The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Leaf Gas Exchange Indices
2.3. Chlorophyll Fluorescence
2.4. Spectral Reflectance Indices
2.5. Sugars
2.6. Biometric Measurements
2.7. Statistical Analysis
3. Results
3.1. Leaf Pigments, Morphology and Biomass
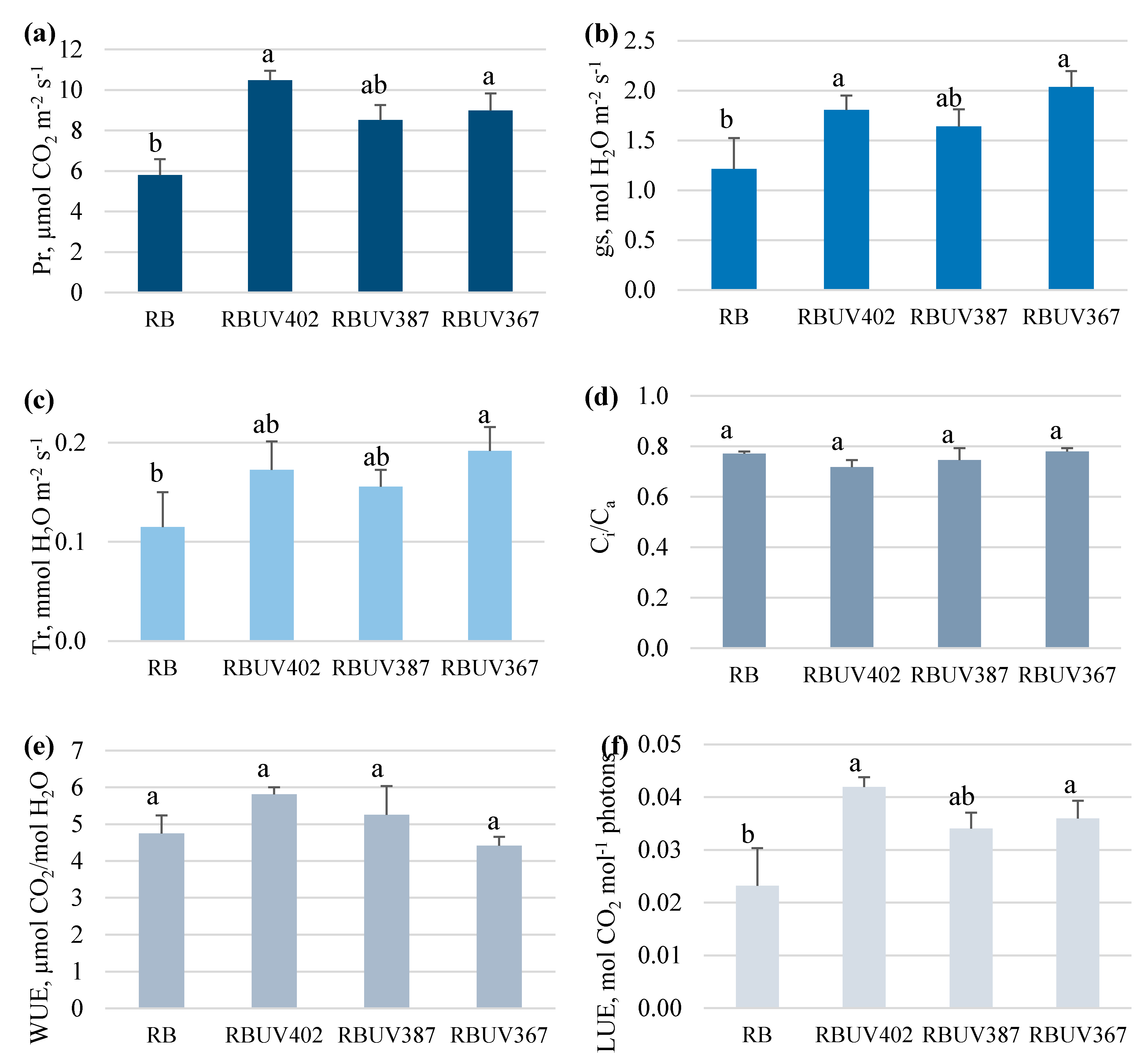
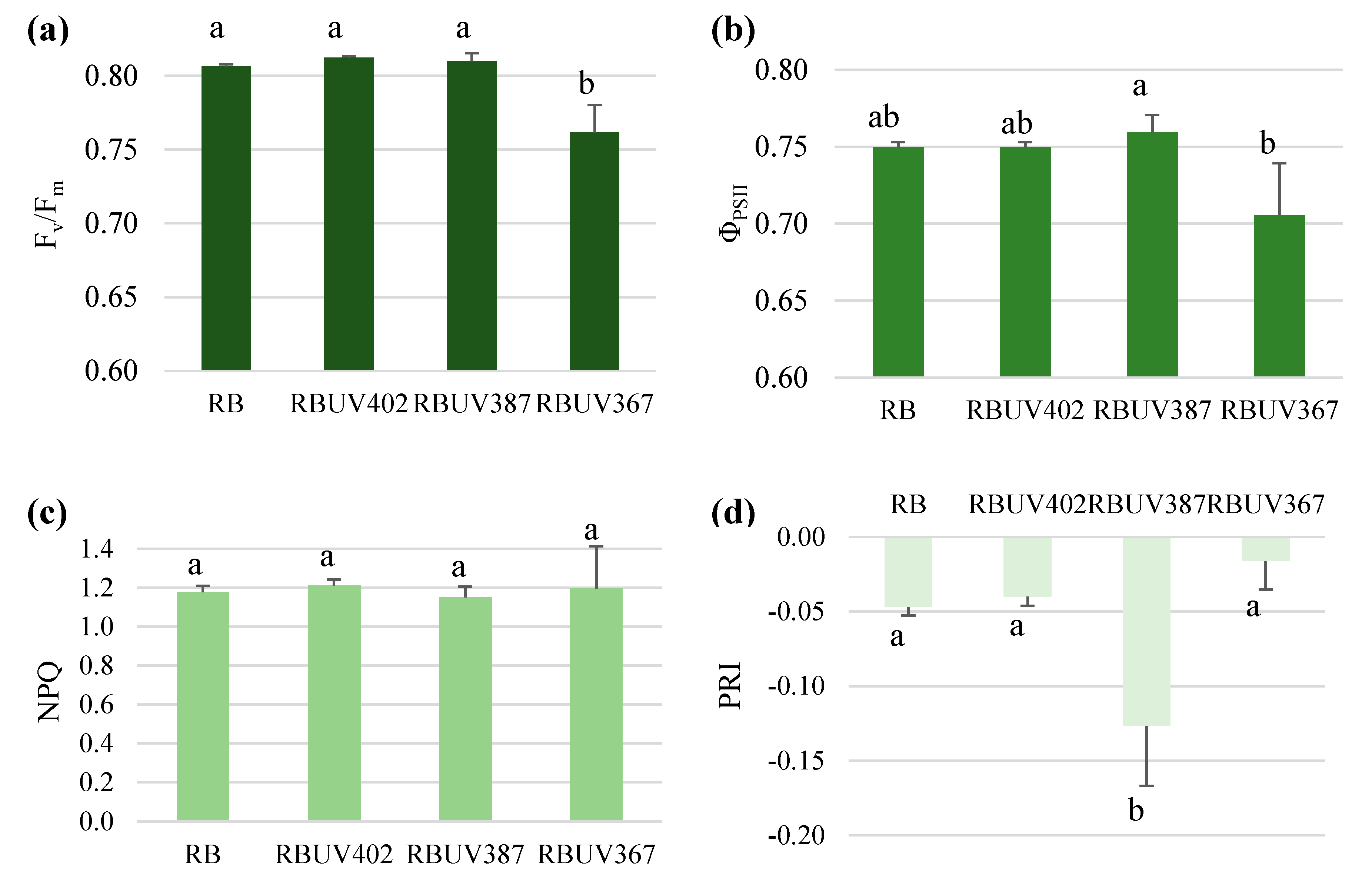
3.2. Photosynthetic Response
3.3. Soluble Sugars
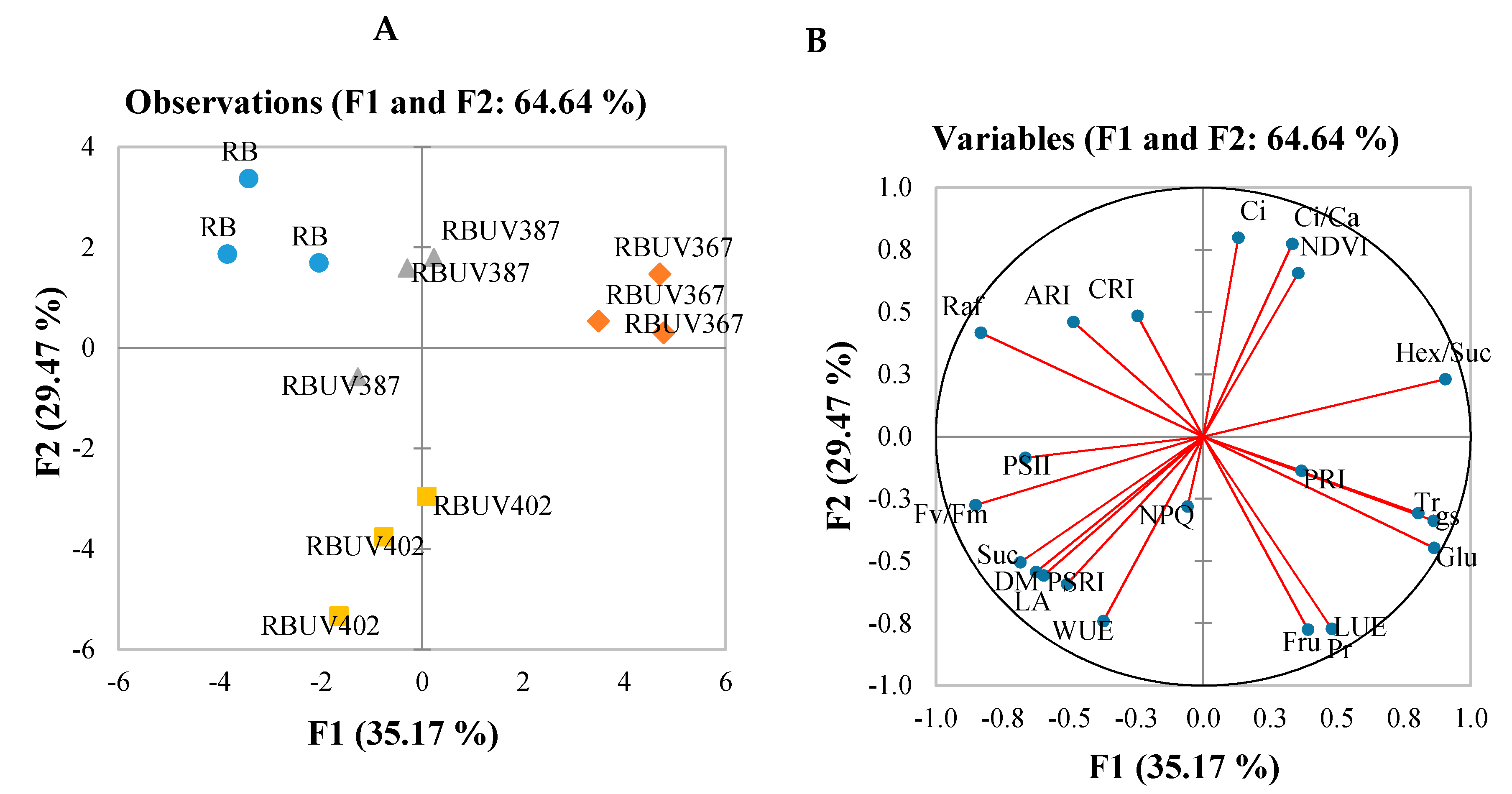
3.4. PCA Scatterplot and Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Vaštakaitė, V. Light-emitting diodes (LEDs) for improved nutritional quality. In Light Emitting Diodes for Agriculture; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; pp. 149–190. [Google Scholar]
- Stutte, G.W.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–541. [Google Scholar] [CrossRef]
- Cocetta, G.; Casciani, D.; Bulgari, R.; Musante, F.; Kołton, A.; Rossi, M.; Ferrante, A. Light use efficiency for vegetables production in protected and indoor environments. Eur. Phys. J. Plus 2017, 132, 43. [Google Scholar] [CrossRef]
- Olle, M.; Viršilė, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Wargent, J.J. UV LEDs in horticulture: From biology to application. Acta Hortic. 2016, 1134, 25–32. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations–perspectives for applications in horticulture. J. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B photoreceptor UVR8: From structure to physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef]
- Barillot, R.; Frak, E.; Combes, D.; Durand, J.-L.; Escobar-Gutiérrez, A.J. What determines the complex kinetics of stomatal conductance under blue less PAR in Festuca arundinacea? Subsequent effects on leaf transpiration. J. Exp. Bot. 2010, 61, 2795–2806. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. (Amst.) 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Klem, K.; Gargallo-Garriga, A.; Rattanapichai, W.; Oravec, M.; Holub, P.; Veselá, B.; Sardans, J.; Peñuelas, J.O. Urban, distinct morphological, physiological, and biochemical responses to light quality in barley leaves and roots. Front. Plant Sci. 2019, 10, 1026. [Google Scholar] [CrossRef]
- Štroch, M.; Meterová, Z.; Vrábl, D.; Karlický, V.; Šigut, L.; Nezval, J.; Špunda, V. Protective effect of UV-A radiation during acclimation of the photosynthetic apparatus to UV-B treatment. Plant Physiol. Biochem. 2015, 96, 90–96. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A -B, or -C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Jankauskienė, J.; Sakalauskienė, S.; Samuolienė, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskienė, J.; Vaštakaitė, V.; et al. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Novičkovas, A.; Duchovskis, P. Response of mustard microgreens to different wavelengths and durations of UV-A LEDs. Front. Plant Sci. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.K.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N.; Ahuja, S.; Singh, B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J. Photochem. Photobiol. B Biol. 2013, 127, 140–152. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Van Iersel, M.W.; Weaver, G.; Martin, M.T.; Ferrarezi, R.S.; Mattos, E.; Haidekker, M. A chlorophyll fluorescence-based biofeedback system to control photosynthetic lighting in controlled environment agriculture. J. Am. Soc. Hortic. Sci. 2016, 141, 169–176. [Google Scholar] [CrossRef]
- Verdaguer, D.; Díaz-Guerra, L.; Fonta, J.; González, J.A.; Llorens, L. Contrasting seasonal morphological and physio-biochemical responses to UV radiation and reduced rainfall of two mature naturally growing Mediterranean shrubs in the context of climate change. Environ. Exp. Bot. 2018, 147, 189–201. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Z.; Chen, C.; Zhang, L.; Zhu, S. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Brons, C.; Olieman, C. Study of the high-performance liquid chromatographic separation of reducing sugars, applied to the determination of lactose in milk. J. Chromatogr. 1983, 259, 79–86. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Khoshimkhujaev, B.; Kwon, J.K.; Park, K.S.; Choi, H.G.; Lee, S.Y. Effect of monochromatic UV-A LED irradiation on the growth of tomato seedlings. Hortic. Environ. Biotechnol. 2014, 55, 287–292. [Google Scholar] [CrossRef]
- Zagajewski, B.; Tømmervik, H.; Bjerke, J.W.; Raczko, E.; Bochenek, Z.; Kłos, A.; Jarocińska, A.; Lavender, S.; Ziółkowski, D. Intraspecific differences in spectral reflectance curves as indicators of reduced vitality in high-arctic plants. Remote Sens. 2017, 9, 1289. [Google Scholar] [CrossRef]
- Britz, S.J.; Caldwell, C.R.; Mirecki, R.; Slusser, J.; Gao, W. Effect of supplemental ultraviolet radiation on the concentration of phytonutrients in green and red leaf lettuce (Lactuca sativa) cultivars. In Proceedings of SPIE 5886, Ultraviolet Ground- and Space-Based Measurements, Models, and Effects V, 58860W; International Society for Optics and Photonics: San Francisco, CA, USA, 2005; Volume 5886, pp. 1–8. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H1-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, Y.G.; Li, X.; Ni, M. Light-regulated stomatal aperture in Arabidopsis. Mol. Plant 2012, 5, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Van Ieperen, W.; Savvides, A.; Fanourakis, D. Red and blue light effects during growth on hydraulic and stomatal conductance in leaves of young cucumber plants. Acta Hortic. 2012, 956, 223–230. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
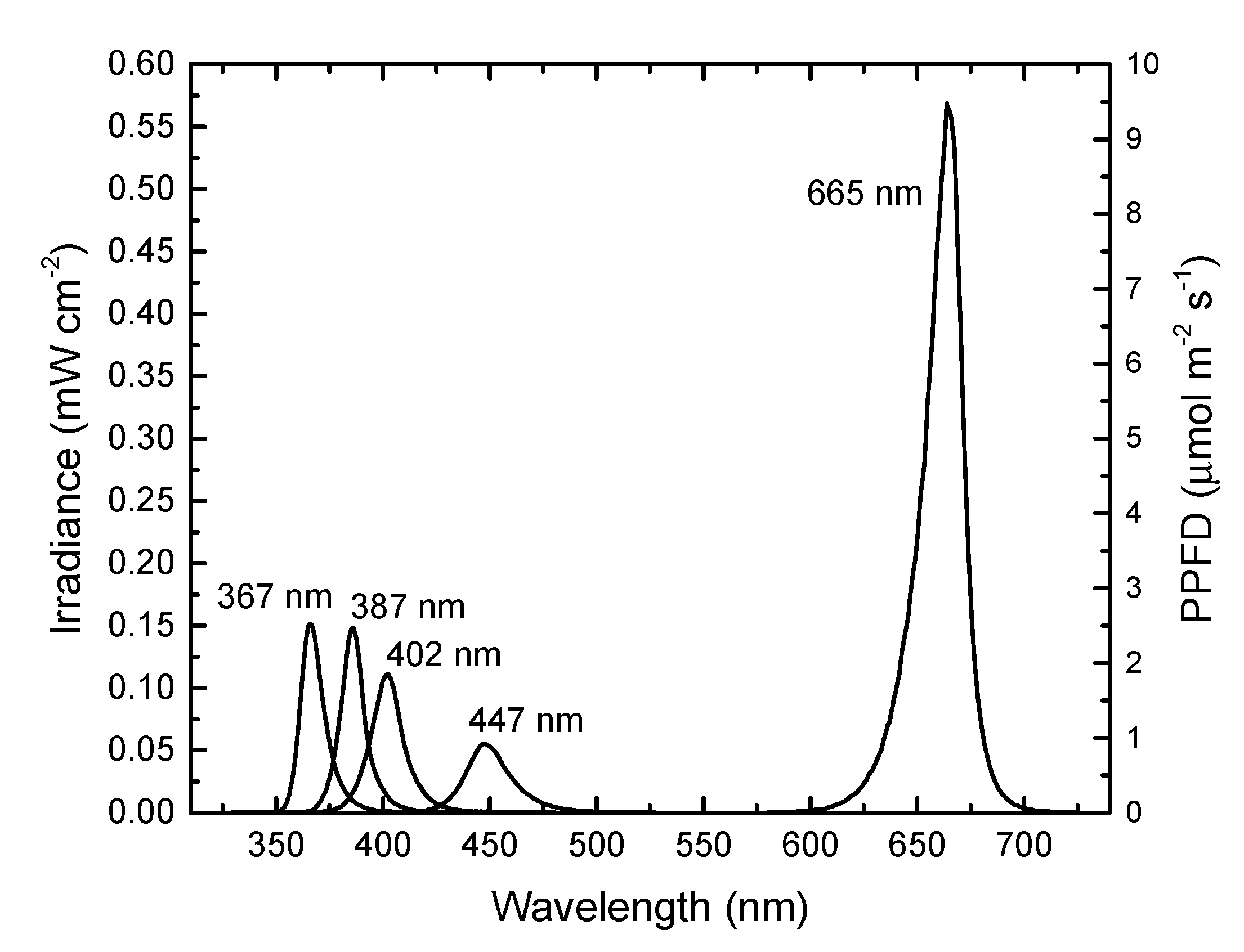

| Treatment | R665 mol m−2 s−1 | B447 mol m−2 s−1 | UV402 mW cm−2 | UV387 mW cm−2 | UV367 mW cm−2 |
|---|---|---|---|---|---|
| RB | 225 | 25 | |||
| RBUV402 | 225 | 25 | 2.2 | ||
| RBUV387 | 225 | 25 | 2.2 | ||
| RBUV367 | 225 | 25 | 2.2 |
| Treatments | CRI | ARI | PSRI | NDVI | DW, g | LA cm2 | |||
|---|---|---|---|---|---|---|---|---|---|
| RB | 0.15b 0.03 | 0.12b 0.02 | 0.03a 0.01 | 0.77a 0.05 | 0.96ab 0.09 | 798.8ab 98.4 | |||
| RBUV402 | 0.08b 0.04 | 0.05c 0.01 | 0.06a 0.04 | 0.65a 0.16 | 1.17a 0.23 | 899.5a 81.1 | |||
| RBUV387 | 0.26a 0.02 | 0.17a 0.02 | 0.03a 0.00 | 0.85a 0.02 | 0.81b 0.11 | 626.0bc 93.4 | |||
| BRUV367 | 0.10b 0.02 | 0.03c 0.01 | 0.01a 0.00 | 0.83a 0.00 | 0.63b 0.02 | 576.5c 59.3 | |||
 |  |  |  | ||||||
| RB | RBUV402 | RBUV387 | RBUV367 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samuoliene, G.; Virsile, A.; Miliauskienė, J.; Haimi, P.; Laužikė, K.; Jankauskienė, J.; Novičkovas, A.; Kupčinskienė, A.; Brazaitytė, A. The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation. Agronomy 2020, 10, 761. https://doi.org/10.3390/agronomy10060761
Samuoliene G, Virsile A, Miliauskienė J, Haimi P, Laužikė K, Jankauskienė J, Novičkovas A, Kupčinskienė A, Brazaitytė A. The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation. Agronomy. 2020; 10(6):761. https://doi.org/10.3390/agronomy10060761
Chicago/Turabian StyleSamuoliene, Giedre, Akvile Virsile, Jurga Miliauskienė, Perttu Haimi, Kristina Laužikė, Julė Jankauskienė, Algirdas Novičkovas, Asta Kupčinskienė, and Aušra Brazaitytė. 2020. "The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation" Agronomy 10, no. 6: 761. https://doi.org/10.3390/agronomy10060761
APA StyleSamuoliene, G., Virsile, A., Miliauskienė, J., Haimi, P., Laužikė, K., Jankauskienė, J., Novičkovas, A., Kupčinskienė, A., & Brazaitytė, A. (2020). The Photosynthetic Performance of Red Leaf Lettuce under UV-A Irradiation. Agronomy, 10(6), 761. https://doi.org/10.3390/agronomy10060761






