Bio-Herbicidal Potential of the Essential Oils from Different Rosmarinus officinalis L. Chemotypes in Laboratory Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oil Extraction and Analyses
2.3. Classification and Chemotype Definition
- -
- single main compound chemotype: one compound representing more than 33% of the total composition and all the rest was less than 20%
- -
- two main compounds chemotype: two compounds together representing more than 50% of the total composition and all the rest was less than 15%
- -
- three main compounds chemotype: three compounds together representing more than 60% and all rest was less than 5%.
2.4. Germination Test
2.5. Post Germination Test
2.6. Statistical Analyses
3. Results
3.1. EOs Content and Chemical Composition
3.2. Germination Test
3.3. Post-Germination Test
3.3.1. Relative Growth Rate
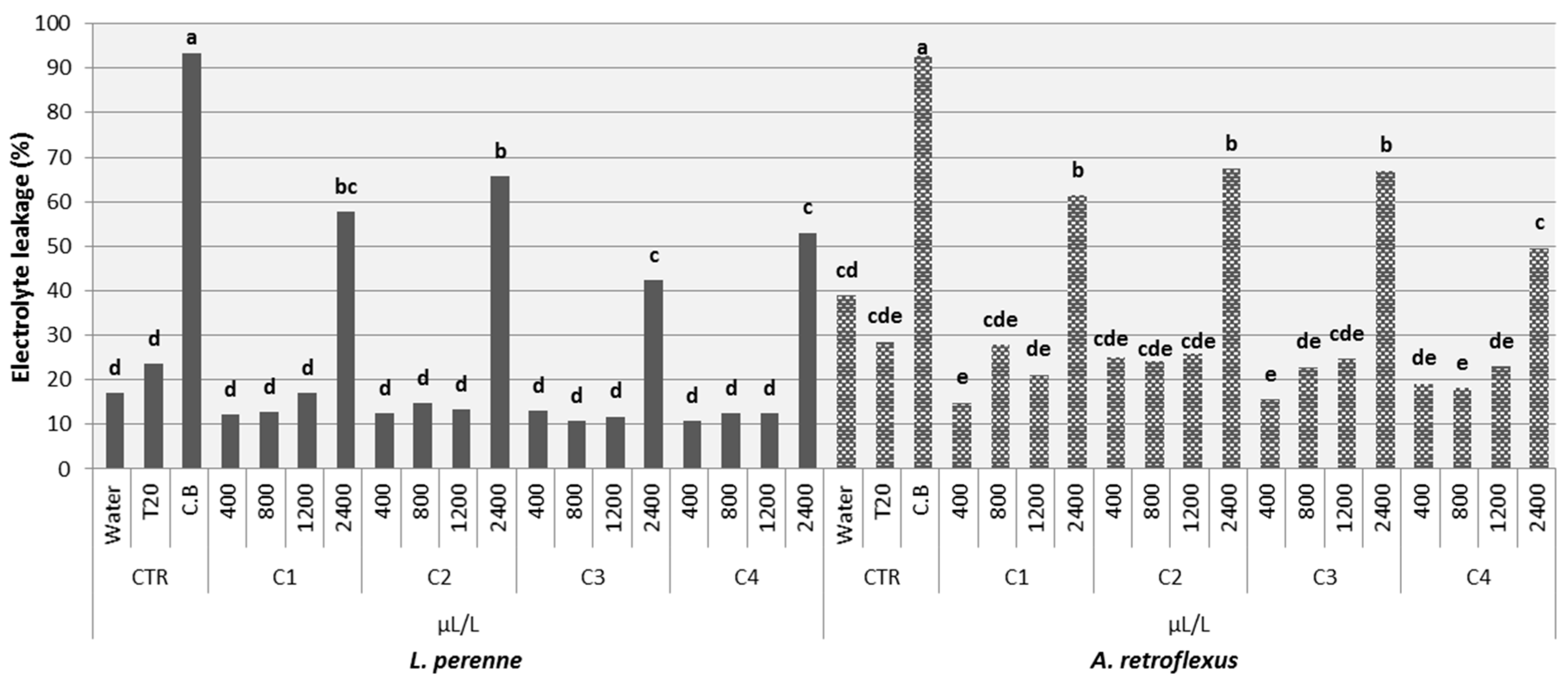
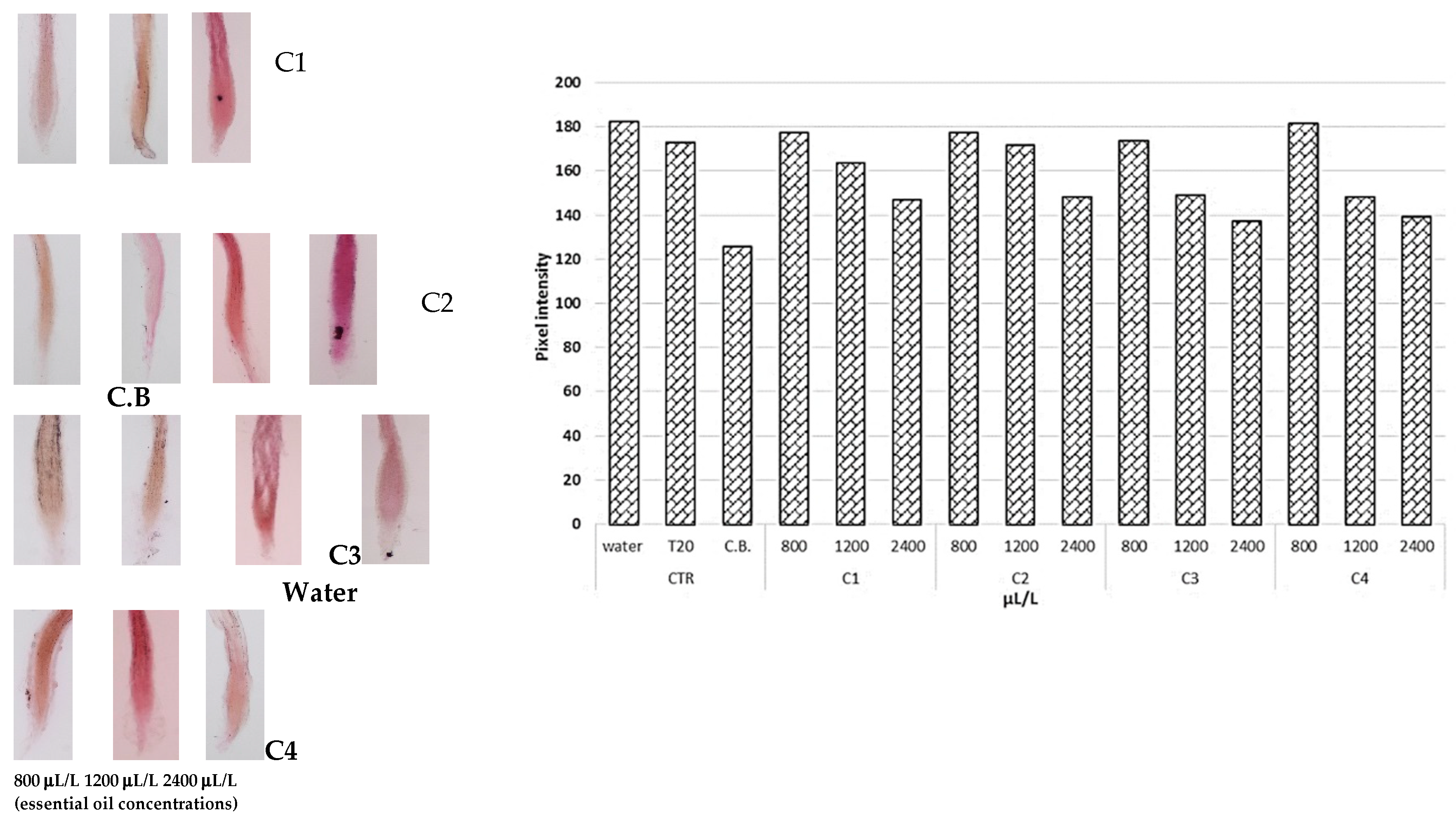
3.3.2. Electrolyte Leakage and Histochemical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimdahl, R.L. Fundamentals of Weed Science, 5th ed.; Zimdahl, R.L., Ed.; Academic Press: Fort Collins, CO, USA, 2018; pp. 17–46. [Google Scholar]
- Van Bueren, E.T.L.; Paul, C.S.; Evert, J. Ecological concepts in organic farming and their consequences for an organic crop ideotype. Neth. J. Agric. Sci. 2002, 50, 1–26. [Google Scholar] [CrossRef]
- Kristiansen, P.; Sindel, B.; Jessop, R. Sustainable Weed Management in Organic Herb and Vegetable Production; RIRDC: Kingston, Australia, 2007; p. 1. [Google Scholar]
- Bastiaans, L.; Paolini, R.; Baumann, D.T. Focus on ecological weed management: What is hindering adoption? Weed Res. 2008, 48, 481–491. [Google Scholar] [CrossRef]
- Bond, W.; Grundy, A.C. Non-chemical weed management in organic farming systems. Weed Res. 2001, 41, 383–405. [Google Scholar] [CrossRef]
- Bàrberi, P. Integrated weed management in organic cropping systems. In Improving Organic Crop Cultivation; Köpke, U., Ed.; Burleigh Dodds: Bonn, Germany, 2018; pp. 323–341. [Google Scholar]
- Ramezani, S.; Saharkhiz, M.J.; Ramezani, F.; Fotokian, M.H. Use of essential oils as bioherbicides. J. Essent 2008, 11, 319–327. [Google Scholar] [CrossRef]
- Flamini, G. Natural Herbicides as a Safer and More Environmentally Friendly Approach to Weed Control: A Review of the Literature Since 2000. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Karachi, Pakistan, 2012; Volume 37, pp. 353–396. ISBN 978-0-444-59514-0. [Google Scholar]
- Benchaa, S.; Hazzit, M.; Nadjia, Z.; Abdelkrim, H. Chemical composition and herbicidal activity of essential oils from two Labiatae species from Algeria. J. Essent. Oil Res. 2019, 31, 335–346. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Mercati, F.; Antonio, S.G.; Abenavoli, M. Calamintha nepeta L. (Savi) as source of phytotoxic compounds: Bio-guided fractionation in identifying biological active molecules. Physiol. Plant 2013, 35, 1–10. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M.J. Phytotoxic activity and variation in essential oil content and composition of Rosemary (Rosmarinus officinalis L.) during different phenological growth stages. Biocatal. Agric. Biotechnol. 2016, 7, 271–278. [Google Scholar] [CrossRef]
- Atak, M.; Mavi, K.; Uremis, I. Bio-Herbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11158. [Google Scholar]
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Seidi, D.M. Phytotoxicity of encapsulated essential oil of rosemary on germination and morphophysiological features of amaranth and radish seedlings. Sci. Hortic. 2019, 243, 131–139. [Google Scholar] [CrossRef]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopathy J. 2010, 25, 1–5. [Google Scholar]
- De Mastro, G.; Fracchiolla, M.; Verdini, L.; Montemurro, P. Oregano and its potential use as bioherbicide. Acta Hortic. 2006, 723, 335–346. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and Tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Işik, D.; Mennan, H.; Çam, M.; Tursun, N.; Arslan, M. Allelopathic Potential of Some Essential Oil Bearing Plant Extracts on Common Lambsquarters (Chenopodium album L.). Rev. Chim. 2016, 67, 455–459. [Google Scholar]
- Islam, A.K.M.M.; Kato-Noguchi, H. Phytotoxic Activity of Ocimum tenuiflorum Extracts on Germination and Seedling Growth of Different Plant Species. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Kordali, S.; Tazegul, A.; Cakir, A. Phytotoxic effects of Nepeta meyeri Benth. Extracts and essential oil on seed germinations and seedling growths of four weed species. Rec. Nat. Prod. 2015, 9, 404–418. [Google Scholar]
- Muller, W.H.; Lorber, P.; Haley, B.; Johnson, K. Volatile growth inhibitors produced by Salvia leucophylla: Effect on oxygen uptake by mitochondrial suspensions. Bull. Torrey Bot. Club. 1969, 96, 89–96. [Google Scholar] [CrossRef]
- Vaughn, F.S.; Spencer, F.G. Volatile monoterpenes as potential parent structures for new herbicides. Weed Sci. 1993, 41, 114–119. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.; Elshamy, A.; El Gendy, A.E.N.; Gaara, A.; Assaeed, A. Volatiles profiling, allelopathic activity, and antioxidant potentiality of Xanthium strumarium leaves essential oil from Egypt: Evidence from chemometrics analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef]
- De Pasquale, C.; La Bella, S.; Cammalleri, I.; Gennaro, M.C.; Licata, M.; Leto, C.; Tuttolomondo, T. Agronomical and postharvest evaluation of the essential oils of Sicilian rosemary (Rosmarinus officinalis L.) biotypes. Acta Hortic. 2019, 1255, 139–144. [Google Scholar] [CrossRef]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Carrubba, A.; Abbate, L.; Sarno, M.; Sunseri, F.; Mauceri, A.; Lupini, A.; Mercati, F. Characterization of 549 Sicilian rosemary (Rosmarinus officinalis L.) germplasm through a multidisciplinary approach. Planta 2020, 251, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. OA 2018, 4, FSO283. [Google Scholar] [CrossRef] [PubMed]
- Elamrani, A.; Zrira, S.; Benjilali, B.; Berrada, M. A study of Moroccan Rosemary oils. J. Essent. Oil Res. 2000, 12, 487–495. [Google Scholar] [CrossRef]
- Porte, A.; Godoy, R.L.O.; Lopes, D.; Koketsu, M.; Gonçalves, S.L.; Torquilho, H.S. Essential oil of Rosmarinus officinalis L. (Rosemary) from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2000, 12, 577–580. [Google Scholar] [CrossRef]
- De Mastro, G.; Ruta, C.; Mincione, A.; Poiana, M. Bio-Morphological and chemical characterization of Rosemary (Rosmarinus officinalis L.) biotypes. ISHS Acta Hortic. 2004, 629, 471–842. [Google Scholar] [CrossRef]
- Zaouali, Y.; Messaoud, C.; Salah, A.B.; Boussaïd, M. Oil composition variability among populations in relationship with their ecological areas in Tunisian Rosmarinus officinalis L. Flavour Frag. J. 2005, 20, 512–520. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Smaeili, S.; Merikhi, M. Essential oil analysis and phytotoxic activity of two ecotypes of Zataria multiflora Boiss. growing in Iran. Nat. Prod. Res. 2010, 24, 1598–1609. [Google Scholar] [CrossRef]
- European Pharmacopoeia 2016. Ph. Eur. 9.0 Council of Europe, Strasbourg. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition (accessed on 22 April 2019).
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Poorter, H. Avoiding Bias in Calculations of Relative Growth Rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, W.; Li, B.; Wang, Y.; Li, K.; Han, C.; Zhang, Y.; Li, X. An endoplasmic reticulum response pathway mediates programmed cell death of root tip induced by water stress in Arabidopsis. New Phytol. 2010, 186, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Fang, I.J.; Trewyn, B.G. Application of Mesoporous Silica Nanoparticles in Intracellular Delivery of Molecules and Proteins. Method Enzymol. 2012, 508, 41–59. [Google Scholar] [CrossRef]
- Kim, S.I.; Kim, H.J.; Lee, H.J.; Lee, K.; Hong, D.; Lim, H.; Cho, K.; Jung, N.; Yi, Y.W. Application of a non-hazardous vital dye for cell counting with automated cell counters. Anal. Bioanal. Chem. 2016, 492, 8–12. [Google Scholar] [CrossRef]
- Ghanmi, M.; Satrani, B.; Aberchane, M.; Ismaili, M.R.; Aafi, A.; Abid, A.E. Nature valorisation et caractérisation des PAM des strates arbustives et herbacées. In Plantes Aromatiques et Médicinales au Maroc: Les Milles et une Vertus; Bayed, G.E., Ed.; Centre de Recherche Forestière: Rabat, Morocco, 2011; p. 28. [Google Scholar]
- Lakušić, D.; Ristić, M.; Slavkovska, V.; Lakušić, B. Seasonal variations in the composition of the essential oils of rosemary (Rosmarinus officinalis, Lamiaceae). Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Hanley, M.E.; Whiting, M.D. Insecticides and arable weeds: Effects on germination and seedling growth. Ecotoxicology 2005, 14, 483–490. [Google Scholar] [CrossRef]
- Dayan, F.E. Factors modulating the levels of the allelochemical sorgoleone in Sorghum bicolor. Planta 2006, 224, 339–346. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd_Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Tran, S.L.; Puhar, A.; Ngo-Camus, M.; Ramarao, N. Trypan blue dye enters viable cells incubated with the pore-forming toxin HlyII of Bacillus cereus. PLoS ONE 2011, 6, e22876. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T. Herbicide effects of essential oils. Weed Sci. 2002, 50, 425–431. [Google Scholar] [CrossRef]
- Ismail, A.; Hamrouni, L.; Hanana, M.; Jamoussi, B. Review on the phytotoxic effects of essential oils and their individual components: News approach for weed management. Int. J. Appl. Biol. Pharm. 2013, 4, 96–114. [Google Scholar]
- Benchaa, S.; Hazzit, M.; Abdelkrim, H. Allelopathic, effect of Eucalyptus citriodora essential oil and its potential use as bioherbicide. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.P.; Kaur, S.; Mittal, S.; Batish, D.R.; Kohli, R.K. Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J. Chem. Ecol. 2009, 35, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, N.; Singh, H.P.; Batish, D.; Kaur, S.; Ahuja, N.; Kohli, R. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 2012, 250, 691–700. [Google Scholar] [CrossRef]
- Soltys, D.; Rudzińska-Langwald, A.; Kurek, W.; Gniazdowska, A.; Sliwinska, E.; Bogatek, R. Cyanamide mode of action during inhibition of onion (Allium cepa L.) root growth involves disturbances in cell division and cytoskeleton formation. Planta 2011, 234, 609–621. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawai, Y.; Tamotsu, S.; Sakai, A. 1,8-Cineole inhibits both proliferation and elongation of BY-2 cultured Tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef]
- Anese, S.; Jatobá, L.J.; Grisi, P.U.; Gualtieri, S.C.J.; Santos, M.F.C.; Berlinck, R.G.S. Bioherbicidal activity of drimane sesquiterpenes from Drimys brasiliensis Miers roots. Ind. Crops Prod. 2015, 74, 28–35. [Google Scholar] [CrossRef]
- Chotsaeng, N.; Laosinwattana, C.; Charoenying, P. Herbicidal activities of some allelochemicals and their synergistic behaviors toward Amaranthus tricolor L. Molecules 2017, 22, 1841. [Google Scholar] [CrossRef] [PubMed]
- Sekine, T.; Appiah, K.S.; Azizi, M.; Fujii, Y. Plant Growth Inhibitory Activities and Volatile Active Compounds of 53 Spices and Herbs. Plants 2020, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mancini, E.; Rolim de Almeida, L.F.; De Feo, V. The antigerminative activity of twenty-seven monoterpenes. Molecules 2010, 15, 6630–6637. [Google Scholar] [CrossRef] [PubMed]




| Accession | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 |
|---|---|---|---|---|---|---|---|---|
| EO * (V/W%) | 1.76 ± 0.04 cd | 1.94 ± 0.08 cd | 1.43 ± 0.09 cd | 4.12 ± 0.32 a | 1.35 ± 0.18 d | 1.76 ± 0.15 cd | 2.15 ± 0.88 c | 2.95 ± 0.80 b |
| Component | Composition (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RI * | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | Average | |
| α-Pinene | 933 | 41.85 | 38.38 | 36.58 | 9.54 | 33.80 | 34.96 | 38.89 | 21.60 | 31.94 |
| Camphene | 948 | 7.70 | 8.21 | 7.72 | 5.68 | 5.87 | 7.56 | 8.32 | 7.28 | 7.27 |
| Verbenene | 953 | 0.15 | 0.15 | |||||||
| β-Pinene | 977 | 0.63 | 0.63 | |||||||
| 3-Octanone | 984 | 0.72 | 0.15 | 0.43 | ||||||
| β-Myrcene | 988 | 3.26 | 2.76 | 3.83 | 1.11 | 3.13 | 5.00 | 1.22 | 2.89 | |
| α-Phellandrene | 1001 | 2.24 | 0.28 | |||||||
| α-Terpinene | 1014 | 3.76 | 0.47 | |||||||
| ρ-Cymene | 1023 | 1.44 | 1.00 | 1.65 | 0.51 | |||||
| 1,8-Cineole | 1029 | 13.70 | 12.09 | 12.85 | 9.53 | 26.24 | 11.66 | 24.54 | 24.89 | 16.93 |
| γ-Terpinene | 1058 | 0.80 | 1.27 | 1.35 | 1.58 | 1.52 | 2.74 | 2.04 | 1.60 | |
| Linalool | 1096 | 1.40 | 1.56 | 2.53 | 3.04 | 1.98 | 0.92 | 1.89 | ||
| Camphor | 1142 | 7.85 | 8.93 | 9.50 | 62.0 | 10.16 | 6.16 | 17.25 | 17.39 | |
| Borneol | 1164 | 3.15 | 4.26 | 5.28 | 7.82 | 4.67 | 3.44 | 3.80 | 4.63 | |
| ρ-Cymen-8-ol | 1187 | 1.49 | 1.75 | 0.93 | 1.79 | 2.18 | 1.85 | 1.53 | 2.16 | 1.70 |
| Verbenone | 1205 | 2.96 | 2.79 | 2.38 | 1.88 | 2.86 | 2.55 | |||
| Bornyl acetate | 1283 | 8.93 | 9.34 | 7.77 | 10.89 | 9.85 | 4.95 | 4.09 | 7.97 | |
| Total | 94.58 | 91.36 | 90.81 | 90.0 | 95.29 | 90.02 | 96.63 | 91.96 | ||
| Code | Accessions | Chemotypes |
|---|---|---|
| C1 | R1, R2, R3, R6 | α-pinene |
| C2 | R4 | camphor |
| C3 | R5 | α-pinene/1,8-cineole |
| C4 | R8 | α-pinene/1,8-cineole/camphor |
| Treatments | L. perenne | A. retroflexus | |||
|---|---|---|---|---|---|
| GI (%) | SVI (mm) | GI (%) | SVI (mm) | ||
| Controls | Concentrations (μL/L) | ||||
| Water | 12.80 a | 4.54 a | |||
| T20 | 7 e | 9.33 ab | 2 d | 2.57 a | |
| C.B | 100 a | 0.00 f | 100 a | 0.00 c | |
| Chemotypes | |||||
| C1 | 400 | 44 bcde | 1.27 bcd | 73 abc | 0.23 bc |
| 800 | 74 bcde | 0.08 def | 86 abc | 0.12 bc | |
| 1200 | 81 bcd | 0.03 def | 82 abc | 0.16 bc | |
| C2 | 400 | 30 cde | 1.97 bcd | 86 abc | 0.10 bc |
| 800 | 56 bcde | 0.54 cde | 100 a | 0.00 bc | |
| 1200 | 96 ab | 0.01 ef | 100 a | 0.00 bc | |
| C3 | 400 | 26 de | 3.03 bc | 64 bc | 0.32 bc |
| 800 | 74 bcde | 0.55 cde | 91 abc | 0.07 bc | |
| 1200 | 89 abc | 0.16 cde | 91 abc | 0.07 bc | |
| C4 | 400 | 22 cde | 2.54 bc | 32 c | 0.67 b |
| 800 | 48 bcde | 1.40 bcd | 95 abc | 0.03 bc | |
| 1200 | 81 bcde | 0.09 def | 86 abc | 0.10 bc | |
| Treatments | Relative Growth Rate (mg/h) | ||
|---|---|---|---|
| L. perenne | A. retroflexus | ||
| Controls | Concentrations (μL/L) | NS | |
| Water | 1.93 | 8.61 a | |
| T20 | 2.92 | 8.72 ab | |
| C.B | 0.00 | 0.00 c | |
| Chemotypes | |||
| C1 | 800 | 0.00 | 0.73 abc |
| 1200 | 1.89 | 4.00 abc | |
| 2400 | 6.74 | 6.14 abc | |
| C2 | 800 | 0.00 | 0.00 c |
| 1200 | 0.00 | 0.00 c | |
| 2400 | 1.82 | 0.00 c | |
| C3 | 800 | 3.43 | 0.00 c |
| 1200 | 1.31 | 0.00 c | |
| 2400 | 1.32 | 0.00 c | |
| C4 | 800 | 2.42 | 5.07 abc |
| 1200 | 4.36 | 2.00 abc | |
| 2400 | 0.66 | 0.00 bc | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mahdi, J.; Tarraf, W.; Ruta, C.; Piscitelli, L.; Aly, A.; De Mastro, G. Bio-Herbicidal Potential of the Essential Oils from Different Rosmarinus officinalis L. Chemotypes in Laboratory Assays. Agronomy 2020, 10, 775. https://doi.org/10.3390/agronomy10060775
El Mahdi J, Tarraf W, Ruta C, Piscitelli L, Aly A, De Mastro G. Bio-Herbicidal Potential of the Essential Oils from Different Rosmarinus officinalis L. Chemotypes in Laboratory Assays. Agronomy. 2020; 10(6):775. https://doi.org/10.3390/agronomy10060775
Chicago/Turabian StyleEl Mahdi, Jihane, Waed Tarraf, Claudia Ruta, Lea Piscitelli, Adel Aly, and Giuseppe De Mastro. 2020. "Bio-Herbicidal Potential of the Essential Oils from Different Rosmarinus officinalis L. Chemotypes in Laboratory Assays" Agronomy 10, no. 6: 775. https://doi.org/10.3390/agronomy10060775
APA StyleEl Mahdi, J., Tarraf, W., Ruta, C., Piscitelli, L., Aly, A., & De Mastro, G. (2020). Bio-Herbicidal Potential of the Essential Oils from Different Rosmarinus officinalis L. Chemotypes in Laboratory Assays. Agronomy, 10(6), 775. https://doi.org/10.3390/agronomy10060775








