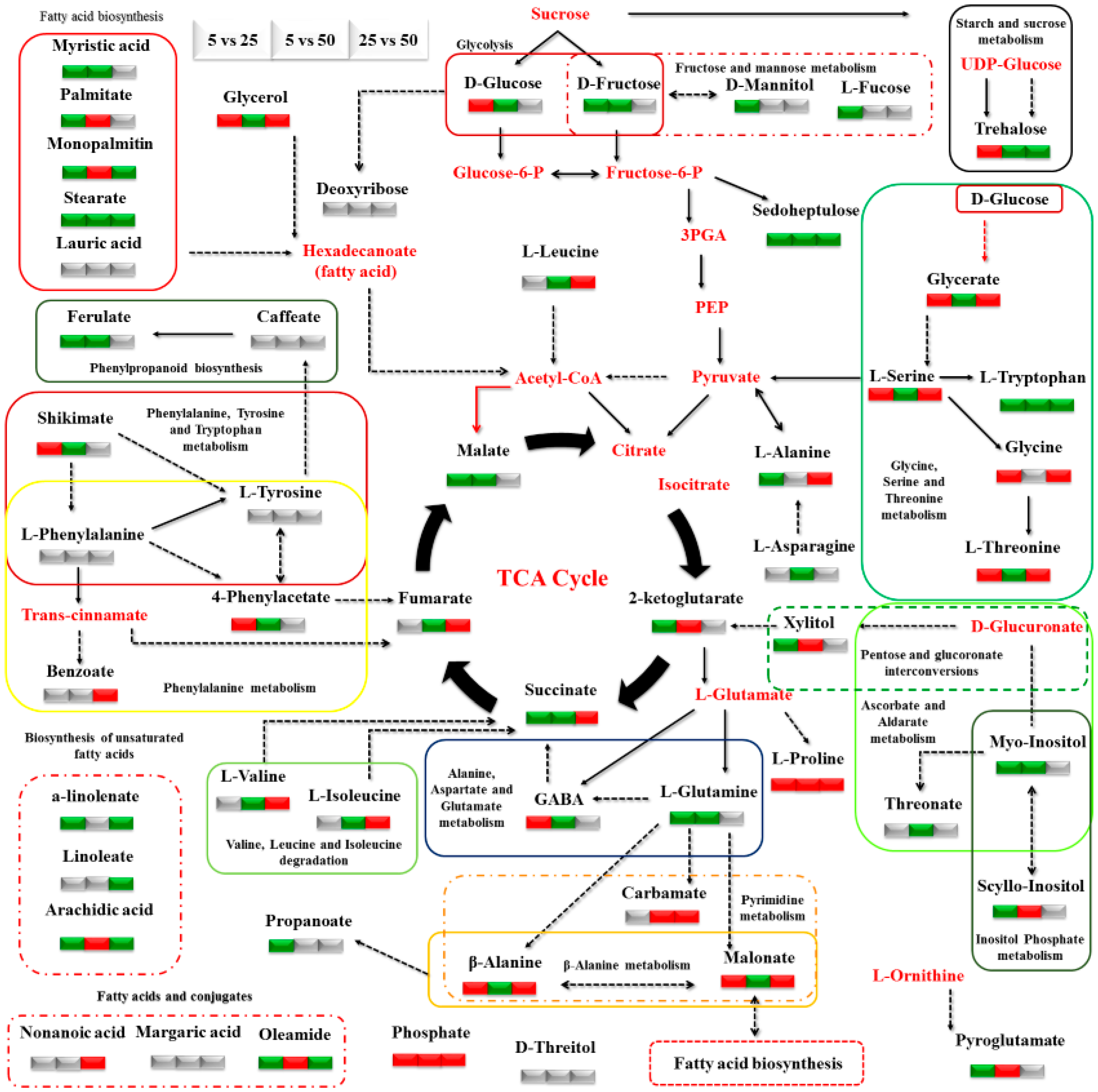
3.2. Impact of N Supply Level and N Source Ratio on the Amino Acid (AA) Pool of Stamnagathi
3.3. Impact of N Supply Level and N Source Ratio on Carbohydrate Content
3.4. Impact of N Supply Level and N Source Ratio on Carboxylic Acids, Fatty Acids, and Selected Stamnagathi Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- von Wiren, N.; Gazzarrini, S.; Gojon, A.; Frommer, W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000, 3, 254–261. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F.; Todaro, E. A survey of nitrate and oxalate content in fresh vegetables. J. Sci. Food Agric. 1999, 79, 1882–1888. [Google Scholar] [CrossRef]
- Bryan, N.S.; Alexander, D.D.; Coughlin, J.R.; Milkowski, A.L.; Boffetta, P. Ingested nitrate and nitrite and stomach cancer risk: An updated review. Food Chem. Toxicol. 2012, 50, 3646–3665. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.A.; Gutierrez, R.A. A systems view of nitrogen nutrient and metabolite responses in Arabidopsis. Curr. Opin. Plant Biol. 2008, 11, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Crawford, N.M.; Coruzzi, G.M.; Tsay, Y.F. Nitrate signaling: Adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010, 13, 266–273. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Vidal, E.A.; Gutierrez, R.A. Integration of local and systemic signaling pathways for plant N responses. Curr. Opin. Plant Biol. 2012, 15, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, Y.S.; Walch-Liu, P.; Neumann, G.; Romheld, V.; von Wiren, N.; Bangerth, F. Root-derived cytokinins as long-distance signals for NO3−-Induced stimulation of leaf growth. J. Exp. Bot. 2005, 56, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.C.; Loef, I.; Bartetzko, L.; Searle, I.; Coupland, G.; Stitt, M.; Osuna, D. Nitrate regulates floral induction in Arabidopsis, acting independently of light, gibberellin and autonomous pathways. Planta 2011, 233, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, C.G.; Hucklesby, D.P.; Emes, M.J. Nitrate reduction and carbohydrate-metabolism in plastids purified from roots of Pisum sativum L. Planta 1989, 177, 359–366. [Google Scholar] [CrossRef]
- Bowsher, C.G.; Boulton, E.L.; Rose, J.; Nayagam, S.; Emes, M.J. Reductant for glutamate synthase is generated by the oxidative pentose-phosphate pathway in non-photosynthetic root plastids. Plant J. 1992, 2, 893–898. [Google Scholar] [CrossRef]
- Foyer, C.H.; Valadier, M.H.; Migge, A.; Becker, T.W. Drought induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol. 1998, 117, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bowsher, C.G.; Emes, M.J.; Cammack, R.; Hucklesby, D.P. Purification and properties of nitrite reductase from roots of pea (Pisum sativum cv. Meteor). Planta 1988, 175, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hoff, T.; Truong, H.N.; Caboche, M. The use of mutants and transgenic plants to study nitrate assimilation. Plant Cell Environ. 1994, 17, 489–506. [Google Scholar] [CrossRef]
- Ferrario-Mery, S.; Hodges, M.; Hirel, B.; Foyer, C.H. Photorespiration-dependent increases in phosphoenolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-alpha-ketoglutarate aminotransferase. Planta 2002, 214, 877–886. [Google Scholar] [CrossRef]
- Lancien, M.; Martin, M.; Hsieh, M.H.; Leustek, T.; Goodman, H.; Coruzzi, G.M. Arabidopsis glt1-T mutant defines a role of NADH-GOGAT in the non-photorespiratory ammonium assimilatory pathway. Plant J. 2002, 29, 347–358. [Google Scholar] [CrossRef]
- Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 1999, 2, 178–186. [Google Scholar] [CrossRef]
- Alseekh, S.; Bermudez, L.; De Haro, L.A.; Fernie, A.R.; Carrari, F. Crop metabolomics: From diagnostics to assisted breeding. Metabolomics 2018, 14, 148. [Google Scholar] [CrossRef]
- Urbanczyk-Wochniak, E.; Fernie, A.R. Metabolic profiling reveals altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2005, 56, 309–321. [Google Scholar] [CrossRef]
- Fernie, A.R. Metabolome characterization in plant system analysis. Funct. Plant Biol. 2003, 30, 111–120. [Google Scholar] [CrossRef]
- Tschoep, H.; Gibon, Y.; Carillo, P.; Armengaud, P.; Szecowka, M.; Nunes-Nesi, A.; Fernie, A.R.; Koehl, K.; Stitt, M. Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ. 2009, 32, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, U.; Masher, M.; Colmsee, C.; Scholz, U.; Bräutigam, A.; Fahnenstich, H.; Sonnewald, U. Maize source leaf adaptation to nitrogen deficiency affects not only nitrogen and carbon metabolism but also control phosphate homeostasis. Plant Physiol. 2012, 160, 1384–1406. [Google Scholar] [CrossRef]
- Amiour, N.; Imbaud, S.; Clement, G.; Agier, N.; Zivy, M.; Valot, B.; Balliau, T.; Armengaud, P.; Quillere, I.; Canas, R.; et al. The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef] [PubMed]
- Chatzigianni, M.; Ntatsi, G.; Theodorou, M.; Stamatakis, A.; Livieratos, I.; Rouphael, Y.; Savvas, D. Functional Quality, Mineral Composition and Biomass Production in Hydroponic Spiny Chicory (Cichorium spinosum L.) Are Modulated Interactively by Ecotype, Salinity and Nitrogen Supply. Front. Plant Sci. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Zeghichi, S.; Kallithraka, S.; Simopoulos, A.P. Nutritional composition of Molokhia (Corchorus olitorius) and stamnagathi (Cichorium spinosum). World Rev. Nutr. Diet. 2003, 91, 1–21. [Google Scholar] [PubMed]
- Petropoulos, S.A.; Fernandes, A.; Ntatsi, G.; Levizou, E.; Barros, L.; Ferreira, I.C.F.R. Nutritional profile and chemical composition of Cichorium spinosum ecotypes. LWT Food Sci. Technol. 2016, 73, 95–101. [Google Scholar] [CrossRef]
- Ntatsi, G.; Aliferis, K.A.; Rouphael, Y.; Napolitano, F.; Makris, K.; Kalala, G.; Katopodis, G.; Savvas, D. Salinity source alters mineral composition and metabolism of Cichorium spinosum. Environ. Exp. Bot. 2017, 141, 113–123. [Google Scholar] [CrossRef]
- Savvas, D.; Adamidis, C. Automated management of nutrient solutions based on target electrical conductivity, pH, and nutrient concentration ratios. J. Plant Nutr. 1999, 22, 1415–1432. [Google Scholar] [CrossRef]
- Kostopoulou, S.; Ntatsi, G.; Arapis, G.; Aliferis, K.A. Assessment of the effects of metribuzin, glyphosate, and their mixtures on the metabolism of the model plant Lemna minor L. applying metabolomics. Chemosphere 2020, 239, 124582. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, D.A.; Aliferis, K.A. The yeast (Saccharomyces cerevisiae) YCF1 vacuole transporter: Evidence on its implication into the yeast resistance to flusilazole as revealed by GC/EI/MS metabolomics. Pestic. Biochem. Physiol. 2020, (in press). [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.; Yun, H.; Back, S.; Fernie, A.R.; Kim, Y.X.; Lee, Y.; Lee, S.; Lee, D.; Kim, J. Changes in mineral nutrient concentrations and C-N metabolism in cabbage shoots and roots following macronutrient deficiency. J. Plant Nutr. Soil Sci. 2018, 181, 777–786. [Google Scholar] [CrossRef]
- Fataftah, N.; Mohr, C.; Hajirezaei, M.R.; von Wirén, N.; Humbeck, K. Changes in nitrogen availability lead to a reprogramming of pyruvate metabolism. Plant Biol. 2018, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Scheible, W.R.; Gonzalez-Fontes, A.; Lauerer, M.; Mueller-Roeber, B.; Caboche, M.; Stitt, M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell 1997, 9, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Scheible, W.R.; Lauerer, M.; Schulze, E.D.; Caboche, M.; Stitt, M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997, 11, 671–691. [Google Scholar] [CrossRef]
- Wang, R.C.; Okamoto, M.; Xing, X.J.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef]
- Scheible, W.R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Palacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically. J. Sci. Food Agric. 2018, 98, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Oka, N.; Shinano, T.; Osaki, M.; Takebe, M. Differences in the metabolite profiles of spinach (Spinacia oleracea L.) leaf in different concentrations of nitrate in the culture solution. Plant Cell Physiol. 2008, 49, 170–177. [Google Scholar] [CrossRef]
- Neuberg, M.; Pavlíková, D.; Pavlík, M.; Balík, J. The effect of different nitrogen nutrition on proline and asparagine content in plant. Plant Soil Environ. 2010, 56, 305–311. [Google Scholar] [CrossRef]
- Barneix, A.J.; Causin, H.F. The central role of amino acids on nitrogen utilization and plant growth. J. Plant Physiol. 1996, 149, 358–362. [Google Scholar]
- Raab, T.K.; Terry, N. Nitrogen source regulation of growth and photosynthesis in Beta vulgaris L. Plant Physiol. 1994, 105, 1159–1166. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, E.V.; Novais, R.F.; Dubay, G.R.; Pereira, G.L.; Araujo, W.L.; Jackson, R.B. Nitrogen supply affects root and shoot amino acid composition in Eucalyptus clones. Aust. J. Crop Sci. 2016, 10, 280–290. [Google Scholar]
- Miranda, R.S.; Gomes-Filho, E.; Prisco, J.T.; Alvarez-Pizarro, J.C. Ammonium improves tolerance to salinity stress in Sorghum bicolor plants. Plant Growth Regul. 2016, 78, 121–131. [Google Scholar] [CrossRef]
- Lam, H.M.; Coschigano, K.T.; Oliveira, I.C.; MeloOliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Bartels, D. Sugar metabolism in the desiccation tolerant grass Oropetium thomaeum in response to environmental stresses. Plant Sci. 2018, 270, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006b, 46, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Matt, P.; Schurr, U.; Krapp, A.; Stitt, M. Growth of tobacco in short day conditions leads to high starch, low sugars, altered diurnal changes of the NIA transcript and low nitrate reductase activity, and an inhibition of amino acid synthesis. Planta 1998, 207, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Morcuende, R.; Stitt, M.; Krapp, A. Regulation of nitrate reductase expression in leaves by nitrate and nitrogen metabolism is completely overridden when sugars fall below a critical level. Plant Cell Environ. 2000, 23, 863–871. [Google Scholar] [CrossRef]
- Sarasketa, A.; González-Moro, M.B.; González-Murua, C.; Marino, D. Nitrogen source and external medium pH interaction differentially affects root and shoot metabolism in Arabidopsis. Front Plant Sci. 2016, 7, 29. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2010, 16, 162–174. [Google Scholar] [CrossRef]
- Obata, A.; Fernie, R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Bouché, N.; Fromm, H. GABA in plants: Just a metabolite? Trends Plant Sci. 2004, 9, 110–115. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzigianni, M.; Aliferis, K.A.; Ntatsi, G.; Savvas, D. Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism. Agronomy 2020, 10, 952. https://doi.org/10.3390/agronomy10070952
Chatzigianni M, Aliferis KA, Ntatsi G, Savvas D. Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism. Agronomy. 2020; 10(7):952. https://doi.org/10.3390/agronomy10070952
Chicago/Turabian StyleChatzigianni, Martina, Konstantinos A. Aliferis, Georgia Ntatsi, and Dimitrios Savvas. 2020. "Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism" Agronomy 10, no. 7: 952. https://doi.org/10.3390/agronomy10070952
APA StyleChatzigianni, M., Aliferis, K. A., Ntatsi, G., & Savvas, D. (2020). Effect of N Supply Level and N Source Ratio on Cichorium spinosum L. Metabolism. Agronomy, 10(7), 952. https://doi.org/10.3390/agronomy10070952






