Distribution of Root-Lesion and Stunt Nematodes, and Their Relationship with Soil Properties and Nematode Fauna in Sugarcane Fields in Okinawa, Japan
Abstract
1. Introduction
2. Materials and Methods
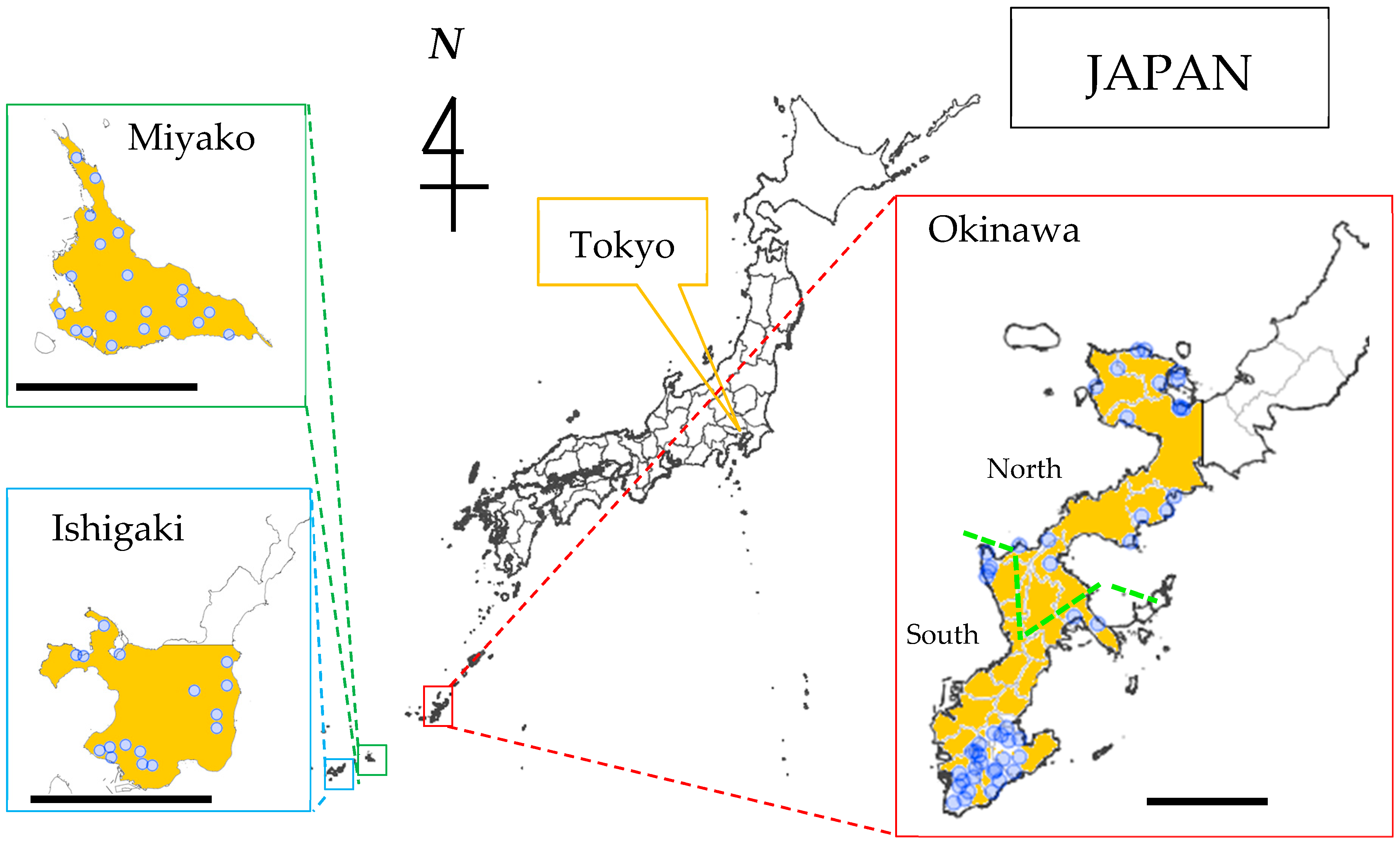
2.1. Study Fields
2.2. Soil Sampling
2.3. Nematode Extraction, Identification and Enumeration
2.4. Physico-Chemical Properties of Soil
2.5. Free-Living Nematode Biodiversity and Maturity Indices
2.6. Statistical Analysis
3. Results
3.1. Nematodes in Sugarcane Fields
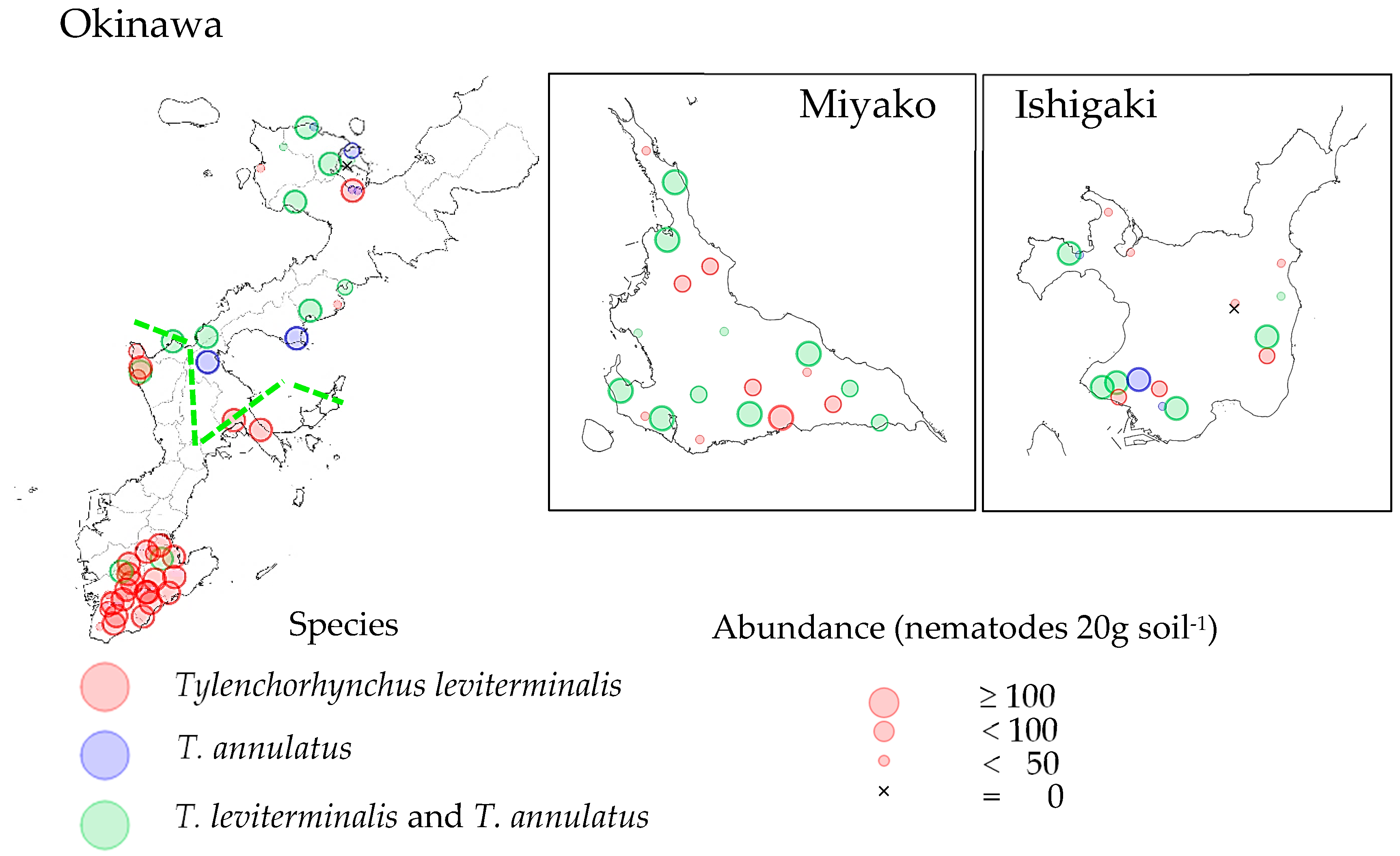
3.2. Spatial Distribution of Pratylenchus and Tylenchorhynchus
3.3. Soil Physico-Chemical Properties
3.4. The Relationship between Soil Properties and Abundance of Pratylenchus and Tylenchorhynchus
3.5. Diversity of Free-Living Nematodes
3.6. Nematode Fauna in Sugarcane Fields
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cadet, P.; Spaull, V.W. Nematode parasites of sugarcane. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 645–674. [Google Scholar] [CrossRef]
- Berry, S.D.; Fargette, M.; Spaull, V.W.; Morand, S.; Cadet, P. Detection and quantification of root-knot nematode (Meloidogyne javanica), lesion nematode (Pratylenchus zeae) and dagger nematode (Xiphinema elongatum) parasites of sugarcane using real-time PCR. Mol. Cell. Probes 2008, 22, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.L.; Stirling, G.R. The role of plant-parasitic nematodes in reducing yield of sugarcane in fine-textured soils in Queensland, Australia. Aust. J. Exp. Agric. 2007, 47, 620–634. [Google Scholar] [CrossRef]
- Cadet, P.; Spaull, V.W. Effect of nematodes on the sustained production of sugarcane in South Africa. Field Crops Res. 2003, 83, 91–100. [Google Scholar] [CrossRef]
- Stirling, G.R.; Blair, B. Nematodes are involved in the yield decline syndrome of sugarcane in Australia. In Proceedings of the XXIV the International Society of Sugar Cane Technologists, Brisbane, Australia, 17–21 September 2001; Hogarth, D.M., Ed.; Australian Society of Sugar Cane Technoloists: Mackay, Australia, 2001; pp. 430–433. [Google Scholar]
- Kawanobe, M.; Miyamaru, N.; Yoshida, K.; Kawanaka, T.; Toyota, K. A field experiment with nematicide treatment revealed potential sugarcane yield loss caused by plant-parasitic nematodes in Okinawa, Japan. Nematol. Res. 2016, 46, 9–16. [Google Scholar] [CrossRef][Green Version]
- Kawanobe, M.; Miyamaru, N.; Yoshida, K.; Kawanaka, T.; Fujita, T.; Toyota, K. Sugarcane yield loss in the ratoon crop carried over from the plant crop damaged by plant-parasitic nematode in a heavy clay field in Okinawa, Japan. Nematol. Res. 2019, 49, 1–7. [Google Scholar] [CrossRef]
- Okinawa Prefecture Sugarcane Production. Available online: https://www.pref.okinawa.jp/site/norin/togyo/kibi/mobile/seisanjisseki.html (accessed on 23 February 2020).
- FOASTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 30 September 2019).
- Kawanobe, M.; Toyota, K.; Seko, T.; Gunjima, K. Nematicidal activity of fipronil against Pratylenchus zeae in sugarcane. J. Nematol. 2019, 51, e2019–e2075. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Neher, D.A. Ecology of plant and free-living nematodes in natural and agricultural soil. Annu. Rev. Phytopathol. 2010, 48, 371–394. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T. Nematode diversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 113–135. [Google Scholar] [CrossRef]
- Mulder, C.; Zwart, D.; Van Wijnen, H.J.; Schouten, A.J.; Breure, A.M. Observational and simulated evidence of ecological shifts within the soil nematode community of agroecosystems under conventional and organic farming. Funct. Ecol. 2003, 17, 516–525. [Google Scholar] [CrossRef]
- Goralczyk, K. Nematodes in a coastal dune succession: Indicators of soil properties? Appl. Soil Ecol. 1998, 9, 465–469. [Google Scholar] [CrossRef]
- Nahar, M.S.; Grewal, P.S.; Miller, S.A.; Stinner, D.; Stinner, B.R.; Kleinhenz, M.D.; Wszelaki, A.; Doohan, D. Differential effects of raw and composted manure on nematode community, and its indicative value for soil microbial, physical and chemical properties. Appl. Soil Ecol. 2006, 34, 140–151. [Google Scholar] [CrossRef]
- Cheng, J.; Karambelkar, B.; Xie, Y. Leaflet: Create Interactive Web Maps with the JavaScript ‘Leaflet’ Library. R Package Version 2.0.3. 2019. Available online: https://CRAN.R-project.org/package=leaflet (accessed on 10 January 2020).
- Okinawa Prefecture Soil Maps. Available online: https://www.pref.okinawa.jp/site/norin/engei/documents/p1-8.pdf (accessed on 24 March 2020).
- Obara, H.; Ohkura, T.; Takata, Y.; Kohyama, K.; Maejima, Y.; Hamazaki, T. Comprehensive soil classification system of Japan first approximation. Bull. Natl. Inst. Agro-Environ. Sci. 2011, 29, 1–73, (In Japanese with English Summary). [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: http://www.data.jma.go.jp/obd/stats/etrn/ (accessed on 23 February 2020).
- GIS Maps. Available online: https://cyberjapandata.gsi.go.jp/xyz/blank/{z}/{x}/{y}.png (accessed on 28 February 2020).
- Geospatial Information Authority of Japan. Available online: https://maps.gsi.go.jp/development/ichiran.html (accessed on 28 February 2020).
- Ingham, R.E.; Trofymow, J.A.; Ingham, E.R.; Coleman, D.C. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecol. Monogr. 1985, 55, 119–140. [Google Scholar] [CrossRef]
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Kawanobe, M.; Miyamaru, N.; Yoshida, K.; Kawanaka, T.; Toyota, K. Plant-parasitic nematodes in sugarcane fields in Kitadaito Island (Okinawa), Japan, as a potential sugarcane growth inhibitor. Nematology 2014, 16, 807–820. [Google Scholar] [CrossRef]
- Kawanobe, M.; Miyamaru, N.; Yoshida, K.; Kawanaka, T.; Toyota, K. Quantification of lesion nematode (Pratylenchus zeae), stunt nematode (Tylenchorhynchus leviterminalis), spiral nematode (Helicotylenchus dihystera), and lance nematode (Hoplolaimus columbus), parasites of sugarcane in Kitadaito, Okinawa, Japan, using real-time PCR. Nematol. Res. 2015, 45, 35–44. [Google Scholar] [CrossRef]
- Teruya, R. Harmful nematodes in Okinawa. Plant Prot. 1971, 25, 458–460. (In Japanese) [Google Scholar]
- Shishida, Y. Nematoda. In Pictorial Keys to Soil Animal of Japan; Aoki, J., Ed.; Tokai University Press: Tokyo, Japan, 1999; pp. 15–38. (In Japanese) [Google Scholar]
- Kawanobe, M.; Toyota, K.; Fujita, T.; Hatta, D. Evaluation of nematicidal activity of fluensulfone against non-target free-living nematodes under field conditions. Agronomy 2019, 9, 853. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Kawanobe, M.; Toyota, K.; Uchihara, H.; Takae, M. Developing a real-time PCR diagnostic method for a potential threat to chrysanthemum, Paratylenchus dianthus. J. Nematol. 2019, 51, e2019–e2043. [Google Scholar] [CrossRef]
- Gee, G.W.; Or, D. Particle-size analysis. In Methods of Soil Analysis, Part 4, Physical Methods, SSSA Book Ser. No. 5; Dane, J.H., Ed.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–289. [Google Scholar]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for chemical analysis of soils. In New Zealand Soil Bureau Scientific Report 80; New Zealand Society of Soil Science: Lower Hutt, New Zealand, 1987; p. 103. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R.-project.org/ (accessed on 30 July 2019).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pebesma, E.J. Multivariable geostatistics in S: The gstat package. Comput. Geosci. 2004, 30, 683–691. [Google Scholar] [CrossRef]
- Graler, B.; Pebesma, E.; Heuvelink, G. Spatio-temporal interpolation using gstat. R J. 2016, 8, 204–218. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 February 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Spaull, V.W.; Cadet, P. Nematodes and nutrients: Association between plant-parasitic nematodes and soil chemicals. In Proceedings of the Annual Congress-South African Sugar Technologists’ Association, South African Sugar Technologists’ Association, Durban, South Africa, 31 July–3 August 2001; pp. 116–117. [Google Scholar]
- Liang, W.; Pinhasi-Adiv, Y.; Shtultz, H.; Steinberger, Y. Nematode population dynamics under the canopy of desert halophytes. Arid Soil Res. Rehab. 2000, 14, 183–192. [Google Scholar] [CrossRef]
- Schmitt, D.P. Population patterns of some stylet-bearing nematodes in a native Iowa prairie. J. Nematol. 1969, 1, 304. [Google Scholar]
- Kheir, A.M.; Shohla, G.S.; Elgindi, D.M. Population behaviour of Tylenchorhynchus clarus infecting Egyptian cotton, Gossypium barbadense, in relation to soil type. J. Plant Dis. Protect. 1977, 84, 663–665. [Google Scholar]
- Norton, D.C.; Frederick, L.R.; Ponchllia, P.E.; Nyhan, J.W. Correlations of nematodes and soil properties in soybean fields. J. Nematol. 1971, 3, 154–163. [Google Scholar] [PubMed]
- Berry, S.D.; Cadet, P.; Spaull, V.W. Nematode pests of sugarcane. In Nematology in South Africa: A View from the 21st Century; Fourie, H., Spaull, V., Jones, R., Daneel, M., De Waele, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 261–284. [Google Scholar]
- Iwahori, H.; Uesugi, K.; Tateishi, Y. Plant-parasitic nematode fauna in upland field crops, fruit trees, and weeds in Okinawa Prefecture, Japan. Kyushu Pl. Prot. Res. 2008, 54, 132–137, (In Japanese with English Summary). [Google Scholar] [CrossRef][Green Version]
- Kimpinski, J.; Willis, C.B. Influence of soil temperature and pH on Pratylenchus penetrans and P. crenatus in alfalfa and timothy. J. Nematol. 1981, 13, 333–338. [Google Scholar] [PubMed]
- Acosta, N.; Malek, R.B. Influence of temperature on population development of eight species of Pratylenchus on soybean. J. Nematol. 1979, 11, 229–232. [Google Scholar] [PubMed]
- Stirling, G.R.; Rames, E.; Stirling, A.M.; Hamill, S. Factors associated with the suppressiveness of sugarcane soils to plant-parasitic nematodes. J. Nematol. 2011, 43, 135–148. [Google Scholar]




| Okinawa-North | Okinawa-South | Miyako | Ishigaki | |||||
|---|---|---|---|---|---|---|---|---|
| Free-living nematodes | 146 | a | 82 | bc | 128 | ab | 54 | c |
| Plant-parasitic nematodes | 372 | a | 273 | ab | 200 | b | 146 | b |
| Pratylenchus | 63 | a | 17 | b | 20 | b | 17 | b |
| Tylenchorhynchus | 148 | ab | 203 | a | 77 | b | 83 | b |
| Helicotylenchus | 153 | a | 21 | b | 64 | b | 23 | b |
| Hoplolaimus | 2 | a | 3 | a | 4 | a | 3 | a |
| Meloidogyne | 0 | a | 0 | a | 3 | b | 0 | a |
| Paratylenchus | 2 | a | 0 | a | 0 | a | 0 | a |
| Rotylenchulus | 1 | a | 27 | ab | 32 | b | 12 | ab |
| Xiphinema | 0 | a | 0 | a | 0 | a | 0 | a |
| Ring nematodes | 3 | ab | 0 | a | 0 | a | 7 | b |
| Okinawa-North | Okinawa-South | Miyako | Ishigaki | |||||
|---|---|---|---|---|---|---|---|---|
| Acrobeloides | 48% | a | 51% | a | 15% | b | 13% | b |
| Other Cephalobidae | 16% | a | 15% | a | 15% | a | 19% | a |
| Rhabditis | 8% | a | 7% | a | 0% | b | 3% | b |
| Other Rhabditidae | 9% | a | 10% | a | 12% | a | 11% | a |
| Other bacterivores | 10% | a | 10% | a | 31% | b | 20% | c |
| Aphelenchus | 3% | a | 2% | a | 3% | a | 1% | a |
| Aphelenchoides | 0% | ac | 0% | a | 3% | b | 2% | bc |
| Filenchus | 3% | a | 3% | a | 4% | a | 13% | b |
| Ditylenchus | 0% | a | 0% | a | 0% | a | 0% | a |
| Dorylaimida | 3% | a | 2% | a | 16% | b | 16% | b |
| Predators | 0% | a | 0% | a | 1% | b | 2% | b |
| Okinawa-North | Okinawa-South | Miyako | Ishigaki | |||||
|---|---|---|---|---|---|---|---|---|
| pH (H2O) | 5.4 | a | 8.1 | b | 6.9 | c | 5.8 | a |
| pH (KCl) | 4.3 | a | 7.0 | b | 5.9 | c | 4.9 | a |
| Soil moisture (%) | 14.1 | a | 14.8 | a | 18.7 | b | 13.8 | a |
| EC(mS m−1) | 13.0 | a | 14.3 | a | 13.3 | a | 13.5 | a |
| Clay (%) | 32.9 | a | 41.4 | b | 61.6 | c | 29.6 | a |
| Silt (%) | 38.4 | a | 49.6 | b | 29.0 | c | 30.2 | c |
| Sand (%) | 28.7 | a | 9.1 | b | 9.3 | b | 40.1 | c |
| Total C (g C kg−1) | 0.93 | ab | 0.85 | a | 1.17 | b | 0.77 | a |
| Total N (g N kg−1) | 0.10 | ab | 0.10 | a | 0.14 | b | 0.09 | a |
| Available P (mg P kg−1) | 214.0 | a | 103.0 | a | 87.2 | a | 113.8 | a |
| Exch. K+ (cmol kg−1) | 0.3 | a | 0.5 | a | 1.0 | b | 0.6 | a |
| Exch. Na+ (cmol kg−1) | 0.1 | a | 0.2 | a | 0.3 | b | 0.1 | a |
| Exch. Ca2+ (cmol kg−1) | 11.2 | a | 35.7 | b | 15.9 | a | 7.2 | a |
| Exch. Mg2+ (cmol kg−1) | 1.6 | ac | 2.7 | b | 2.5 | ab | 1.1 | c |
| CEC (cmol kg−1) | 14.1 | a | 17.9 | b | 17.9 | b | 11.2 | a |
| Okinawa-North | Okinawa-South | Miyako | Ishigaki | |||||
|---|---|---|---|---|---|---|---|---|
| Shannon-Wiener H′ | 2.07 | ab | 1.93 | a | 2.43 | bc | 2.60 | c |
| Evenness J′ | 0.90 | ab | 0.84 | a | 1.05 | bc | 1.13 | c |
| Number of free-living nematode species | 6.94 | ab | 6.58 | a | 7.50 | ab | 7.81 | b |
| Species richness (Margalef index) | 1.50 | a | 1.56 | a | 1.61 | ab | 2.05 | b |
| Simpson′s D | 0.34 | ab | 0.38 | a | 0.23 | bc | 0.19 | c |
| Maturity Index (MI) | 1.90 | a | 1.87 | a | 2.20 | b | 2.17 | b |
| MI (cp2–5) | 1.73 | a | 1.69 | a | 2.08 | b | 2.02 | b |
| MI (Cephalobidae adjusted) | 1.26 | a | 1.21 | a | 1.90 | b | 1.84 | b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawanobe, M.; Sugihara, S.; Miyamaru, N.; Yoshida, K.; Nonomura, E.; Oshiro, H.; Toyota, K. Distribution of Root-Lesion and Stunt Nematodes, and Their Relationship with Soil Properties and Nematode Fauna in Sugarcane Fields in Okinawa, Japan. Agronomy 2020, 10, 762. https://doi.org/10.3390/agronomy10060762
Kawanobe M, Sugihara S, Miyamaru N, Yoshida K, Nonomura E, Oshiro H, Toyota K. Distribution of Root-Lesion and Stunt Nematodes, and Their Relationship with Soil Properties and Nematode Fauna in Sugarcane Fields in Okinawa, Japan. Agronomy. 2020; 10(6):762. https://doi.org/10.3390/agronomy10060762
Chicago/Turabian StyleKawanobe, Masanori, Soh Sugihara, Naoko Miyamaru, Koichi Yoshida, Eito Nonomura, Hiroaki Oshiro, and Koki Toyota. 2020. "Distribution of Root-Lesion and Stunt Nematodes, and Their Relationship with Soil Properties and Nematode Fauna in Sugarcane Fields in Okinawa, Japan" Agronomy 10, no. 6: 762. https://doi.org/10.3390/agronomy10060762
APA StyleKawanobe, M., Sugihara, S., Miyamaru, N., Yoshida, K., Nonomura, E., Oshiro, H., & Toyota, K. (2020). Distribution of Root-Lesion and Stunt Nematodes, and Their Relationship with Soil Properties and Nematode Fauna in Sugarcane Fields in Okinawa, Japan. Agronomy, 10(6), 762. https://doi.org/10.3390/agronomy10060762






