1. Introduction
The process of flowering starts with the formation of flower buds which is regulated by the interaction and coordination of endogenous and environmental factors, leading to the development of the flower. The floral bud induction, differentiation and organogenesis represent the main steps which require the transition from an undifferentiated meristematic apex to the flower bud. During the ‘floral induction’, in the apical meristem qualitative changes occur throughout a sequence of morphological, biochemical and molecular genetic mechanisms [
1,
2]. The induction can also be viewed as a process during which previously repressed information is being transformed and a meristematic apex is fated to become reproductive. The role of genes, hormonal balance and presence of sufficient assimilates is supposed to be included in the induction [
3]. This is an irreversible phase that evolves with the progressive appearance of floral organs (sepals, petals, anthers, pistil). The floral organogenesis is under the control of an exceptional complex of gene networks that encode transcription factors of the MADS domain family [
4]. The floral organ identity factors also control the expression of many genes involved in the metabolism or the response to different phytohormones, including auxin, cytokinins, gibberellins, and jasmonic acid. These hormones have been shown to mediate diverse processes during flower development, ranging from the control of meristematic activity, to pattern formation and organ maturation [
5]. In deciduous fruit species of the temperate climatic regions, these processes occur through the summer season that precedes anthesis [
6].
During the growth of floral whorls, the development of vascular traces within buds also takes place. The transition from procambial tissues to xylem cells, dead cells with lignified walls producing an empty conduit, forms the vascular elements [
7]. Xylem vessels progressively develop from the base of the bud axis up to the floral primordium, reaching the rudimentary sepals, petals, anther filaments, and finally pistil. This latter stage, which occurs at the resumption of bud growth, coincides with the re-establishment of the vascular continuity between bud axis and floral primordium [
8,
9]. In dormant buds, during the autumn–winter season, the xylem differentiation progresses at the bud axis level so that the floral primordium remains isolated and protected from frost damage [
10]. It has been ascertained that a regular evolution of xylogenesis should assure the acropetal transport of water and fundamental elements (i.e., potassium, boron) which play a significant role in determining the ‘bud quality’, namely the capacity to bloom and setting fruit [
11,
12]. This is a presuppose for contributing to constant crop productivity which may be negatively affected by abiotic stresses. The xylogenesis is a process particularly sensitive to environmental factors such as temperature, solar radiation, and water availability. They interact with inductive signals such as metabolic substances and phytohormones that play a regulatory role in the control of primary vascular differentiation [
13,
14]. In particular, the effect of drought on xylogenesis has been studied on several species which, under water-limited conditions, show an increased number of vessels and reduced lumina [
15]. Different periods of water stress have modified the regularity of crucial phases of the annual cycle of trees, such as floral differentiation and bud growth evolution, causing significant yield reductions and financial losses [
16,
17].
Apricot is one of the most important fruit species cultivated in Italy, mainly in the Southern regions [
18], whose cultivation might be compromised as a consequence of the current phenomenon of climate changes. Indeed, the large canopies that are characteristic of woody fruit trees like apricot (
Prunus armeniaca L.) represent a great evaporative surface with low levels of stem hydraulic conductivity [
19]. As a matter of fact, the occurrence of extreme climate events, such as an increase of temperatures and heavy or low and irregularly distributed rainfall throughout the year, are increasingly frequent. Moreover, for the current century, climate models predict remarkable changes in the Mediterranean region by rising air temperatures, evapotranspiration rates and 25–30% decrease of rainfall [
20,
21]. Thus, the future climate scenario in which water is increasingly scarce imposes specific investigations on apricot varietal panorama in order to identify the most adaptive genotypes to conditions of drought or deficit irrigation. This investigation aimed to assess, in one of the most widespread Italian apricot cultivars, the influence of summer-autumn water deficit and re-watering treatments on floral morphogenesis, xylem vessel differentiation and quality of flower buds.
2. Materials and Methods
2.1. Plant Material and Irrigation Regime
Experimental trials were conducted at the Department of Agriculture, Food and Environment, University of Pisa, Italy (43°42’ N 10°25’ E), from summer to springtime (2016–2017). Two-year-old apricot trees (Prunus armeniaca L.) of the mid-ripening cultivar ‘Portici’, grafted onto Myrabolan 29C rootstock, were used. Trees were grown in plastic pots (70 L) containing a soil mixture (50% clay loam, 25% peat, and 25% vermiculite).
Healthy trees were divided into four equivalent groups which were submitted to different irrigation regimes: (i) fully irrigated plants (control) maintaining soil water content around 90% of field capacity; (ii) stressed plants by 30 days of imposed drought in June (S1), July (S2) and October (S3). Drought was imposed reducing daily water supply around 30% of control, maintaining midday xylem water potential (MDΨS) around −1.5 MPa and stomatal conductance around 0.1 mol H2O m−2 s−1. To avoid rainfall water supply and evaporation from the soil surface, the pots of stressed plants were covered with plastic film.
After the deficit periods, stressed plants were subjected to a regular re-watering. MDΨS was measured in the hottest part of the day using a Scholander type pressure chamber (Technogas, Pisa, Italy). Pressurisation rate was 0.2 MPa every 30 s. MDΨS was measured on one leaf per plants previously wrapped in aluminium foil and encased in polyethene bags at least 1 h before measurement. Leaves were cut off halfway along the stalk and immediately processed.
Gas exchange measurements at saturating light (namely at >1200 μmol m−2 s−1 over the PAR waveband) were carried out after 15 days of water stress, on medial leaves, at 11:00 h on three leaves per plant, using a portable infrared gas analyser Li-Cor 6400 (Li-Cor Inc., Lincoln, NE, USA) operating at 39 ± 0.5 Pa ambient CO2.
This study was arranged as a completely randomised design and each treatment consisted of seven replicate trees.
2.2. Histological Analysis
On fully irrigated and stressed plants, samples were periodically collected in order to determine: (i) the evolution of floral differentiation and (ii) the acropetal progression of primary xylem differentiation along the flower bud axis.
2.2.1. Floral Differentiation
To establish the evolution of floral bud differentiation, prior, during and after the imposed water stress of June (S1) and July (S2), samples of lateral nodes at leaf axil (
n = 25 per treatment, for each sampling time) were periodically collected from the median portion of long shoots, until the end of summer. They were fixed in a FAA solution (45% ethyl alcohol, 5% glacial acetic acid, 10% formaldehyde; 8:1:1
v/
v) for histological observations [
22]. Slices of meristematic apices (7 µm) were stained with 0.05% Toluidine Blue (Sigma-Aldrich, St. Louis, Missouri, USA) and observed under an optical microscope (Fluophot, Nikon, Shinjuku, Japan) whit polarised white light. The floral differentiation stage was assessed according to Andreini and Bartolini [
23]: stage A = undifferentiated meristematic apex constituted by ‘tunica’ (external zone of the meristematic apex constituted by three layers of cells) and ‘corpus’ (under the tunica constituted by mother cells); stage B = receptacle primordium arrangement; stage C = sepal primordia; stage D = petal primordia; stage E = stamen primordial and first appearance of pistil.
2.2.2. Xylem Differentiation
From November to February, flower buds (
n = 20 per treatment, for each sampling time) were excised with a small portion of twig (comprising the pulvinar juncture) and collected from the median-apical part of long fruiting shoots (FS, 30–40 cm), and fixed as described above. Paraffin section analysis was performed as described by Andreini et al. [
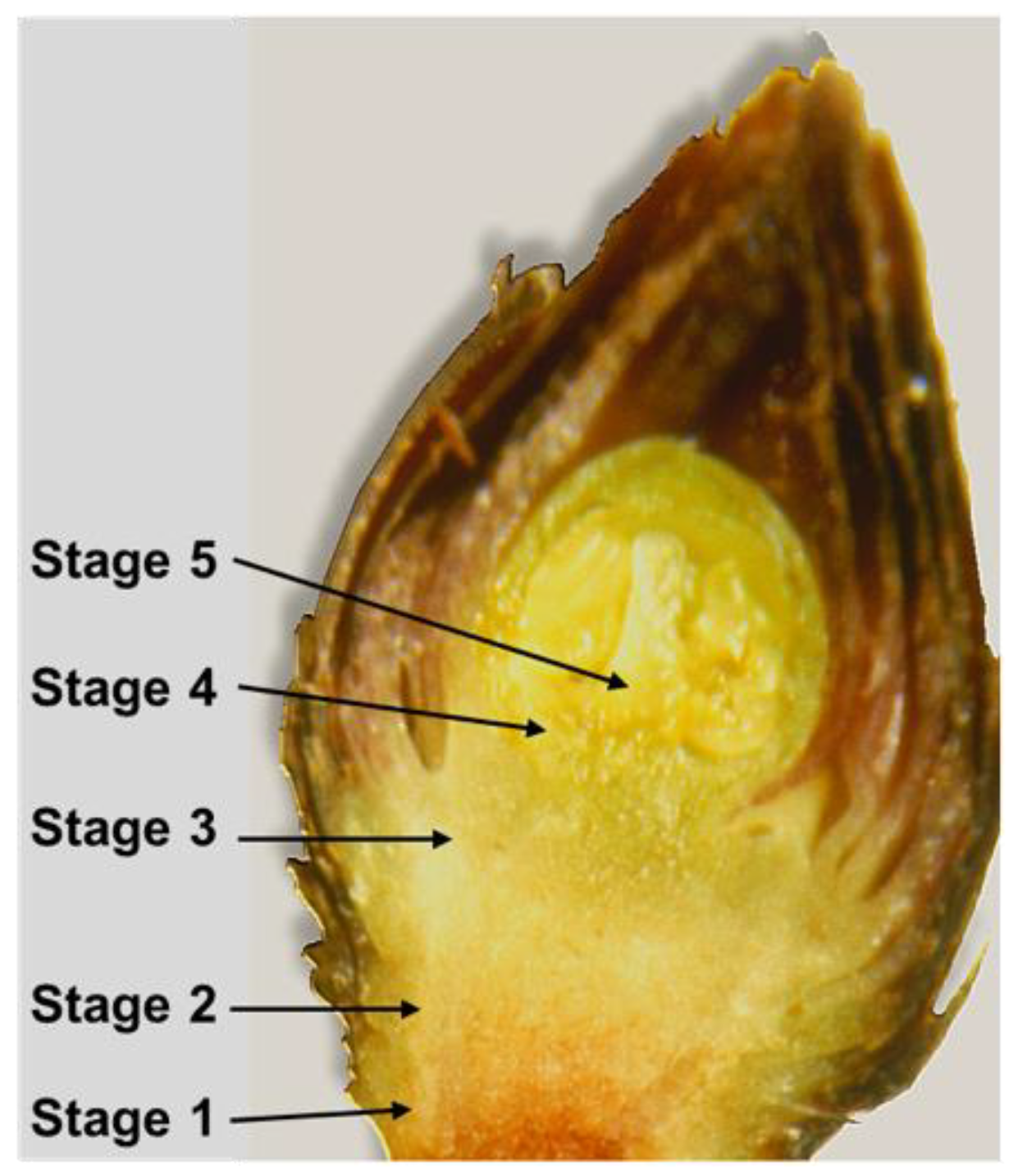
9] using an acridine orange solution as stain. The acropetal progression of primary xylem differentiation along the flower bud axis was detected under an epifluorescent microscope (Fluophot, Nikon, Shinjuku, Japan) according to the following stages: stage 1 = at the base of the axis; stage 2 = at 1/2 of the axis; stage 3 = at 3/4 of the axis; stage 4 = at the base of the ovary; stage 5 = inside the pistil (
Figure 1).
Representative selected sections of meristematic apices and flower buds were photographed with a digital camera (Olympus C-2000 z, Tokio, Japan) equipped to the microscope.
2.3. Biological Analysis
2.3.1. Monitoring of Flower Buds
From November to March, flower buds were periodically sampled from FS to determine: (i) the time of endodormancy release: it was evaluated by the ‘forcing test’ according to Viti et al. [
24]. This test is able to assess the effect of warm temperatures (23 ± 1 °C) on dormancy release, after trees received a certain amount of natural chilling temperatures. The end of endodormancy was established on the basis of fresh weight increase (25–30%) between after- and pre-forcing state (
n = 45 per treatment in three replicates). (ii) the appearance of flower anomalies: it was evaluated by longitudinally dissected buds which were observed under a stereomicroscope (Leica DMC 4500, Fremont, CA, USA) in order to assess the tissue browning and necrosis of ovary, pistil, stamen or whole bud (
n = 30 per treatment in three replicates).
2.3.2. Flowering Performance
At full blooming time, BBCH scale (code 65) according to Wenden et al. [
25], the flowering entity was estimated on the basis of the initial number of flower buds per FS and expressed by a score of 1–3 representing the following percentage classes: 1 = low (<20%), 2 = medium (20–60%); 3 = abundant (>60%).
2.4. Weather Parameters
Daily temperatures and precipitation were considered to describe the environmental conditions during the experimental period. Hourly minimum and maximum temperatures, acquired by an automatic data-loggers (Tynitag Plus
®, West Sussex, UK, 2003), were used to estimate the chilling received by the trees during the autumn-winter season. The chilling amount, a key factor involved in endodormancy release of flower buds, was expressed as Chill Units (CU) according to Richardson et al. [
26]. The rainfall records were acquired from the meteorological station of the Hydrological Service of Tuscany, Tuscany, Italy [
27] located at the experimental site.
2.5. Data Analysis
Statistical analyses were performed by the package GraphPad Prism 8 (GraphPad SoftwareInc., San Diego, CA, USA). Prior to analyses, data were log, square-root and square-transformed to satisfy normality and homoscedasticity assumptions. A paired t-test was used to determine differences on water status and physiological indicators among treated and control. Analysis of variance (ANOVA) was carried out on morpho-biological parameters, and means (±SD, Standard Deviation) were separated by Tukey test (p ≤ 0.05).
3. Results and Discussion
3.1. Weather Parameters
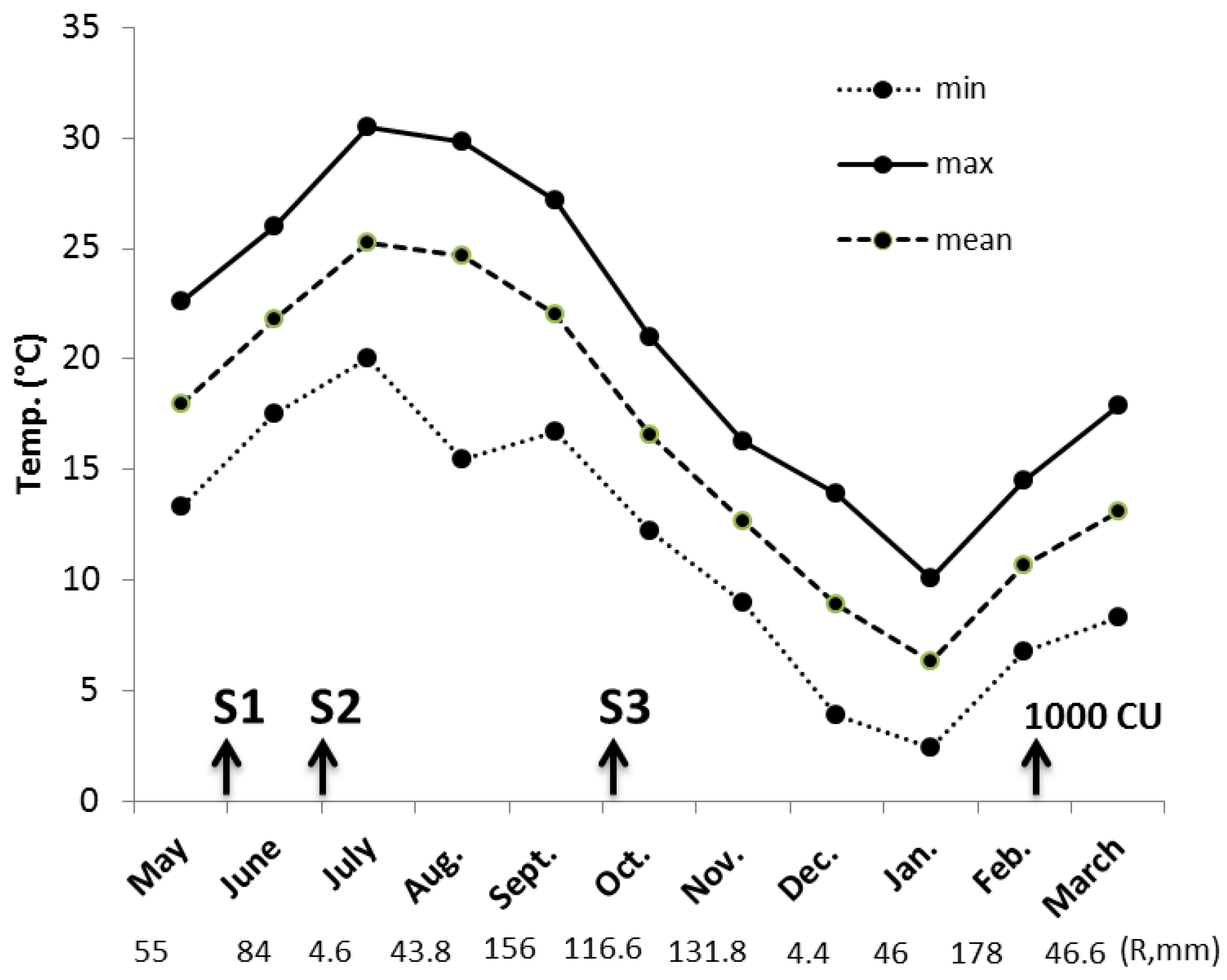
The air temperatures and rainfall events recorded over the experimental period are shown in
Figure 2. During the summer months of imposed droughts, the air means temperatures were about 22 and 25 °C in June (S1) and July (S2), respectively. The highest values of maximum temperatures were recorded in June (29–30 °C, after about 15 d from the beginning of water privation) and July (33–34 °C, after about 10 d from the beginning of water privation). The mean temperatures of August were similar to July, but with lower minimum temperatures. Summer 2016 was defined as being more “normal” than previous hot summers (i.e., 2015, 2011) and was similar to the 1981–2010 thirty-year period [
26]. During October, in correspondence with the third period of imposed water stress (S3), the mean temperatures were about 16 °C. In this autumn season (September–November) the average temperature was around 0.8 °C higher than 1981–2010, and during the winter, fluctuating conditions were observed. In particular, December was a little hotter and January 2017 was rather cold, the eighth coldest January since 1955 (
http://www.lamma.rete.toscana.it). However, only one freezing episode (−2 °C) occurred in the first ten days of January, which was not harmful since flower buds were still in a state of endodormancy.
Under our environmental conditions, it was ascertained that January is important for achieving significant CU amounts for inducing the endodormancy break of apricot flower buds. Temperatures occurring in 2017 were effective for attaining, in mid-February, the threshold of 1000 CU that has been found useful for most apricot cultivars characterized by medium chilling requirement like ‘Portici’ [
24].
As regards rainfall, the summer was characterized by a particularly rainy June (80 mm) and a droughty July (about 5 mm). In the autumn season (September–November), regular precipitation occurred, but it never rained, and rained very little, in December and January, respectively.
3.2. Influences of Water Stress on Carbon Assimilation among Different Periods of Stress
Leaf water potential decreased up to the established stress level, causing moderate stress to plants in each stress period as also confirmed by gas exchange analysis (
Table 1). Leaf photosynthesis is one of the first physiological processes to be influenced by drought stress conditions [
28,
29]. The decrease of CO
2 assimilation rate during water stress could be due to a reduced mesophyll/stomatal conductance or other metabolic alterations [
30,
31]. In addition, trees that experience water stress make a series of strategies to save water as decrease of leaf transpiration or increase the leaf osmotic potential through the accumulation of osmolytes such as sucrose or sorbitol [
32,
33].
In our experiment, regardless of the period, water stress decreased the A390 considerably in both S1, S2 and S3 trees respect to relative controls. However, a higher decrease in A390 among stress periods with respect to relative controls was measured in S1 plants (58%). The higher decrease in CO2 assimilation rate during June period is related to high values of A390 detected in controls, probably due to: (i) lesser environmental limitations to photosynthesis such as high temperatures; (ii) younger mature leaf stage respect to July and October. Stomatal conductance fell concomitantly with the CO2 assimilation rate limiting the intercellular CO2 concentration (Ci). This suggests that stomatal adjustments, mainly limited photosynthetic processes on apricot leaves during water stress, also limiting the leaf transpiration. Stressed trees revealed their suffering state by higher values of leaf drop than full watered trees. Indeed, while in control trees, the leaf drop was about 12%, the water shortage caused percentages more than two-fold higher. However, when trees were re-watered (regardless of the stress period recovered their CO2 assimilation rate with similar control values (data not shown), suggesting that stressed apricots were able to recover their photosynthetic processes after the stress.
3.3. Floral Differentiation
In apricot, as well as in other
Prunoideae, the floral initiation and organogenesis take place during the summer season and is usually completed before leaf fall, in the year before anthesis [
34]. Histological analysis showed that triple meristematic apices constituted most of the sampled nodes. At the end of the differentiation phase, a ‘bud complex’ was formed showing a typical arrangement of stone fruits: one central vegetative bud flanked by one to several flower buds [
35].
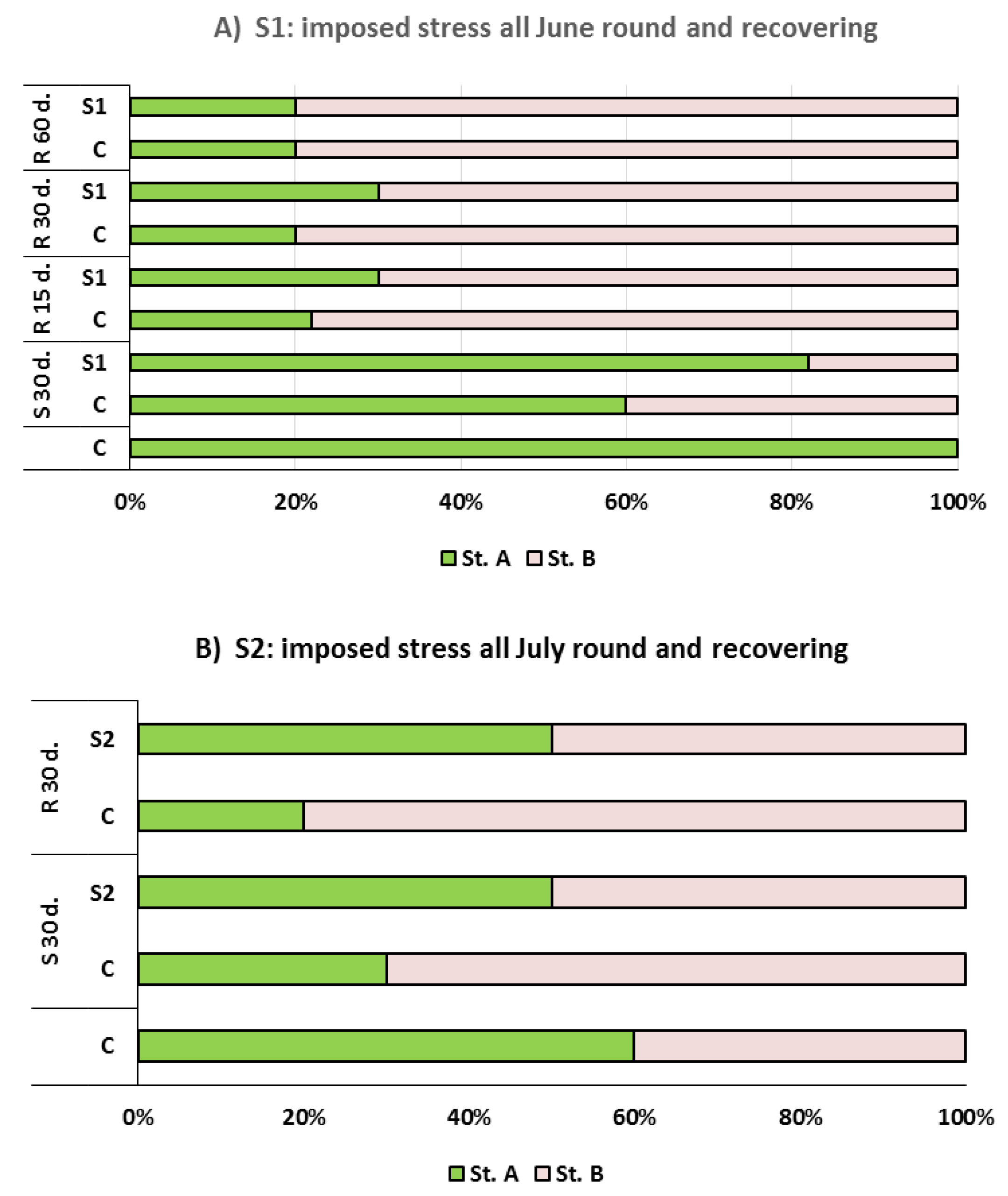
Both summer water stress periods affected the floral differentiation leading to a different temporary shutdown. In the first drought treatment (S1;
Figure 3A), histological analysis revealed that, the day before starting treatments, no meristematic apices showed sign of differentiation being at stage A. At this stage, apices were constituted by two distinctive zones: The external ‘tunica’ organized in three layers of cells, and the internal ‘corpus’ with mother cells (
Figure 4A). During the following 30 d, morphological changes of meristematic apices took place, indicating the transition to floral phase (stage B) with the appearance of the receptacle (
Figure 4B). Differences between control and stressed plants (S1) were observed. Indeed, stage B was reached in 40% and 15% of meristematic apices from control and S1, respectively. The re-watering regime of S1, from July 1st onwards, promoted a fast recovering of the differentiation process, just 15 d after the regular water supply. At this time, comparing the stage B rate reached in control and S1 meristems, similar values were found (70–80%). At the end of August, 60 d after re-watering, the similarity between control and S1 trees persisted, and 20% of apices showed morphological features of undifferentiation (stage A). The ability to quickly recover the inductive stimulus for flower initiation was observed in other fruit species such as citrus and blackcurrant subjected to early summer water stress (June) followed by re-watering [
36,
37].
In the second drought treatment (S2), trees were subjected to water stress all July round (
Figure 3B). After 30 d of treatment, in S2 samples the percentage of apices at stage B was lower than control in which a normal progression of differentiation was observed. This occurrence was denoted by a strong increase at stage B, passing from 40% at the end of June to 70% at the end of July. On the other hand, 50% of apices sampled from S2 were at stage A. Such condition also persisted 30 d. after stress treatment ending, suggesting the re-watering not able to induce a recovering of the differentiation process. Thus, the lack of water supply on July may severely compromise the regularity of floral induction. This condition would have been further aggravated by high temperatures (>30 °C) which occurred in the period of mid-summer. Studies conducted in open fields, under comparable environmental conditions, demonstrated that a critical period exists just in correspondence of July when regular irrigations induced a significant advance of floral differentiation in young apricot trees (cv. ‘San Castrese’ on Myrabolan 29C rootstock) (pers. comm.).
3.4. Xylem Vessel Differentiation
Sampling started in November, at the dormant phenological stage (A
o–A
2) of the flower buds [
38]. At that time, rudimentary sepals, petals, stamen and pistil were fully differentiated. During early growth, buds are connected to the stem through a parenchymatous zone, traversed by a procambial strand constituted by elongated cells characterized by densely stained cytoplasm and lack of lignified secondary wall thickenings [
7]. The differentiation process consists of the transition from this meristematic tissue to xylem cells, namely dead cells with lignified walls producing an empty conduit through which water flows [
10]. In apricot, five progressive stages of the acropetal primary xylem differentiation along the flower bud axis have been identified [
11]. In our study, the final stages 4 and 5, usually detected during the ecodormancy phase, were not found. Indeed, flower buds were collected up to the endodormancy release, and the presence of stages 1–3, corresponding to xylem vessels from the base up t¾ of the bud axis, was observed (
Figure 5).
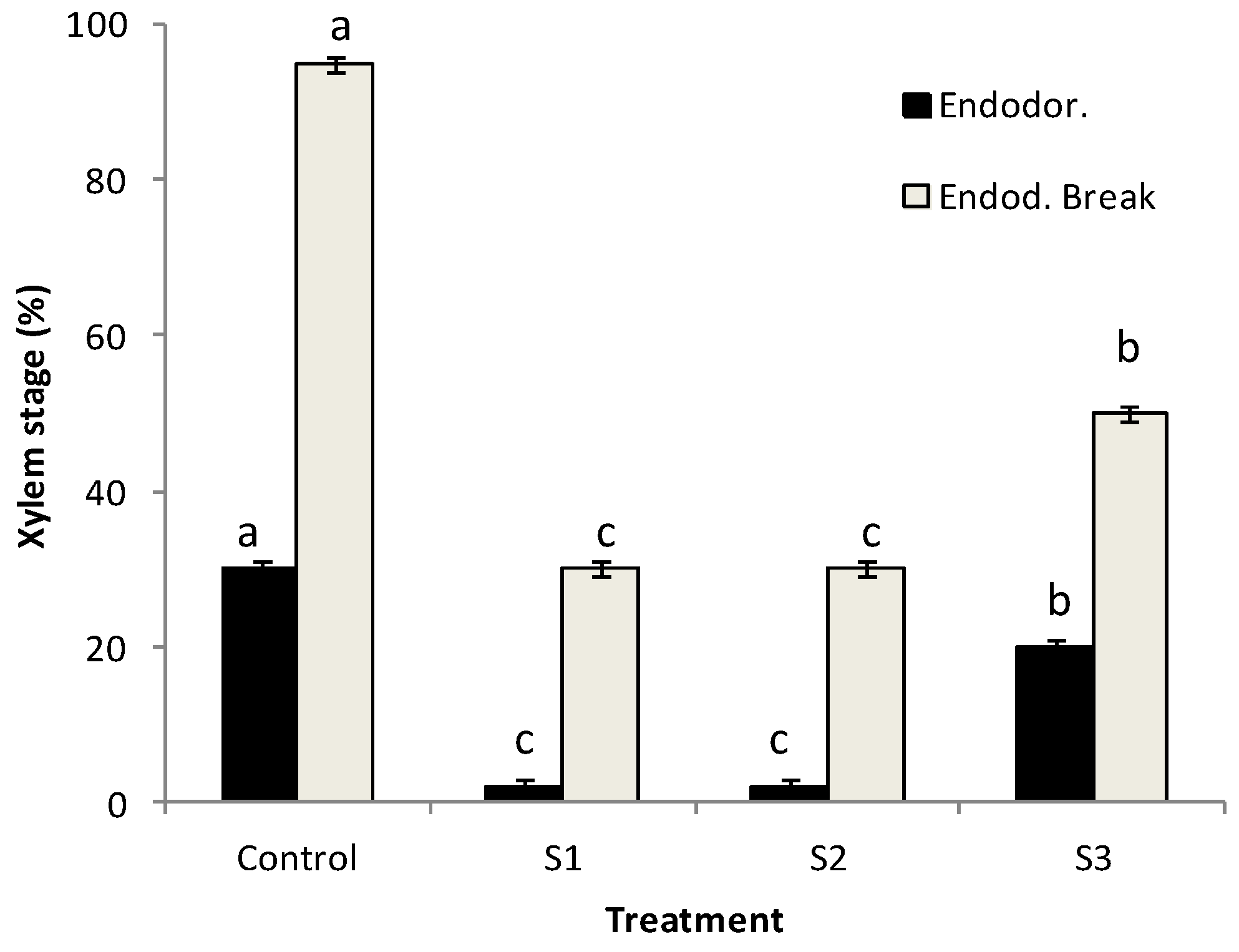
During the endodormancy phase, flower bud samples from S1 and S2 stressed trees were found at stage 1 (xylem vessels at the base of the flower bud axis). A less severe impact was observed in buds collected from autumn-stressed trees (S3), which showed stages 2 and 3 in almost 20% of analysed samples. At the end of endodormancy, more than 90% of analysed control buds showed stage 2–3 while in stressed ones the percentage ranged between 25 (S1, S2) and 50% (S3). As a consequence of prolonged and severe stress periods, the xylem process was not able to have a compensatory capacity for growth even after trees were subjected to a regular water supply regime. Under drought-prone areas, a delay in the initiation of xylogenesis was observed suggesting soil water availability to be one of the main drivers when xylem formation starts [
39,
40]. Drought stress can affect xylogenesis through its effects on the cambium and the developing xylem, given that xylem cell expansion is a turgor-driven process depending on cellular water uptake and solute accumulation [
41,
42].
All drought treatments (S1, S2, S3) caused a significant slower xylem progression than control trees in which, a more regular xylem differentiation was observed (
Figure 6).
Obviously, the formation and differentiation of xylem are related to multiple elements and, in addition to water, other factors can be involved. Experimental trials, carried out on apricot trees in an open field, showed that the xylogenesis process has been found particularly sensitive to low PAR (photosynthetically active radiation) levels [
22]. Stressors, such as drought and shading conditions, could interact with inductive signals such as metabolic substances and phytohormones that play a regulatory role in the control of primary vascular differentiation [
13]. Different levels of auxin, together with other signalling molecules such as cytokinin and growth factors, might act as a patterning agent for differentiation of vascular tissue [
14]. A shift of the xylogenesis process could compromise the availability of nutritional elements throughout the xylem supply, particularly in correspondence with bud growth reactivation A correlation between an increase of certain elements (i.e., potassium and boron) by the acropetal xylem transport and bud swelling was observed in several
Prunus species [
11,
43].
3.5. Monitoring of Flower Buds
The bud growth and the appearance of floral anomalies were periodically analysed, in order to investigate the influence of imposed summer and autumn water stress in the performance of flower buds. As regards the endodormancy process, in any of treatments, there was no effect of irrigation deficit, and its break occurred within the first decade of February. At this time, about 1000 CU were accumulated (
Figure 2), allowing the fulfilment of the chilling requirement (CR), confirming ‘Portici’ as a cultivar with medium CR [
44]. On the other hand, differences in flower bud size among treatments were found (
Table 2).
In general, the bud weight substantially increased at the end of endodormancy. Trees subjected to summer water deficits (S1, S2) had bigger buds in accordance with authors who have found that summer drought conditions increased the size of buds in the next season [
45]. The mechanism by which the summer drought increased the size of buds may be mediated by an increase in abscisic acid (ABA) under drought, which arrests plant growth and induces earlier bud formation. The production of ABA in response to abiotic stress and as a root-to-shoot signalling molecule has been reported for several crops [
46].
The imposed water stress during October (S3) negatively affected the bud size. In this period, the water shortage could trigger strong competition among buds that, in early autumn, are completing the floral organogenesis [
34]. As a consequence, flower buds may suffer an irregular growth, revealing morphological anomalies and/or inner signs of browning and tissue necrosis [
24]. This occurrence was verified analysing the floral anomalies periodically from autumn onwards. The studied cultivar was characterized by a late appearance of anomalies, in agreement with findings related to apricot genotypes with medium CR [
24]. At the pre-flowering stage (BBCH 56–57), differences among treatments were recorded. Almost the totality of the examined flower buds collected from control trees had a morphologically well-developed floral organ, with only 2% being anomalous buds. In contrast, abnormal flower buds from stressed trees ranged between 16% and 22%, with the highest values for S2 and S3 (
Table 2). The most frequent anomalies were ascribed to necrosis at the ovary or pistil level, symptoms indicative of a suffering status of leaves subjected to water stress which caused a slowdown of photosynthetic capacity affecting the availability of assimilates for making an optimal growth of reproductive organs. It was observed that the amount of starch reserves in the transmitting tissue of the style and the number of xylem vessels surrounding the transmitting tissue were a requisite for apricot flowers that finally set fruits [
47]. In particular, negative influences at long distance have been found by postharvest water stress, which induced a significant decrease in fruit set the following year [
48].
The blooming time occurred, as usual, during the first decade of March without differences among treatments. On the other hand, trees subjected to water shortage showed a reduced amount of flowering in comparison with control. The last treatment in October produced the lowest flowering score which statistically differed from the other treatments (
Table 2). This finding suggests the importance of apricot trees also having adequate water availability in early autumn. This period could represent another crucial step affecting the regular growth of flower buds that, for ensuring good-enough fruit setting, have to hold functional floral organs. Considering that under the Mediterranean environmental conditions autumn seasons appear to be dryer, late water supply could be a key strategy for improving the quality of flower buds.
4. Conclusions
Data presented herein about the influence of extensive summer–autumn water deficit periods evidenced negative short- and long-term impacts on fundamental phases of the annual cycle of apricot trees, cultivar ‘Portici’. Leaves under water stress strongly reduced the photosynthetic processes due to the rise of leaf stomatal limitations, which also limits leaf transpiration. Although after stress, apricots were able to re-establish photosynthetic patterns, significant changes on floral differentiation, xylem vessels progression and quality of flower buds were found.
During summer, floral differentiation was differently conditioned by the water stress treatments which always caused a shutdown of the process. Trees stressed in June were able to recover the differentiation process after a regular re-watering, showing a status of physiological plasticity to the inductive stimulus for flower initiation. On the other hand, in correspondence with the second drought period, imposed all July round, the recovery ability of trees was not observed, compromising the regularity of floral differentiation.
The xylogenesis process was affected by summer and autumn water deprivations. Indeed, under stress conditions, a slower xylem progression within flower buds up to the break of endodormancy was observed. This occurrence, limiting the acropetal flow of water and mineral elements during organogenesis and growth of buds, could prejudice the quality of flower buds. As a consequence, an anomalous growth of floral organs will produce non-functional flowers, leading to a poor fruit set.
This study particularly highlights the importance of water availability also in early autumn, ensuring the best conditions for obtaining healthy flower buds to avoid reduction or loss of the next year’s crop. Considering that in Mediterranean countries, dry and hot autumn–winter seasons are increasing, an adequate water supply is becoming crucial, as well as the adoption of reduced irrigation strategies in apricot culture.