Abstract
Alternative grazing systems that incorporate cover crops may be useful to achieve a longer grazing season and maximize forage production. However, little is known about their impact on soil properties, especially in the presence or absence of cattle grazing in the early spring. The aim of this study was to evaluate the interacting effects of cropping systems with and without cattle grazing in rotation with corn or soybean on the balance and dynamics of soil fertility and enzyme activity. This study was conducted as a system experiment between 2015 and 2019 in Minnesota and Pennsylvania, USA. The experimental design was a randomized complete block design with four replications. Treatments included presence or absence of cattle grazing and two types of cropping systems (pasture-rye-soybean-pasture [P-R-SB-P] and pasture-wheat/vetch-corn-pasture [P-W/V-C-P]. Soil samples were collected six times during the study. Soil properties analyzed were soil pH, organic matter, salinity, K, Ca, Mg, cation exchange capacity (CEC), P, β-glucosidase, alkaline phosphatase, aryl-sulfatase, fluorescein diacetate hydrolysis, ammonium, nitrate, permanganate oxidizable carbon (POXC), N%, C%, S%, and C:N ratio. Grazing increased glucosidase activity, available Ca, Mg, NO3−, NH4+, soil pH, soil C%, S%, and the C:N ratio. In the P-W/V-C-P cropping system, soil pH, available Ca, NO3−, and sulfatase activity were found to increase compared with the P-R-SB-P cropping system. In contrast, soil OM, available K, Mg, CEC, glucosidase, phosphatase, POXC, and total C%, N%, and S% were greater in the P-R-SB-P cropping system compared with the P-W/V-C-P cropping system. The results of this study suggested that rotational grazing can increase soil quality and microbial decomposition under the P-W/V-C-P cropping system, and that this result was greater than under the P-R-SB-P cropping system, leading to a faster nutrient cycling. These results show promise for producers who are seeking methods to diversify their farming operation and reduce the need for external inputs.
1. Introduction
Enhancing soil ecosystem services, including regulating, supplying, and supporting services, is a high priority for the development of sustainable agricultural systems [1,2]. Organic agricultural systems primarily rely on ecological principles to maintain soil ecosystem services including biodiversity and natural soil fertility [3,4,5]. Furthermore, it is well established that organic farming practices are able to maintain and provide a more diverse set of soil ecosystem services than conventional farming systems [6,7,8,9]. The growing concerns on how global warming may affect global food production systems has spurred interest on how to use agriculture to enhance atmospheric sequestration of greenhouse gases, such as carbon dioxide (CO2), on terrestrial ecosystems [10]. Some forms of CO2 sequestration include the use of agricultural practices such as conservation tillage, cover crops, crop rotation, and fertilization [10,11]. Together those practices could optimize biomass growth, minimize reliance of fossil fuel-based fertilizer, and increase the amount of carbon being returned to soil ecosystems.
Unfavorable growing conditions, due to unpredictable weather over the last decade, have created many challenges for farmers located in the Midwestern region of the US [11]. The area is predominately cropped to corn (Zea mays L.) and soybean (Glycine max (L.) Merr.) and the vast majority of the land is under a conventional system with high reliance on chemical inputs. The soils under these agricultural systems typically include long periods of fall/winter fallow with low annual rate of carbon (C) return [10,11]. It has been estimated that the potential for agricultural land to support cover crops is 60% in some Midwestern regions; however, only 18.8% of this area is planted with reduced or conventional tillage following corn [12]. These systems are known to promote microbial activity and decomposition of soil organic matter (SOM), with little to no increase in soil organic carbon (SOC) [13]. In addition, these practices also contribute to the rapid deterioration of soil physical and chemical properties [14], further increasing the dependency on chemical inputs for adequate management. Considering the strong prevalence of corn and soybean as the main crop in conventional production systems, the absence of a soil cover is becoming a limiting factor [11,15].
Sustainability of agricultural systems can be achieved by enhancing the C-balance by increasing plant biomass production on the land [16]. In this sense, cover crops are an agricultural tool that can supply significant amounts of C-rich residues to the soil, thus modifying the quantity and quality of SOM [17] and improving soil fertility [14,18]. Moreover, planting cover crops is an effective method to reduce both nitrogen (N) leaching and soil erosion from agricultural lands [16]. The ability of certain cover crops to accumulate biomass even during the cold season, makes some winter cover crops such as rye (Secale cereale L.), hairy vetch (Vicia villosa Roth), and winter wheat (Triticum aestivum L.) an attractive option. Rye is a popular winter cover crop in the upper Midwest due to its adaptability to low temperatures, superior growth and N uptake compared to other species [19]. Hairy vetch is a winter legume that is widely adapted to most areas of the eastern United States [20]. Hairy vetch has a low C:N ratio (usually 10:1 to 15:1) that results in rapid biomass decomposition, with the majority of N mineralization occurring within the first 4 to 8 weeks after termination in the spring [21]. It can produce more than 150 kg N ha−1 when planting and termination dates are optimized [20,22]. Wheat is also a popular winter cover crop in the upper Midwest, as it matures later than rye but begins growing earlier in the spring than perennial forages. Having biomass on the land for an extended period of time not only provides a cover for bare soils but also presents an additional benefit to the land that is primarily used for grain production and cattle grazing. Therefore, incorporating winter cover crops may offer additional available forage for grazing cattle earlier than what is possible with perennial pastures in the spring [23].
Producers plant winter cover crops in the late-summer or fall with the target of establishing the crop in that same year, with re-growth in the spring—though this is often a challenge in the upper Midwestern states. The crops used as cover crops in the Midwest have been selected for their cold hardy characteristics and therefore grow in cooler temperatures more efficiently than most perennial grass species, making them an ideal forage source for grazing [24]. This may be a useful strategy because one of the main obstacles organic beef producers face is lack of supply of pasture-based feed [25]. Furthermore, grazing winter cover crops may help organic producers meet the soil-building plan and daily dry matter intake requirements mandated by the United States Department of Agriculture (USDA)—National Organic Program (NOP) [26].
Alternative grazing systems, which incorporate winter cover crops, may be useful for achieving a longer grazing season and maximizing forage production [25]. The role of cover crops in the upper Midwest has been studied by many researchers [27,28,29]. However, little is known about their impact on soil properties, especially with or without cattle grazing in early spring. We hypothesize that cover crop inclusion in different cropping systems, with or without cattle grazing, can improve soil quality and fertility, thus affecting nutrient availability and microbial activity in the long term. The aim of this study was to evaluate the interacting effects of winter cover crops and cattle grazing in rotation with corn or soybean on the balance and dynamics of soil fertility and soil enzyme activity.
2. Materials and Methods
2.1. Experimental Design and Baseline Soil Sampling
This field-scale study of an integrated crop-livestock system experiment, described by Phillips et al. [25] and Nazareth et al. [30], was conducted between 2015 and 2019 at the University of Minnesota West Central Research and Outreach Center (WCROC) (Morris, MN, USA) and at the Rodale Institute (Kutztown, PA, USA). The experimental design was a randomized complete block design with four replications with a factorial arrangement of treatments. Treatments included presence or absence of cattle grazing and two types of model cropping systems (pasture-rye-soybean-pasture [P-R-SB-P] and pasture–mix of wheat and vetch-corn-pasture [P-W/V-C-P]. The pastureland included perennial forbs, grasses, and legumes, such as alfalfa (Medicago sativa L.), chicory (Cichorium intybus L.), meadow brome grass (Bromus biebersteinii L.), meadow fescue (Festuca pratensis L.), orchard grass (Dactylis glomerate L.), perennial ryegrass (Lolium perenne L.), red clover (Trifolium pretense L.), and white clover (Trifolium repens L.). The cover crops of winter wheat (WW) and winter rye (WR) were selected due to their success and popularity as cover crops in the upper Midwest, and for their grazing quality. The experiment began in 2015 on existing pastures, which were tilled and planted to WW and WR in separate blocks on 10 September 2015. These forage crops were grazed during 2016 (grazing details below). Using MN as an example, the WR stubble remained until a soybean crop was planted in the WR block on 20 May 2017. Hairy vetch was drilled into WW stubble on 15 August 2016 and then the WW block was planted to corn on 20 May 2017. Harvest of the corn and soybean occurred on 20 October 2017. The corn and soybean blocks were planted back to pasture on 20 April 2018. Manure from cattle during the grazing season fertilized pastures in this study, without additional commercial fertilizer or irrigation. No additional fertilizer was applied to the corn, soybean or pasture crops.
The 29 cattle for the study in Minnesota were derived from the organically managed cattle from the WCROC [31]. The Rodale Institute purchased 12 organic cattle for the experiment. During the grazing part of the experiment, a metal exclusion cage, measuring 6.1 m × 6.1 m, which prevented cattle grazing (the non-grazed portion of the study), was established in each replicated paddock. Crops and cattle were raised according to USDA-AMS organic regulations as set forth in the National Organic Program (NOP) rules [25,32].
At each site, the designated pasture area selected for the 4-year experiment was sampled prior to cover crop planting. Soil samples were taken (baseline sampling) from 0–15 cm depths. Samples were collected randomly within each plot as an attempt to minimize variability using a metal soil probe, 2.54 cm diameter, and 8 cores were collected from each plot and combined into one composite sample. Soil samples were air dried after collection and ground to pass through a 2-mm sieve and saved for biological and chemical tests. Because the use of moist or dry soil can affect biological activity, dried and wet soils for the enzyme analysis in this study produced similar results, as verified on a set of subsamples prior to the start of the study (unpublished data). Soil pH and salinity were analyzed using 1:1 saturated paste soil extract methods described by Watson and Brown [33] and Whitney [34], respectively; SOM was analyzed using the ignition method described by Combs and Nathan [35], extractable K, calcium (Ca), magnesium (Mg), and cation exchange capacity (CEC) were determined according to Warncke and Brown [36]; available P was determined using the Bray-1 extractant [37]. β-glucosidase (E.C. 3.2.1.21), alkaline phosphatase (E.C. 3.1.3.1) and arylsulfatase (E.C. 3.1.6.1) activities were determined according to Tabatabai [38]. Fluorescein diacetate hydrolysis (FDA) was analyzed using a modified protocol adapted from Adam and Duncan [39]. For all enzymes, the reactions were measured against a control from the same soil sample to account for p-nitrophenol released from activity not related to enzymes. Both absorbance values were plotted on a calibration curve of known standards, and the difference was used to represent the enzyme activity (ug kg−1 h−1). Ammonium was analyzed after extraction in 2M KCl using the sodium salicylate method as described by Nelson [40]. Nitrate was determined after extraction in 2M KCL using the vanadium method [41]. Permanganate oxidizable carbon (POXC) was analyzed using the method described by Culman et al. [42]. Total N, C, and S percentage was determined on the vario MAX cube CNS analyser (Elementar Analysensysteme, Ronkonkoma, NY, USA). The results are shown in Table 1 and Table 2.

Table 1.
Soil chemical attributes in 0–15 cm before the field trial at the Minnesota site.

Table 2.
Soil chemical attributes in 0–15 cm layer before the field trial at Pennsylvania site.
2.2. Cattle Grazing of Paddocks
Each pasture was divided into seven 0.57-ha paddocks in order to implement rotational grazing methods, with a stocking rate of 4 or 5 steers per paddock. Grazing was initiated when forage height reached 15 cm at both sites in April 2016. Steers were randomly assigned to graze either WR or WW, and remained in their groups throughout the grazing season, separated by paddocks using temporary fencing. As an example, in Minnesota, starting from the north end of the seven paddocks, steer groups rotationally grazed until 13 June 2016 with free-choice mineral supplements for seven weeks. Steers moved to a new paddock every three days and grazed the same paddock three times during the study. Briefly, steers grazed on WR and WW had similar (p = 0.88) average daily gains (ADG; 0.87 kg d−1) from birth until harvest, which are similar to results in Bjorklund et al. [43] who reported an ADG range of 0.62–0.82 kg/d for grass-fed and organic steers of similar breeds in the current study. Furthermore, steers grazed on WR (0.33 kg d−1) and WW (0.32 kg d−1) had similar (p = 0.64) ADG from the first day of grazing to the last day of grazing [25]. Steers were prevented from grazing inside exclusion cages throughout the course of the experiment.
2.3. Soil Sampling during the Experiment
Soil samples were taken five times during the study at each location: (1) baseline measures before cover crop seed planting in Summer 2015 (MN: June; PA: July); (2) after cattle grazing in Fall 2016 (MN: September; PA: August); (3) after corn and soybean planting in Spring 2017 (MN: May; PA: April); (4) after corn and soybean harvest in Fall 2017 (MN: November; PA: December); (5) after perennial pasture planting in Spring 2019 (MN: May; PA: May). Additional samples were taken prior to cattle grazing in May 2016 at the MN site and after perennial pasture planting in November 2018 at the PA site. Soil samples were taken from 0–15 cm depths. Soil samples were collected randomly across the paddock/plot, taking care to avoid areas that had clear signs of urine or manure deposition. As with the baseline sampling, 8 probes were taken per paddock (2016), corn/soybean plot (2017), and pasture plot (2018–2019) and combined into one composite sample for analysis. The soil properties analyzed were soil pH, organic matter, salinity, K, Ca, Mg, cation exchange capacity, Bray-1 P, β-glucosidase, alkaline phosphatase, aryl sulfatase, fluorescein diacetate hydrolysis, ammonium, nitrate, permanganate oxidizable carbon, N%, C%, S%, and C:N ratio, following procedures mentioned previously.
2.4. Statistical Analysis
Data were analyzed by linear mixed models with repeated measures using the GLIMMIX procedure of SAS 9.4 (SAS Institute Inc.: Cary, NC, USA). Main fixed factors included grazing (2 levels: grazing and no grazing), cover crop (2 levels: rye and wheat), rotation, and sampling date (4 levels: Summer 2015, Spring 2017, Fall 2017, and Spring 2019), as well as their interactions. Separate models were built for each outcome. Main effects were deemed significant when the when the p-value for mean difference was p ≤ 0.05. In the presence of an interaction, pairwise mean comparisons were made using the lines option and differences are discussed when p ≤ 0.05. Data were analyzed separately by location due to different sampling dates in each experimental site.
3. Results
Weather patterns over the course of the study in Minnesota and Pennsylvania are shown in Figure 1. At the Minnesota site, statistical analysis showed that soil salinity, Ca, Mg, CEC, glucosidase activity, sulfatase activity, C%, and C:N ratio were significantly affected by the main effect of sampling date (Table 3). Soil pH, OM, K, Ca, Mg, CEC, glucosidase activity, phosphatase activity, sulfatase activity, POXC, N%, C%, and S% were significantly affected by the main effect of cropping systems (Table 3). Bray-1 P and NO3− content were significantly affected by the interaction between sampling date x cover crop (Table 3). Ammonium content was significantly affected by the interaction effect between grazing x sampling date x cover crop (Table 3).
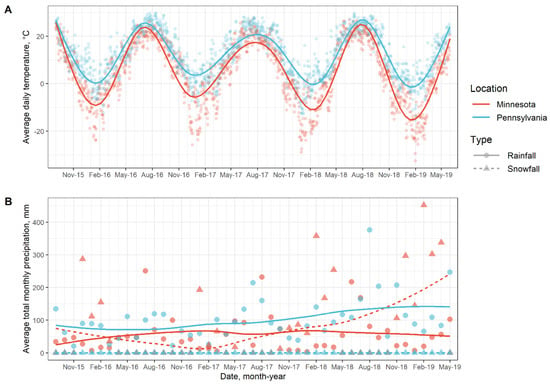
Figure 1.
Weather patterns over the course of the study in Minnesota and Pennsylvania.

Table 3.
Summary of statistical analysis for pH, OM, salinity, K, Ca, Mg, cation exchange capacity (CEC), Bray-1 P, glucosidase, phosphatase, sulfatase, fluorescein diacetate hydrolysis (FDA), NH4+, NO3−, permanganate oxidizable carbon (POXC), N%, C%, S%, and C:N ratio as a function of sampling date, cropping system and grazing in Minnesota and Pennsylvania from 2015 to 2018.
At the Pennsylvania site, statistical analysis showed that soil OM, bray-1 P, phosphatase, sulfatase, and FDA were significantly affected by the main effect of sampling date (Table 3). Ammonium content was significantly affected by the interaction between sampling date x grazing (Table 3). The pH, salinity, Ca, Mg, CEC, glucosidase, NO3−, POXC, C%, S%, and C:N ratio were significantly affected by the interaction effect between grazing x sampling date x cover crop (Table 3).
3.1. Minnesota
Soil salinity was found to be higher in the last sampling in 2016 and also in the samplings collected in 2017, being always higher in samples collected in the fall than in the spring (Figure 2A). Calcium content was lowest in the first and sixth samplings compared with the other samplings (Figure 2B). Magnesium content fluctuated throughout the sampling times and was greatest in the fall of 2017 and both sampling collected in 2016 (Figure 2C). Cation exchange capacity followed the same behavior observed for Ca and Mg, which was expected since these metals represent more than 60% of the metals occupying the binding sites in the CEC (Figure 2D). Activity of the enzymes β-Glucosidase and aryl-sulfatase varied significantly during the study and were usually greater in samples collected early in the spring than in samples collected later in the fall within each year (Figure 2E,F). Carbon percentage also fluctuated throughout the study and tended to be higher after cover crop (19 May 2016) growth or corn harvest (2 November 2017) (Figure 3A). C:N ratio was lowest in the fourth sampling compared to all of the other samplings (Figure 3B).
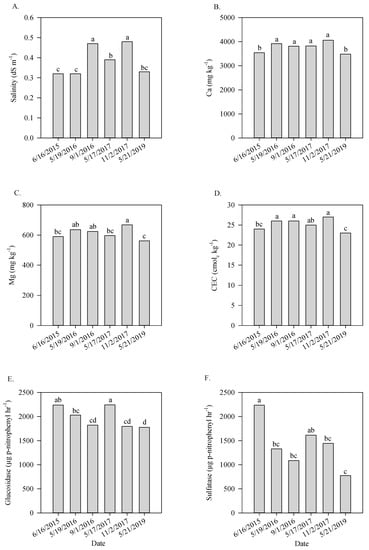
Figure 2.
Salinity (A), Ca (B), Mg (C), CEC (D), glucosidase (E), and sulfatase (F) as a function of sampling date in Minnesota site. Means followed by different letters are significantly different (p-value ≤ 0.05). CEC = cation exchange capacity; Glucosidase = β-Glucosidase; Sulfatase = Aryl sulfatase.
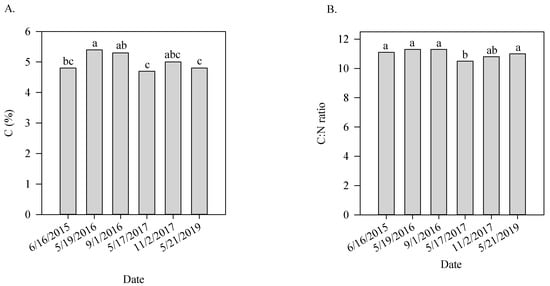
Figure 3.
C (A) and C:N ratio (B) as a function of sampling date in Minnesota site. Means followed by different letters are significantly different (p-value ≤ 0.05).
Soil pH, Ca, and aryl-sulfatase activity were greater in plots under the P-R-SB-P cropping system; however, OM, K, Mg, CEC, glucosidase, phosphatase, POXC, N, C, and S were greater in the plots under the P-W/V-C-P cropping system (Table 4). Potassium concentrations were greater in the P-R-SB-P cropping system in the baseline measures than in the P-W/V-C-P cropping system by about 176 ppm (Table 1). While at the end of the study the differences were 113 ppm (Table 2).

Table 4.
pH, OM, K, Ca, Mg, CEC, glucosidase, phosphatase, sulfatase, POXC, N, C, and S as a function of cropping systems in the Minnesota site.
Bray-1 P behaved differently between the two cropping systems as well as during the time the study was conducted (Table 5). In the cropping system, P-R-SB-P Bray-1 P levels in the soil tended to remain the same averaging around 19 mg P kg−1 (Table 5). In contrast, there was a significant difference in soil Bray-1 P levels in the P-W/V-C-P cropping system. In this case, samples collected in the fall 2016 had the highest P levels (38 mg P kg−1) compared with other sampling times (average 9.1 mg P kg−1) (Table 5). Nitrate levels showed a consistent increase after the spring in 2016 where levels increased from 3.3 to 46.3 and 1.0 to 28.8 mg N kg−1 in the P-R-SB-P and P-W/V-C-P cropping systems (Table 5). At Minnesota, extractable NH4+ showed high variability, but levels tended to stay close to initial levels (Table 6). The most noticeable significant differences were observed in the sampling taking place on 17 May 2017 and 2 November 2017 for the P-R-SB-P cropping system when NH4+ levels (22.3 and 27.9 mg N kg−1, respectively) were significantly higher than at any other sampling time (average 6.8 mg N kg−1) in this system (Table 6). For the P-W/V-C-P cropping system NH4+ levels were greatest at the sampling taking place on 2 November 2017 (23.9 mg N kg−1) compared with the other samplings (average 6.8 mg N kg−1) (Table 6). In addition, at both cropping systems NH4+ levels were the lowest in samples collected on 1 September 2016, likely a reflection of crop growth and nutrient removal (Table 6).

Table 5.
Bray-1 P and NO3− as a function of the interaction between sampling date and cropping systems in Minnesota site.

Table 6.
NH4+ as a function of the interaction between sampling date, cropping systems, and grazing in Minnesota site.
3.2. Pennsylvania
Soil organic matter levels were neither affected by cropping system nor by grazing, being mostly affected by time of sampling (Table 7). Organic matter levels in samples collected on the first sampling (5.8%) were greater than those collected on the second (5.0) and fourth (4.7) samplings (Table 7). Bray-1 P was greater in the first and second samplings and tended to decrease as the study went on (Table 8). Alkaline phosphatase and aryl-sulfatase activity were greater in the third sampling (3167 and 1959 µg p-nitrophenyl h−1, respectively) and lowest in the last sampling (2021 and 1060 µg p-nitrophenyl h−1, respectively) compared to any other samplings (Table 7). Fluorescein diacetate hydrolysis was greater in the first sampling compared to all other samplings (Table 7). Ammonium concentration in soil samples varied across sampling and was highest in the last sampling than during the study (Figure 4). Furthermore, samples collected at the 11/30/2018 sampling time showed that grazed plots had higher levels of NH4+ (6.0 mg N kg−1) in the soil than exclusion plots (0.2 mg N kg−1) (Figure 4). This trend was not observed at all sampling times and it could be due to the complex mechanism related to ammonium oxidation into nitrate [26]. Urine or feces deposition can affect the rate at which ammonium is converted into nitrate [26]. Therefore, it is possible that the results observed for the sampling taking place on 30 November 2018 were close to deposition of urine and/or feces and that is why we only observed this effect at this sampling time.

Table 7.
OM, Bray-1 P, phosphatase, sulfatase, and FDA as a function of sampling date in Pennsylvania site.

Table 8.
pH, salinity, Ca, Mg, CEC, and glucosidase as a function of the interaction between sampling date, cropping systems and grazing in Pennsylvania site.
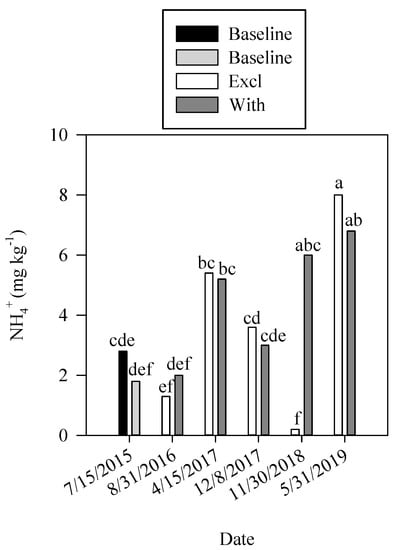
Figure 4.
NH4+ as a function of the interaction between sampling date and grazing in Pennsylvania site. Means followed by different letters are significantly different (p-value ≤ 0.05).
Although there were several significant differences in soil pH, the most biological meaningful trend was that soil pH decreased from the beginning of the study (pH 6.5) to the end of the study (6.1) by 0.4 units (Table 8). Soil salinity varied during the study without any clear-cut trend (Table 8). Under the P-R-S-P cropping system, extractable soil Ca and Mg behaved similarly. Calcium and Mg levels in samples collected on 31 August 2016 and 8 December 2017 were found to be greatest in plots that were grazed (1335 and 1446 mg Ca kg−1 and 137 and 133 mg Mg kg−1, respectively) than in the exclusion plots (1249 and 1293 mg Ca kg−1 and 113 and 121 mg Mg kg−1, respectively) (Table 8). Overall, Ca and Mg levels tended to decrease from the beginning (1192 mg Ca kg−1 and 113 mg Mg kg−1) of the study to the end (889 mg Ca kg−1 and 91 mg Mg kg−1) of the study (Table 8). Soil CEC followed a similar behavior to Ca and Mg and was highest at the beginning of the study (9.3 cmolc kg−1) and lowest at the end of the study (7.8 cmolc kg−1) (Table 8). β-Glucosidase activity was greater in the samples collected at the 4/15/2017 sampling time than at any other sampling times (Table 8). No clear-cut trend was observed for the effect of grazing on β-Glucosidase activity (Table 8). Soil NO3− content also varied significantly during the course of the study without any clear-cut trend (Table 9). In general, NO3− was greater during a cash crop year or the year after (Table 9). The highest NO3− levels were observed for the P-W/V-C-P cropping system in 2017 (average 19 mg kg−1) compared with the other sampling times and cropping system (average 7 mg kg−1) (Table 9).

Table 9.
NO3−, POXC, C, S, and C:N ratio as a function of the interaction between sampling date, cropping systems, and grazing in Pennsylvania site.
Permanganate oxidizable carbon was greater at the beginning of the study (average 6.5 mg kg−1 in 2015) and decreased as the study went on (average 6.1 mg kg−1 in 2019) (Table 9). Carbon and S percentage, and C:N ratio in the soil changed randomly during the course of the study without a clear-cut trend (Table 9).
4. Discussion
Early responses of soil enzymatic activity and soil fertility to differing cropping systems under cattle grazing have been difficult to document. However, the results of this study demonstrated that grazing had a significant impact on many soil properties, e.g., increased glucosidase activity, available Ca, Mg, NO3−, NH4+, soil pH, soil C%, S%, and also C:N ratio. The effects of cover crop use on soil quality were even more evident in this research. The results of this study showed that the soil properties measured varied according to which cropping system was adopted. In the P-W/V-C-P cropping system, soil pH, available Ca, NO3−, and sulfatase activity were found to increase compared with the P-R-SB-P cropping system. In contrast, soil OM, available K, Mg, CEC, glucosidase, phosphatase, POXC, and total C%, N%, and S% were greater in the P-R-SB-P cropping system compared with the P-W/V-C-P cropping system. Although increased available nutrients in the soil can benefit crops, some might pose risks to the environment, e.g., increased NO3− can impair water quality. Therefore, the combination of grazing with cover crops is beneficial because, under correct management practices, negative aspects of one practice can be ameliorated by the other practice. In this case, grazing increased NO3− but the use of a P-R-SB-P rotation enabled mitigation of the increased NO3−.
The results of our study showed that, under the P-R-SB-P cropping system, soil OM and glucosidase and phosphatase activity levels were greater, suggesting that although more OM was available in this system, greater enzymatic activity by microorganisms was needed for decomposition. It is possible that the quality of the OM being added in the P-W/V-C-P cropping system was of greater quality for the microbial community since this system had lower enzyme activities and also lower total C%, N%, and S%, suggesting greater OM decomposition. Similarly, other studies have reported that cover crops can increase soil OM and improve soil structure, fertility, and soil biological activity [44,45,46]. In addition, the quality and quantity of plant residue entering the soil can significantly influence soil microorganisms and soil microbial processes [47,48,49]. Both crop residue and OM quality have the potential to increase functional diversity in soil microbial communities [44,50,51]. It should be expected that overall plant growth and development could be affected by the different cropping system tested [52] and, consequently, nutrient cycling in the soil would affect soil fertility and enzyme activity as observed in this study. Muñoz et al. [53] showed that the change of C:N ratio in soil indicated different degrees of microbial decomposition, considering that C depletion was a product of microbial activity. Therefore, it is possible that microbial decomposition under the P-W/V-C-P cropping system was greater than under the P-R-SB-P cropping system.
Cover crops would be expected to use soil water and N during the period from April through June, when the plants were accumulating biomass. The increase in cover crop biomass, above and below ground, could explain the greater C%, β-glucosidade, and aryl-sulfatase activity, as needed for biomass decomposition, as well as the greater NO3− and NH4+ levels with the P-R-SB-P cropping system during the spring sampling at the Minnesota site. Additionally, it is known that plant biomass increases lead to increased root density, which could have led to the increases in the soil enzyme activities and microbial respiration [54], reinforcing the importance of the cover crops in organic systems. After planting soybean or corn, a reduction in the C:N ratio, due to tillage practices should be expected, which was observed at the Minnesota site, speeding up C decomposition. Soil microorganisms play a central role in decomposition and respiration, and influences C storage in soil [55]. Therefore, the enzymatic activity would be greater at this sampling date. The results of this study did show increased β-glucosidase, phosphatase, and aryl-sulfatase soon after soybean or corn planting at the Pennsylvania site. Soil enzymes are important components of the biochemical functioning of soils as they take part in OM decomposition and nutrient cycling [54,55,56,57]. These enzymes most likely act in an extracellular manner and are involved in the hydrolytic reactions that convert inorganic compounds from organic sources and they are considered as microbiological activity indexes in soils [54].
Considering the increase in soil enzyme activities after cover crops and grazing at the Minnesota site, it can be concluded that soil microbiological activity was beneficial for corn and soybean crops. The results of this study showed that it is possible to intensify land use in organic cropping systems and maintain adequate soil fertility and microbial activity. However, corn and soybean crops can remove large amounts of nutrients [58,59] and water use due to a robust root system [60]. Furthermore, a well-developed root system will also increase nutrient absorption by plants, and consequently greater P, exchangeable bases, and OM (upon decomposition), reducing soil pH and enzymatic activity, as verified at the Pennsylvania site. In addition, the activity of soil microorganisms is suppressed due to low temperature and high soil water saturation, reducing oxygen availability [15], decreasing microbial biomass and soil pH [60]. This could explain the increased soil salinity, Ca, Mg, and CTC, and also NH4+ and P level with the P-W/V-C-P cropping system during the fall season at the Minnesota site.
Although cattle grazing provided a slight benefit for enzymatic activity and soil fertility, due to an increase in Ca and Mg levels in Pennsylvania and NH4+ in both sites, it had no negative impact on soil fertility and quality. Therefore, grazing has the potential to increase nutrient availability and nutrient cycling in the soil, and if crops are unavailable to remove or immobilize excess nutrients, non-point source pollution can result. In addition, the organic cattle system can be environmentally favorable, as it can help minimize N losses to groundwater, neighboring habitats, or mitigating greenhouse gases [61,62], leading to an increasingly sustainable agricultural production system.
5. Conclusions
The results of this study showed that grazing had a significant impact on many of the soil properties measured, including increased available nutrients and total C. Cover crop use was found to help minimize the potential negative impacts generated by grazing within a crop rotation system which includes grain crops. Further research is needed to determine best management practices that would facilitate the incorporation of grazing and cover crops for organic grain producers.
Author Contributions
Conceptualization, K.D., B.H., P.H.P.; methodology, K.D., B.H., P.H.P.; validation, K.D., B.H., P.H.P.; investigation, F.S.G., K.D., B.H., P.H.P., A.S., H.P.; data curation, K.D., B.H., P.H.P., A.S., H.P.; writing—original draft preparation, F.S.G., P.H.P.; writing—review and editing, F.S.G., K.D., B.H., P.H.P., H.P.; supervision, K.D., B.H., P.H.P.; project administration, K.D., B.H., P.H.P.; funding acquisition, K.D., B.H., P.H.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Organic Research and Extension Initiative, grant no. 2014-51300-22541 from the USDA National Institute of Food and Agriculture.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Statement
Researchers conducted the study at the University of Minnesota West Central Research and Outreach Center, Morris, MN (WCROC) organic dairy in Morris, Minnesota. The University of Minnesota Institutional Animal Care and Use Committee approved all animal care and management (Animal Subjects Code number 1411-32060A). The Pennsylvania study was conducted on a private farm.
References
- Blanco-Canqui, H.; Francis, C.A.; Galusha, T.D. Does organic farming accumulate carbon in deeper soil profiles in the long term? Geoderma 2017, 288, 213–221. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.; Mäder, P.; Bünemann, E.K.; De Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Mirsky, S.B.; Teasdale, J.R.; Spargo, J.T.; Doran, J. Organic grain cropping systems to enhance ecosystem services. Renew. Agric. Food Syst. 2013, 28, 145–159. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.; De Deyn, G.B.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Ponisio, L.C.; M’Gonigle, L.K.; Mace, K.C.; Palomino, J.; De Valpine, P.; Kremen, C. Diversification practices reduce organic to conventional yield gap. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141396. [Google Scholar] [CrossRef] [PubMed]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.B.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Dorn, B.; Jossi, W.; Van Der Heijden, M.G.A. Cover crops support ecological intensification of arable cropping systems. Sci. Rep. 2017, 7, 41911. [Google Scholar] [CrossRef]
- Schrama, M.; De Haan, J.; Kroonen, M.; Verstegen, H.; Van Der Putten, W. Crop yield gap and stability in organic and conventional farming systems. Agric. Ecosyst. Environ. 2018, 256, 123–130. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Capurro, J.E.; Martínez, J.M. Winter cover crops in soybean monoculture: Effects on soil organic carbon and its fractions. Soil Tillage Res. 2016, 161, 95–105. [Google Scholar] [CrossRef]
- Basche, A.D.; Archontoulis, S.V.; Kaspar, T.C.; Jaynes, D.B.; Parkin, T.B.; Miguez, F. Simulating long-term impacts of cover crops and climate change on crop production and environmental outcomes in the Midwestern United States. Agric. Ecosyst. Environ. 2016, 218, 95–106. [Google Scholar] [CrossRef]
- Kladivko, E.J.; Kaspar, T.C.; Jaynes, D.B.; Malone, R.W.; Singer, J.; Morin, X.K.; Searchinger, T. Cover crops in the upper midwestern United States: Potential adoption and reduction of nitrate leaching in the Mississippi River Basin. J. Soil Water Conserv. 2014, 69, 279–291. [Google Scholar] [CrossRef]
- De Baets, S.; Van De Weg, M.; Lewis, R.; Steinberg, N.; Meersmans, J.; Quine, T.; Shaver, G.R.; Hartley, I. Investigating the controls on soil organic matter decomposition in tussock tundra soil and permafrost after fire. Soil Biol. Biochem. 2016, 99, 108–116. [Google Scholar] [CrossRef]
- Dou, X.; He, P.; Cheng, X.; Zhou, W. Long-term fertilization alters chemically-separated soil organic carbon pools: Based on stable C isotope analyses. Sci. Rep. 2016, 6, 19061. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.; Hastings, A.; Chadwick, D.; Jones, D.L.; Evans, C.; Jones, M.; Rees, R.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Prabhakara, K.; Hively, D.; Mccarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 88–102. [Google Scholar] [CrossRef]
- Eze, S.; Palmer, S.M.; Chapman, P. Soil organic carbon stock in grasslands: Effects of inorganic fertilizers, liming and grazing in different climate settings. J. Environ. Manag. 2018, 223, 74–84. [Google Scholar] [CrossRef]
- Rakkar, M.K.; Blanco-Canqui, H. Grazing of crop residues: Impacts on soils and crop production. Agric. Ecosyst. Environ. 2018, 258, 71–90. [Google Scholar] [CrossRef]
- A Martinez-Feria, R.; Dietzel, R.; Liebman, M.; Helmers, M.J.; Archontoulis, S.V. Rye cover crop effects on maize: A system-level analysis. Field Crop. Res. 2016, 196, 145–159. [Google Scholar] [CrossRef]
- Spargo, J.T.; Cavigelli, M.A.; Mirsky, S.B.; Meisinger, J.J.; Ackroyd, V.J. Organic supplemental nitrogen sources for field corn production after a hairy vetch cover crop. Agron. J. 2016, 108, 1992–2002. [Google Scholar] [CrossRef]
- Poffenbarger, H.J.; Mirsky, S.B.; Weil, R.R.; Maul, J.E.; Kramer, M.; Spargo, J.T.; Cavigelli, M.A. Biomass and nitrogen accumulation of hairy vetch-cereal rye cover crop mixtures as influenced by species proportions. Agron. J. 2015, 107, 2069–2082. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mirsky, S.B.; Spargo, J.T.; Cavigelli, M.A.; Maul, J.E. Reduced-tillage organic corn production in a hairy vetch cover crop. Agron. J. 2012, 104, 621–628. [Google Scholar] [CrossRef]
- E Drewnoski, M.; Parsons, J.; Blanco, H.; Redfearn, D.; Hales, K.; Macdonald, J. Forages and pastures symposium: Cover crops in livestock production: Whole-system approach. Can cover crops pull double duty: Conservation and profitable forage production in the Midwestern United States? J. Anim. Sci. 2018, 96, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Allen, V.; Hou, F.; Chen, J.; Brown, C.P. Steers grazing a rye cover crop influence growth of rye and no-till cotton. Agron. J. 2013, 105, 1571–1580. [Google Scholar] [CrossRef]
- Phillips, H.N.; Heins, B.; Delate, K.; Turnbull, R. Impact of grazing dairy steers on winter rye (Secale cereale) versus winter wheat (Triticum aestivum) and effects on meat quality, fatty acid and amino acid profiles, and consumer acceptability of organic beef. PLoS ONE 2017, 12, e0187686. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.; Baier, A.H. Guide for Organic Livestock Producers; National Center for Appropriate Technology (NCAT): Butte, MO, USA; USDA: Washington, DC, USA, 2012.
- Lehman, R.M.; Taheri, W.I.; Osborne, S.L.; Buyer, J.; Douds, D.D. Fall cover cropping can increase arbuscular mycorrhizae in soils supporting intensive agricultural production. Appl. Soil Ecol. 2012, 61, 300–304. [Google Scholar] [CrossRef]
- Silva, E.M. Screening five fall-sown cover crops for use in organic no-till crop production in the Upper Midwest. Agroecol. Sustain. Food Syst. 2014, 38, 748–763. [Google Scholar] [CrossRef]
- Appelgate, S.R.; Lenssen, A.; Wiedenhoeft, M.H.; Kaspar, T.C. Cover crop options and mixes for Upper Midwest corn-soybean systems. Agron. J. 2017, 109, 968–984. [Google Scholar] [CrossRef]
- Nazareth, J.; Shaw, A.; Delate, K.; Turnbull, R. Food safety considerations in integrated organic crop–livestock systems: Prevalence of Salmonella spp. and E. coli O157:H7 in organically raised cattle and organic feed. Renew. Agric. Food Syst. 2019, 1–9. [Google Scholar] [CrossRef]
- Heins, B.; Hansen, L.; Hazel, A.; Seykora, A.; Johnson, D.; Linn, J. Birth traits of pure Holstein calves versus Montbeliarde-sired crossbred calves. J. Dairy Sci. 2010, 93, 2293–2299. [Google Scholar] [CrossRef]
- USDA-AMS. The program handbook United States. Available online: https://www.ams.usda.gov/rules-regulations/organic/handbook (accessed on 13 April 2020).
- Watson, M.E.; Brown, J.R. pH and lime requirement. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 13–16. [Google Scholar]
- Whitney, D.A. Soil salinity. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 59–60. [Google Scholar]
- Combs, S.M.; Nathan, M.V. Soil organic matter. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 53–58. [Google Scholar]
- Warncke, D.; Brown, J.R. Potassium and other basic cations. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 31–33. [Google Scholar]
- Frank, K.; Beegle, D.; Denning, J. Phosphorus. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 21–26. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis, Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Adam, G.; Duncan, H. Development of sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Nelson, D.W. Determination of ammonium in KCl extracts of soils by the salicylate method. Comm. Soil Sci. Plant Anal. 1983, 14, 1051–1062. [Google Scholar] [CrossRef]
- Gelderman, R.H.; Beegle, D. Nitrate-nitrogen. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; pp. 17–20. [Google Scholar]
- Culman, S.W.; Freeman, M.; Snapp, S.S. Procedure for the determination of permanganate oxidizable carbon. Kellogg Biological Station-Long Term Ecological Research Protocols, Hickory Corners, MI. Available online: http://lter.kbs.msu.edu/protocols/133 (accessed on 13 April 2020).
- Bjorklund, E.; Heins, B.; DiCostanzo, A.; Chester-Jones, H. Growth, carcass characteristics, and profitability of organic versus conventional dairy beef steers. J. Dairy Sci. 2014, 97, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Scotti, R.; Conte, P.; Berns, A.E.E.; Alonzo, G.; A Rao, M. Effect of organic amendments on the evolution of soil organic matter in soils stressed by intensive agricultural practices. Curr. Org. Chem. 2013, 17, 2998–3005. [Google Scholar] [CrossRef][Green Version]
- Mbuthia, L.W.; Acosta-Martinez, V.; Debruyn, J.M.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N.S. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Govaerts, B.; Mezzalama, M.; Unno, Y.; Sayre, K.D.; Luna-Guido, M.; Vanherck, K.; Dendooven, L.; Deckers, J. Influence of tillage, residue management, and crop rotation on soil microbial biomass and catabolic diversity. Appl. Soil Ecol. 2007, 37, 18–30. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: Fourteen years on. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Yang, H.; Feng, J.; Zhai, S.; Dai, Y.; Xu, M.; Wu, J.; Shen, M.; Bian, X.; Koide, R.T.; Liu, J. Long-term ditch-buried straw return alters soil water potential, temperature, and microbial communities in a rice-wheat rotation system. Soil Tillage Res. 2016, 163, 21–31. [Google Scholar] [CrossRef]
- Thompson, K.A.; Deen, B.; Dunfield, K.E. Impacts of surface-applied residues on N-cycling soil microbial communities in miscanthus and switchgrass cropping systems. Appl. Soil Ecol. 2018, 130, 79–83. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Muñoz, C.; Monreal, C.; Schnitzer, M.; Zagal, E. Influence of Acacia caven (Mol) coverage on carbon distribution and its chemical composition in soil organic carbon fractions in a Mediterranean-type climate region. Geoderma 2008, 144, 352–360. [Google Scholar] [CrossRef]
- Fterich, A.; Mahdhi, M.; Mars, M. Impact of grazing on soil microbial communities along a chronosequence of Acacia tortilis subsp. raddiana in arid soils in Tunisia. Eur. J. Soil Biol. 2012, 50, 56–63. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.-J.; Chang, S.; Kan, H.; Lin, L. Impact of frazing on soil carbon and microbial biomass in typical steppe and desert steppe of Inner Mongolia. PLoS ONE 2012, 7, e36434. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar-Cepeda, C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2017, 54, 11–19. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Laboski, C.A.; Naeve, S.L.; Conley, S.P. Dry matter and nitrogen uptake, partitioning, and removal across a wide range of soybean seed yield levels. Crop. Sci. 2017, 57, 2170–2182. [Google Scholar] [CrossRef]
- Sawyer, J.E.; Woli, K.P.; Barker, D.; Pantoja, J.L. Stover removal impact on corn plant biomass, nitrogen, and use efficiency. Agron. J. 2017, 109, 802–810. [Google Scholar] [CrossRef]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency—A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Klaus, V.H.; Kleinebecker, T.; Prati, D.; Gossner, M.M.; Alt, F.; Boch, S.; Gockel, S.; Hemp, A.; Lange, M.; Müller, J.; et al. Does organic grassland farming benefit plant and arthropod diversity at the expense of yield and soil fertility? Agric. Ecosyst. Environ. 2013, 177, 1–9. [Google Scholar] [CrossRef]
- Egan, G.; Zhou, X.; Wang, D.; Jia, Z.; Crawley, M.; Fornara, D.A. Long-term effects of grazing, liming and nutrient fertilization on the nitrifying community of grassland soils. Soil Biol. Biochem. 2018, 118, 97–102. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
