Witchweed’s Suicidal Germination: Can Slenderleaf Help?
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Crotalaria Root Exudate
2.3. Striga Germination Bioassays
2.4. Post germination Analysis of Striga-Crotalaria Interactions
3. Results
3.1. Crotalaria Root Exudates Stimulate Germination of S. hermonthica Seeds
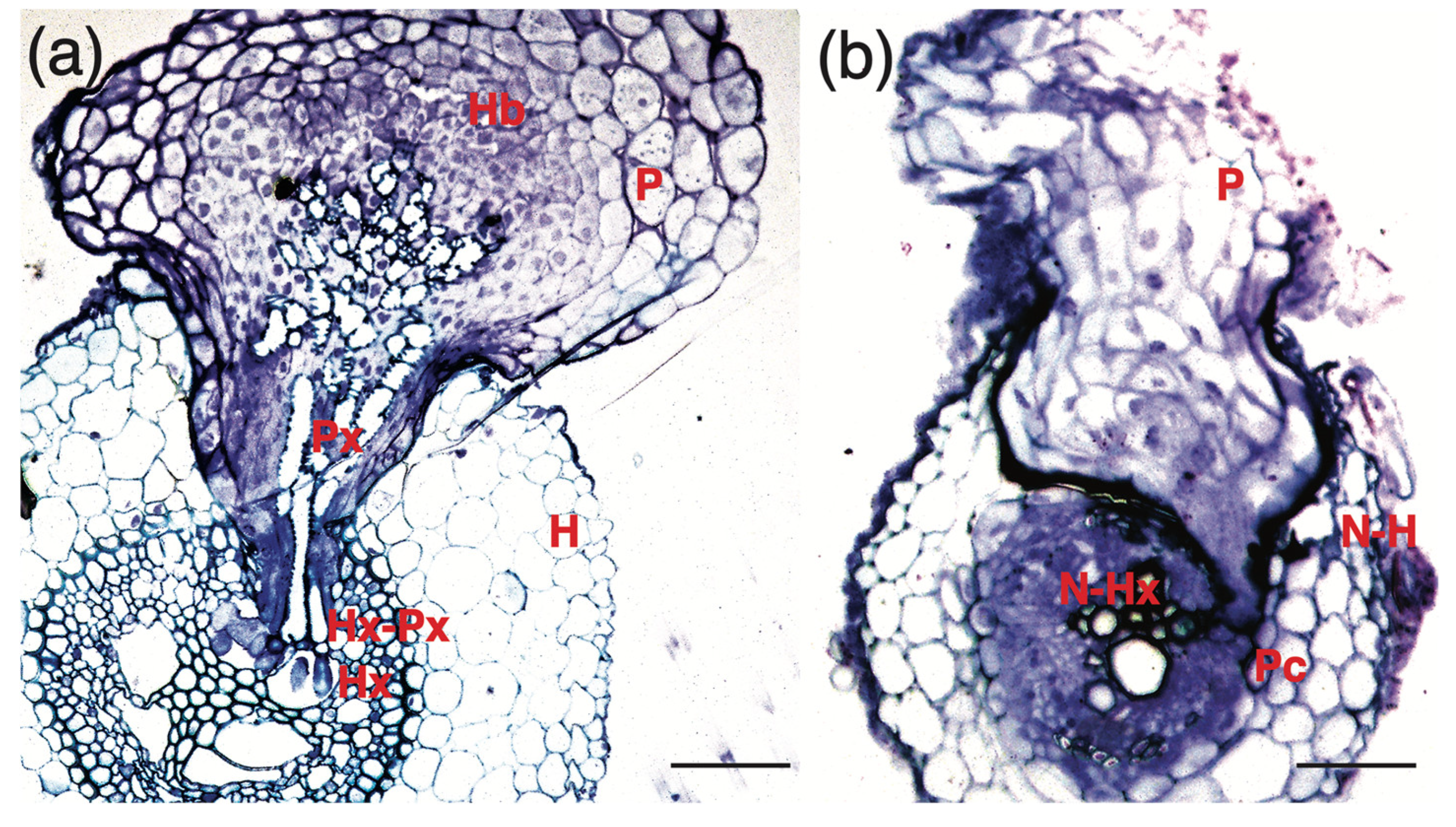
3.2. Crotalaria Blocks Striga Penetration at Multiple Levels up to the Pericycle
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamed, K.I.; Musselman, L.J. Taxonomy of Agronomically Important Striga and Orobanche Species. In Progress on Farmer Training in Parasitic Weed Management; Labrada, R., Ed.; FAO: Rome, Italy, 2008; pp. 7–14. [Google Scholar]
- Ejeta, G. The Striga Scourge in Africa: A Growing Pandemic. In Integrating New Technologies for Striga Control; Ejeta, G., Gressel, J., Eds.; World Scientific Publishing Company: Singapore, 2007; pp. 3–16. [Google Scholar]
- Berner, D.K.; Kling, J.G.; Singh, B.B. Striga Research and Control. A perspective from Africa. Plant Dis. 1995, 79, 652–660. [Google Scholar] [CrossRef]
- Atera, E.A.; Itoh, K.; Onyango, J.C. Evaluation of Ecologies and Severity of Striga Weed on Rice in sub-Saharan Africa. Agric. Biol. J. N. Am. 2011, 2, 753–760. [Google Scholar] [CrossRef]
- Matusova, R.; Rani, K.; Verstappen, F.W.A.; Franssen, M.C.R.; Beale, M.H.; Bouwmeester, H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. Are Derived from the Carotenoid Pathway. Plant Physiol. 2005, 139, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S.; Kannan, C. Suicidal Germination for Parasitic Weed Control. Pest Manage. Sci. 2016, 72, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Kountche, B.A.; Jamil, M.; Yonli, D.; Nikiema, M.P.; Ania, D.B.; Asami, T.; Zwanenburg, B.; Al-Babili, S. Suicidal Germination as a Control Strategy for Striga hermonthica (Benth.) in Smallholder Farms of sub-Saharan Africa. Plants People Planet 2019, 1, 107–118. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.O.; Hassanali, A.; Pickett, J.A.; Wadhams, L.J. Assessment of Different Legumes for the Control of Striga hermonthica in Maize and Sorghum. Crop Sci. 2007, 47, 730–734. [Google Scholar] [CrossRef]
- Midega, C.A.O.; Khan, Z.R.; Amudavi, D.M.; Pittchar, J.; Pickett, J.A. Integrated Management of Striga hermonthica and Cereal Stemborers in Finger Millet (Eleusine coracana (L.) Gaertn.) through Intercropping with Desmodium intortum. Int. J. Pest Manag. 2010, 56, 145–151. [Google Scholar] [CrossRef]
- Fischler, M. Impact Assessment of Push–Pull Technology Developed and Promoted by ICIPE and Partners in Eastern Africa; International Centre of Insect Physiology and Ecology: Nairobi, Kenya, 2010. [Google Scholar]
- Hooper, A.M.; Hassanali, A.; Chamberlain, K.; Khan, Z.; Pickett, J.A. New Genetic Opportunities from Legume Intercrops for Controlling Striga spp. Parasitic Weeds. Pest Manage. Sci. 2009, 65, 546–552. [Google Scholar] [CrossRef]
- Abukutsa-Onyango, M.O. Response of Slenderleaf (Crotalaria brevidens benth) to inorganic nitrogen application. AJFAND 2016, 7, 1–10. [Google Scholar]
- Samba, R.T.; Sylla, S.N.; Neyra, M.; Gueye, M.; Dreyfus, B.; Ndoye, I. Biological Nitrogen Fixation in Crotalaria species estimated using the 15N isotope dilution method. Afri. J. Biotechnol. 2002, 1, 17–22. [Google Scholar] [CrossRef][Green Version]
- Yoshida, S.; Shirasu, K. Multiple Layers of Incompatibility to the Parasitic Witchweed, Striga hermonthica. New Phytol. 2009, 183, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Waweru, D.N.; Kuria, E.K.; Bradley, J.M.; Scholes, J.D.; Runo, S. Tissue Culture Protocols for the Obligate Parasitic Plant Striga hermonthica and Implications for Host-parasite Co-cultivation. Plant Cell Tiss. Organ Cult. 2019, 138, 247–256. [Google Scholar] [CrossRef]
- Fujioka, H.; Samejima, H.; Mizutani, M.; Okamoto, M.; Sugimoto, Y. How does Striga hermonthica Bewitch its Hosts? Plant Signal. Behav. 2019, 14, 1605810. [Google Scholar] [CrossRef]
- Berner, D.K.; Winslow, M.D. Striga Research Methods: A manual; The Pan-African Striga Control Network (PASCON); IITA: Ibadan, Nigeria, 1997. [Google Scholar]
- Hudson, J.P. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Cambridge University Press: Cambridge, UK, 1967; Volume 3, p. 104. [Google Scholar]
- Mbuvi, D.A.; Masiga, C.W.; Kuria, E.; Masanga, J.; Wamalwa, M.; Mohamed, A.; Odeny, D.A.; Hamza, N.; Timko, M.P.; Runo, S. Novel Sources of Witchweed (Striga) Resistance from Wild Sorghum Landraces. Front. Plant Sci. 2017, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- Mutinda, S.M.; Masanga, J.; Mutuku, J.M.; Runo, S.; Alakonya, A. KSTP 94, an Open-pollinated Maize Variety Has Postattachment Resistance to Purple Witchweed (Striga hermonthica). Weed Sci. 2018, 66, 525–529. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Conner, H.M.; Terry, M.S. Carnoy fixation: Practical and theoretical considerations. Histochemie 1968, 16, 361–371. [Google Scholar] [CrossRef]
- Runo, S.; Kuria, E.K. Habits of a highly successful cereal killer, Striga. PLoS Pathog. 2018, 14, e1006731–e1006736. [Google Scholar] [CrossRef]
- Odhiambo, J.A.; Vanlauwe, B.; Tabu, I.M.; Kanampiu, F.; Khan, Z. Effect of Intercropping Maize and Soybeans on Striga hermonthica Parasitism and Yield of Maize. Arch. Phytopathol. Plant Protect. 2011, 44, 158–167. [Google Scholar] [CrossRef]
- Botanga, C.J.; Alabi, S.O.; Echekwu, C.A.; Lagoke, S.T.O. Genetics of Suicidal Germination of Striga hermonthica (Del.) Benth by Cotton. Crop Sci. 2003, 43, 483–488. [Google Scholar] [CrossRef]
- Toh, S.; Holbrook-Smith, D.; Stogios, P.J.; Onopriyenko, O.; Lumba, S.; Tsuchiya, Y.; Savchenko, A.; McCourt, P. Structure-function Analysis Identifies Highly Sensitive Strigolactone Receptors in Striga. Science 2015, 350, 203–207. [Google Scholar] [CrossRef]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural Requirements of Strigolactones for Hyphal Branching in AM Fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
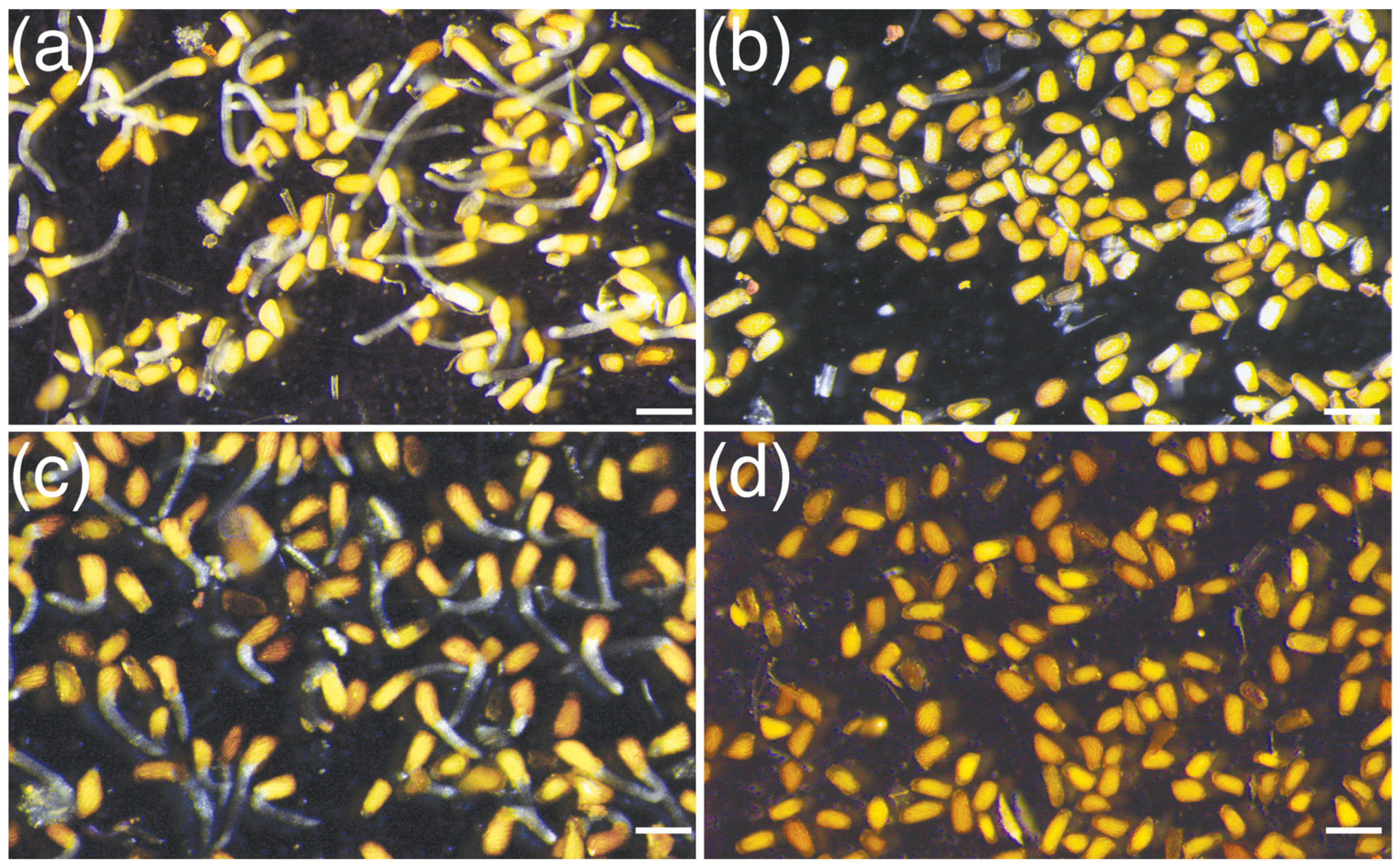

| S/NO | Landrace | Species | Germination Frequency (%) | Radicle Length (mm) |
|---|---|---|---|---|
| 1 | GR24 | Synthetic Analog | 76.2 ± 4.20 a | 0.55 ± 0.06 bcd |
| 2 | BHMBY0213 | Crotalaria brevidens | 54.6 ± 3.01 b | 0.21 ± 0.02 jk |
| 3 | BMGR0234 | Crotalaria brevidens | 51.9 ± 0.40 b | 0.50 ± 0.05 bcdef |
| 4 | MKKMG0011 | Crotalaria ochroleuca | 44.9 ± 1.31 c | 0.43 ± 0.00 cdefg |
| 5 | MKS0226 | Crotalaria ochroleuca | 37.5 ± 1.13 d | 0.35 ± 0.02 bfghij |
| 6 | MVG0003 | Crotalaria ochroleuca | 36.7 ± 1.52 de | 0.42 ± 0.04 cdefgh |
| 7 | MMGSR0029 | Crotalaria ochroleuca | 36.5 ± 1.52 de | 0.24 ± 0.02 ijk |
| 8 | MKSM0204 | Crotalaria ochroleuca | 35.2 ± 0.06 def | 0.78 ± 0.08 a |
| 9 | MSY0110 | Crotalaria ochroleuca | 34.8 ± 1.69 defg | 0.39 ± 0.04 defghi |
| 10 | MHMBY0207 | Crotalaria ochroleuca | 34.2 ± 1.87 defg | 0.24 ± 0.03 ijk |
| 11 | MKS0221 | Crotalaria ochroleuca | 33.75 ± 1.75 cde | 0.41 ± 0.04 cdefjhi |
| 12 | BKSM0203 | Crotalaria brevidens | 32.5 ± 1.28 defgh | 0.16 ± 0.03 kl |
| 13 | MMGR0234 | Crotalaria ochroleuca | 32.1 ± 0.79 defgh | 0.61 ± 0.01 b |
| 14 | MKKMG0111 | Crotalaria ochroleuca | 30.8 ± 1.08 efghi | 0.72 ± 0.01 a |
| 15 | MBG0086 | Crotalaria ochroleuca | 30.1 ± 0.52 fghij | 0.43 ± 0.02 cdefgh |
| 16 | MHMBY0215 | Crotalaria ochroleuca | 28.9 ± 0.64 fghij | 0.56 ± 0.03 bc |
| 17 | BMGR0239 | Crotalaria brevidens | 28.6 ± 0.21 ghijk | 0.51 ± 0.01 bcde |
| 18 | BSY0113 | Crotalaria brevidens | 27.1 ± 0.11 hijk | 0.78 ± 0.02 a |
| 19 | BVG0001 | Crotalaria brevidens | 25.4 ± 1.02 ijkl | 0.25 ± 0.10 hijk |
| 20 | BHMBY0198 | Crotalaria brevidens | 24.7 ± 1.75 ijklm | 0.14 ± 0.04 kl |
| 21 | BKSM0197 | Crotalaria brevidens | 23.7 ± 1.52 jklmn | 0.15 ± 0.03 kl |
| 22 | BKS0220 | Crotalaria brevidens | 23.7 ± 1.52 jklmne | 0.41 ± 0.03 cdefghi |
| 23 | BKKMG0091 | Crotalaria brevidens | 22.4 ± 0.52 klmn | 0.06 ± 0.00 l |
| 24 | MBS0065 | Crotalaria ochroleuca | 22.3 ± 0.71 klmn | 0.16 ± 0.00 kl |
| 25 | MVG0125 | Crotalaria ochroleuca | 22.0 ± 1.37 klmn | 0.27 ± 0.06 ghijk |
| 26 | MSY0216 | Crotalaria ochroleuca | 19.8 ± 1.11 lmno | 0.28 ± 0.01 ghijk |
| 27 | BVG0004 | Crotalaria brevidens | 19.8 ± 0.71 lmno | 0.36 ± 0.00 efghi |
| 28 | MBS0064 | Crotalaria ochroleuca | 18.4 ± 1.79 mno | 0.05 ± 0.00 l |
| 29 | BKKMG0129 | Crotalaria brevidens | 17.9 ± 0.05 no | 0.28 ± 0.00 ghijk |
| 30 | MKSM0218 | Crotalaria ochroleuca | 15.5 ± 0.28 o | 0.29 ± 0.05 ghijk |
| 31 | Water | 0 p | 0 m |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwakha, F.A.; Budambula, N.L.M.; Neondo, J.O.; Gichimu, B.M.; Odari, E.O.; Kamau, P.K.; Odero, C.; Kibet, W.; Runo, S. Witchweed’s Suicidal Germination: Can Slenderleaf Help? Agronomy 2020, 10, 873. https://doi.org/10.3390/agronomy10060873
Mwakha FA, Budambula NLM, Neondo JO, Gichimu BM, Odari EO, Kamau PK, Odero C, Kibet W, Runo S. Witchweed’s Suicidal Germination: Can Slenderleaf Help? Agronomy. 2020; 10(6):873. https://doi.org/10.3390/agronomy10060873
Chicago/Turabian StyleMwakha, Fridah A., Nancy L.M. Budambula, Johnstone O. Neondo, Bernard M. Gichimu, Eddy O. Odari, Peter K. Kamau, Calvins Odero, Willy Kibet, and Steven Runo. 2020. "Witchweed’s Suicidal Germination: Can Slenderleaf Help?" Agronomy 10, no. 6: 873. https://doi.org/10.3390/agronomy10060873
APA StyleMwakha, F. A., Budambula, N. L. M., Neondo, J. O., Gichimu, B. M., Odari, E. O., Kamau, P. K., Odero, C., Kibet, W., & Runo, S. (2020). Witchweed’s Suicidal Germination: Can Slenderleaf Help? Agronomy, 10(6), 873. https://doi.org/10.3390/agronomy10060873





