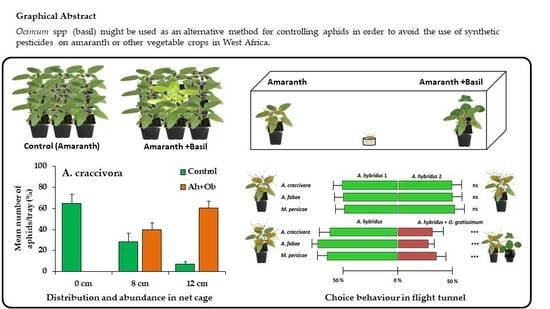
Aphid Behavior on Amaranthus hybridus L. (Amaranthaceae) Associated with Ocimum spp. (Lamiaceae) as Repellent Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Aphids Rearing
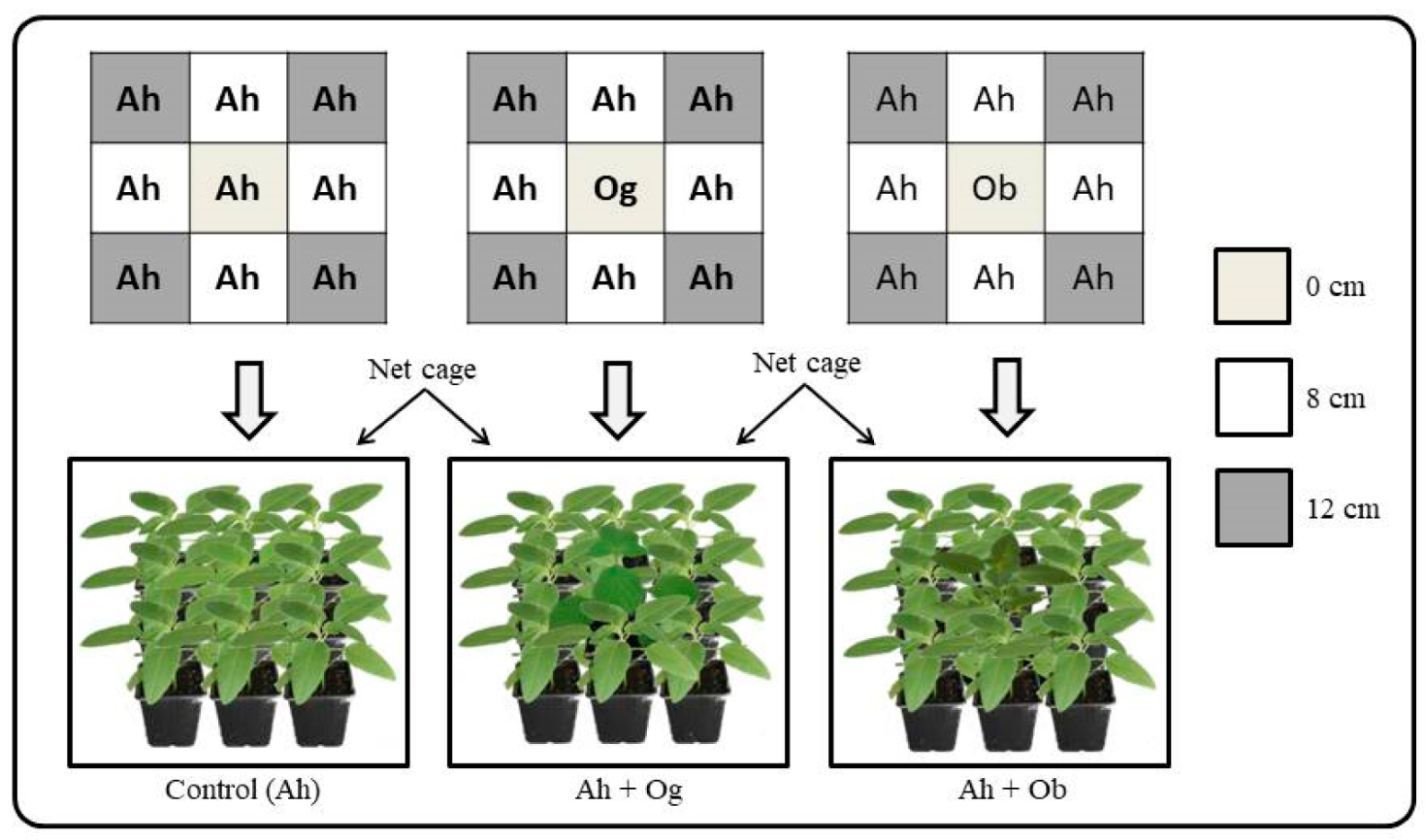
2.2. Impact of Ocimum on the Dispersion of Unwinged Aphids
2.3. Impact of Ocimum on the Behavior of Winged Aphids in the Dual-Choice Test
2.4. Statistical Analysis
3. Results
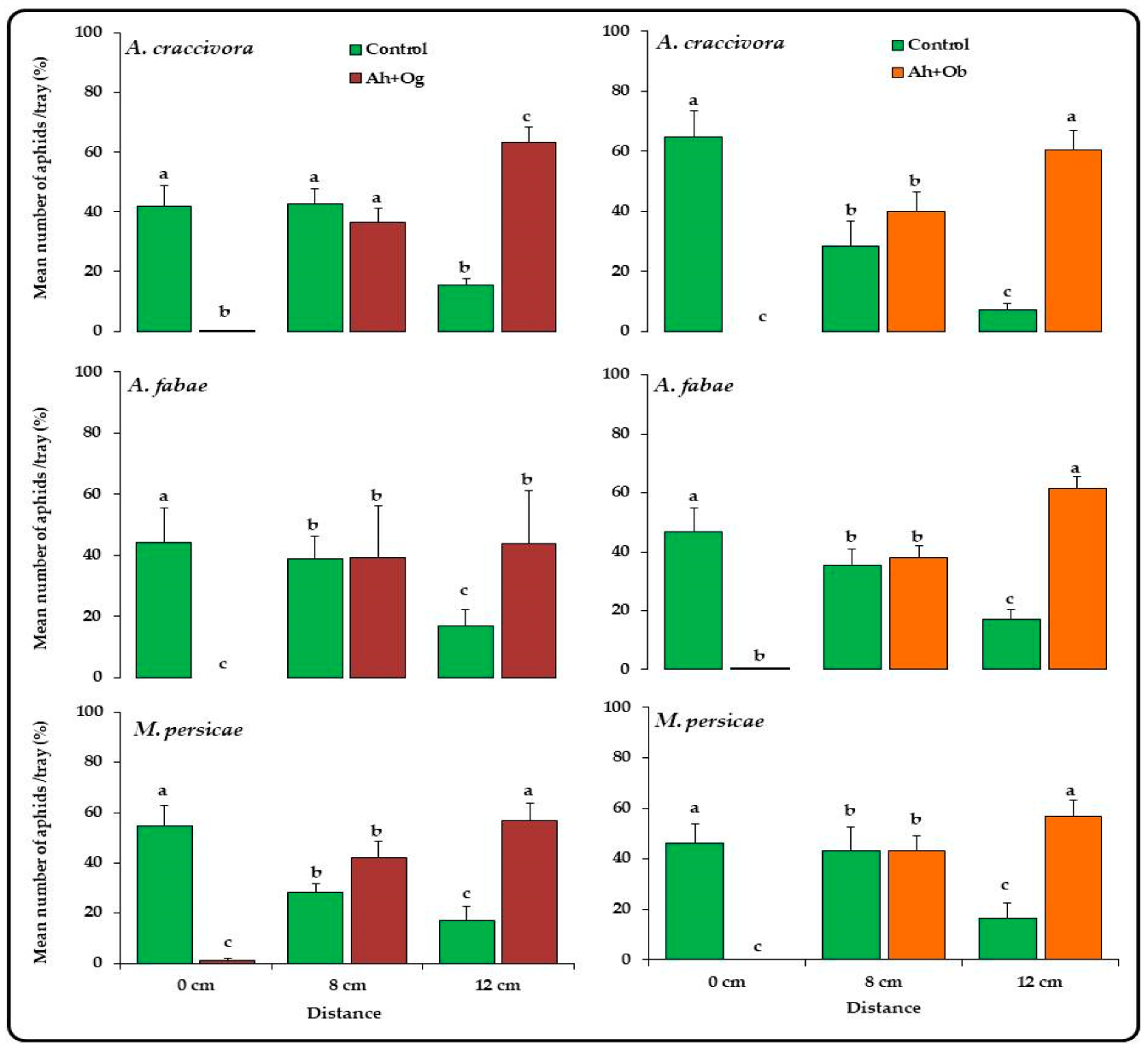
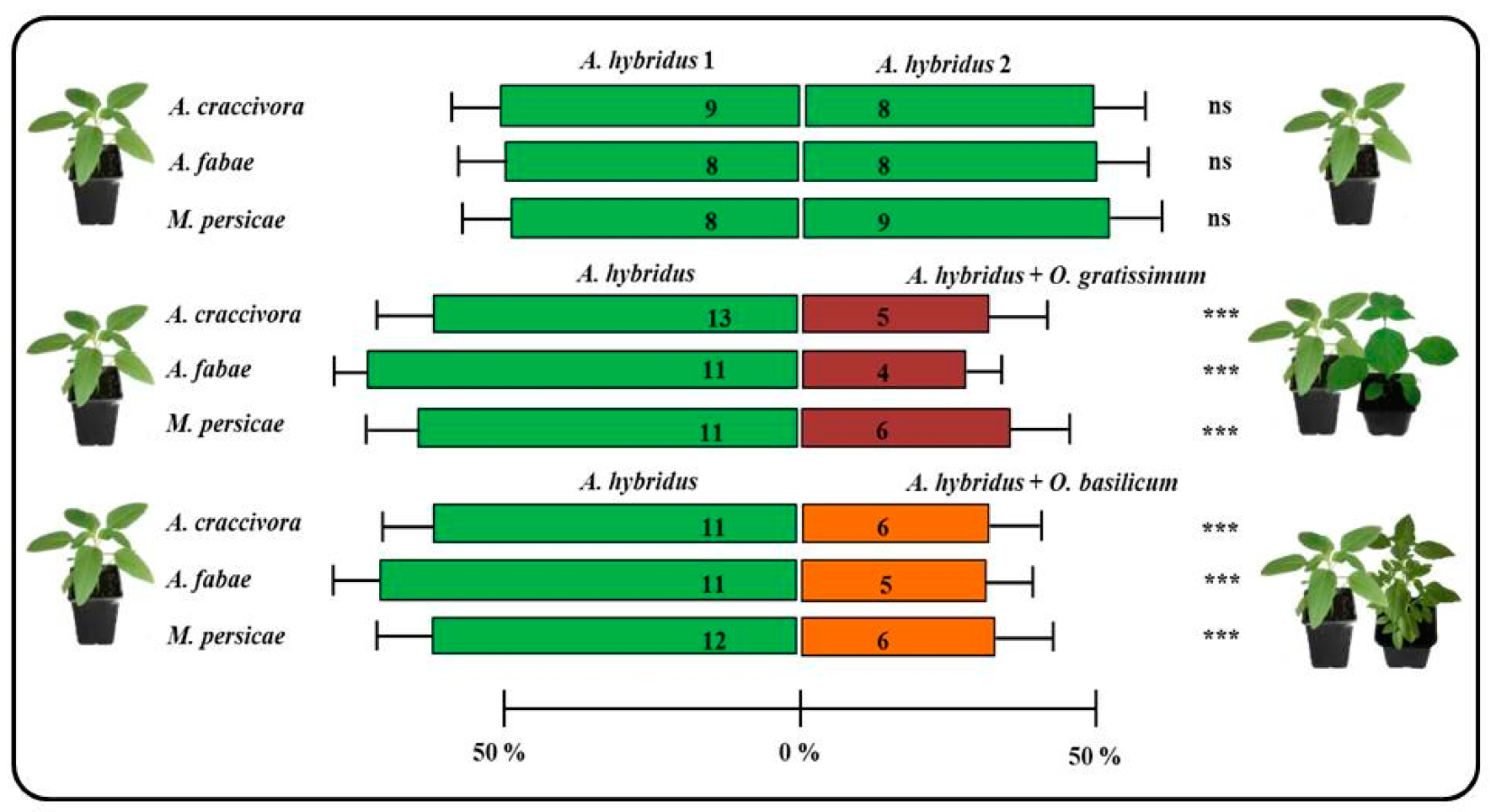
3.1. Effect of Ocimum Species on the Abundance of Unwinged Aphids and Repellent Activity
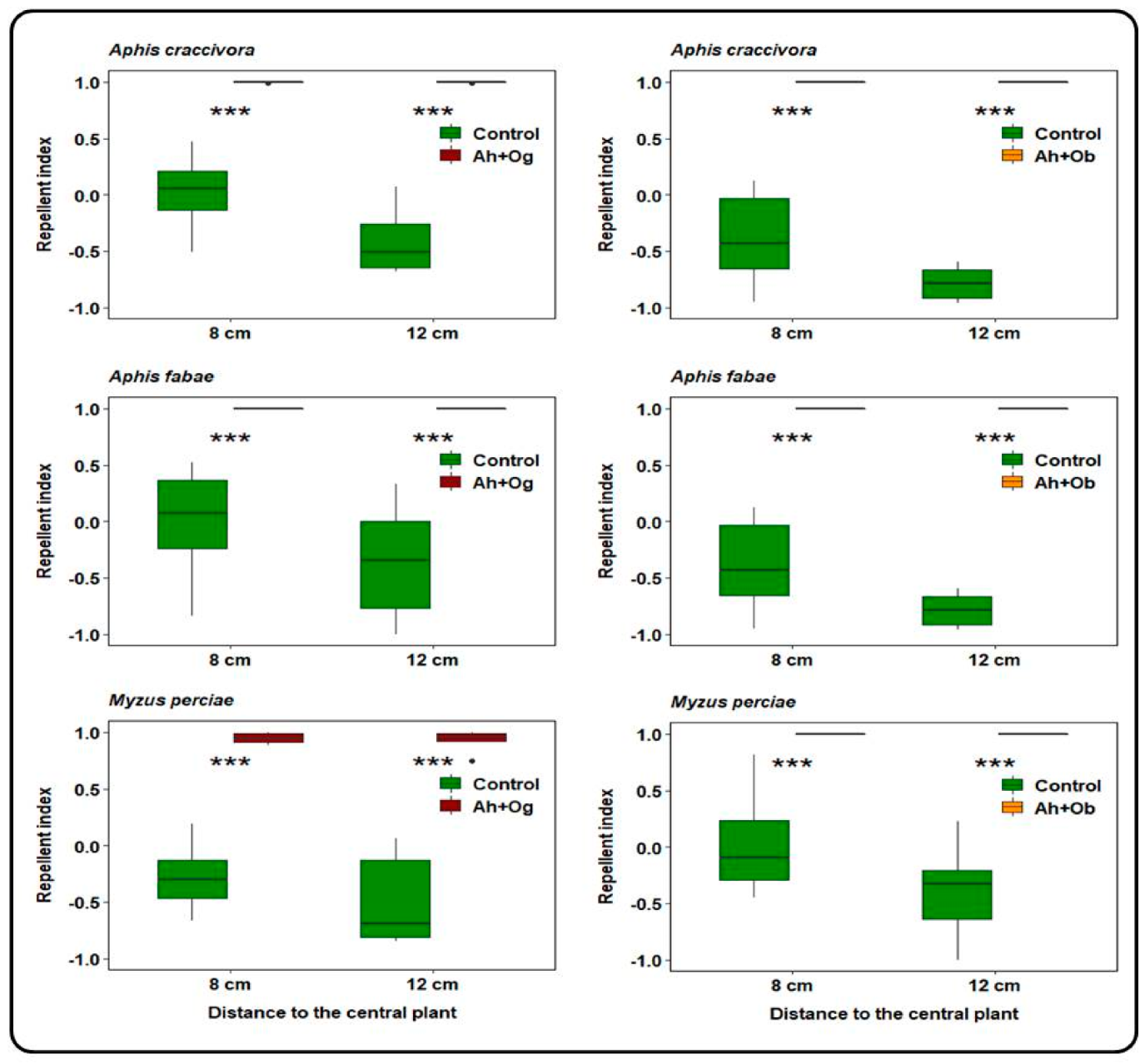
3.2. Repellent Activity of Ocimum on Winged Aphids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatia, V.; Uniyal, P.L.; Bhattacharya, R. Aphid resistance in Brassica crops: Challenges, biotechnological progress and emerging possibilities. Biotechnol. Adv. 2011, 29, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Snyder, W.E.; Hamilton, G.C.; Saona, C.R. Companion planting and insect pest control. In Weed and Pest Control—Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; InTech: Rijeka, Croatia, 2013; pp. 1–30. ISBN 978-953-51-0984-6. [Google Scholar] [CrossRef]
- Fouad, E.A.; Abou-Yousef, H.M.; Abdallah, I.S.; Kandil, M.A. Resistance monitoring and enzyme activity in three field populations of cowpea aphid (Aphis craccivora) from Egypt. Crop Prot. 2016, 81, 163–167. [Google Scholar] [CrossRef]
- Lundström, N.L.P.; Zhang, H.; Brännström, Å. Pareto-efficient biological pest control enable high efficacy at small costs. Ecol. Model. 2017, 364, 89–97. [Google Scholar] [CrossRef]
- Erdoğan, P.; Yildirim, A. Insecticidal activity of three different plant extracts on the green peach aphid [(Myzus persicae Sulzer) (Hemiptera: Aphididae)]. J. Entomol. Res. Soc. 2016, 18, 27–35. [Google Scholar]
- Petrakis, E.A.; Kimbaris, A.C.; Perdikis, D.C.; Lykouressis, D.P.; Tarantilis, P.A.; Polissiou, M.G. Responses of Myzus persicae (Sulzer) to three Lamiaceae essential oils obtained by microwave-assisted and conventional hydrodistillation. Ind. Crops Prod. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Abtew, A.; Subramanian, S.; Cheseto, X.; Kreiter, S.; Garzia, G.; Martin, T. Repellency of plant extracts against the legume flower Thrips Megalurothrips sjostedti (Thysanoptera: Thripidae). Insects 2015, 6, 608–625. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Xu, Y.; Huang, X.; Zhang, L.; Mu, W. Effects of intercropping tea with aromatic plants on population dynamics of arthropods in Chinese tea plantations. J. Pest Sci. 2017, 90, 227–237. [Google Scholar] [CrossRef]
- Kianmatee, S.; Ranamukhaarachchi, S.L. Pest repellent plants for management of insect pests of chinese kale, Brassica oleracea L. Int. J. Agric. Biol. 2007, 9, 64–67. [Google Scholar]
- Adeniyi, S.A.; Orjiekwe, C.L.; Ehiagbonare, J.E.; Arimah, B.D. Preliminary phytochemical analysis and insecticidal activity of ethanolic extracts of four tropical plants (Vernonia amygdalina, Sida acuta, Ocimum gratissimum and Telfaria occidentalis) against beans weevil (Acanthscelides obtectus). Int. J. Phys. Sci. 2010, 5, 753–762. [Google Scholar]
- Ma, Y.F.; Xiao, C. Push-pull effects of three plant secondary metabolites on oviposition of the potato tuber moth. J. Insect Sci. 2013, 13, 128. [Google Scholar] [CrossRef][Green Version]
- Kiradoo, M.M.; Srivastava, M. A comparative study on the efficacy of two Lamiaceae plants on egg-laying performance by the pulse beetle Callosobruchus chinensis Linn. (Coleoptera: Bruchidae). J. Biopestic. 2010, 3, 590–595. [Google Scholar]
- Koech, P.; Mwangi, R. Repellent activities of Ocimum basilicum, Azadirachta indica and Eucalyptus citriodora extracts on rabbit skin against Aedes aegypti. J. Entomol. Zool. Stud. 2013, 1, 84–91. [Google Scholar]
- Akono Ntonga, P.; Baldovini, N.; Mouray, E.; Mambu, L.; Belong, P.; Grellier, P. Activity of Ocimum basilicum, Ocimum canum, and Cymbopogon citratus essential oils against Plasmodium falciparum and mature-stage larvae of Anopheles funestus s.s. Parasite 2014, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Shadia, E.; Abd, E.; Elsayed, A.O.; Aly, S.S. Chemical composition of Ocimum americanum essential oil and its biological effects against, Agrotis ipsilon, (Lepidoptera: Noctuidae). Res. J. Agric. Biol. Sci. 2007, 3, 740–747. [Google Scholar]
- Parolin, P.; Bresch, C.; Poncet, C.; Suay-Cortez, R.; Van Oudenhove, L. Testing basil as banker plant in IPM greenhouse tomato crops. Int. J. Pest Manag. 2015, 61, 235–242. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bawin, T.; Boullis, A.; Heukin, S.; Lognay, G.; Verheggen, F.J.; Francis, F. Oviposition deterrent activity of basil plants and their essentials oils against Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. 2017. [Google Scholar] [CrossRef]
- Schader, C.; Zaller, J.G.; Köpke, U. Cotton-Basil intercropping: Effects on pests, yields and economical parameters in an organic field in Fayoum, Egypt. Biol. Agric. Hortic. 2005, 23, 59–72. [Google Scholar] [CrossRef]
- Basedow, T.; Hua, L.; Aggarwal, N. The infestation of Vicia faba L. (Fabaceae) by Aphis fabae (Scop.) (Homoptera: Aphididae) under the influence of Lamiaceae (Ocimum basilicum L. and Satureja hortensis L.). J. Pest Sci. 2006, 79, 149–154. [Google Scholar] [CrossRef]
- Yarou, B.B.; Assogba-Komlan, F.; Tossou, E.; Mensah, A.C.; Simon, S.; Verheggen, F.J.; Francis, F. Efficacy of Basil-Cabbage intercropping to control insect pests in Benin, West Africa. Commun. Agric. Appl. Biol. Sci. 2017, 82, 157–166. [Google Scholar]
- Beizhou, S.; Jie, Z.; Jinghui, H.; Hongying, W.; Yun, K.; Yuncong, Y. Temporal dynamics of the arthropod community in pear orchards intercropped with aromatic plants. Pest Manag. Sci. 2011, 67, 1107–1114. [Google Scholar] [CrossRef]
- Beizhou, S.; Tang, G.; Sang, X.; Zhang, J.; Yao, Y.; Wiggins, N. Intercropping with aromatic plants hindered the occurrence of Aphis citricola in an apple orchard system by shifting predator–prey abundances. Biocontrol Sci. Technol. 2013, 23, 381–395. [Google Scholar]
- Tang, G.B.; Song, B.Z.; Zhao, L.L.; Sang, X.S.; Wan, H.H.; Zhang, J.; Yao, Y.C. Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agrofor. Syst. 2013, 87, 273–285. [Google Scholar] [CrossRef]
- Das, S. Amaranthus: A Promising Crop of Future; Springer Nature: Singapore, 2016; pp. 42–46. [Google Scholar] [CrossRef]
- Sæthre, M.-G.; Godonou, I.; Hofsvang, T.; Tepa-Yotto, G.; James, B. Aphids and their natural enemies in vegetable agroecosystems in Benin. Int. J. Trop. Insect Sci. 2011, 31, 103–117. [Google Scholar] [CrossRef]
- Singh, G.; Singh, R. Distribution and economic importance of Aphis (Aphis) craccivora Koch, 1854 (Aphidini: Aphidinae: Aphididae: Hemiptera) and its food plants in India. Int. J. Recent Adv. Multidiscip. 2017, 4, 2274–2286. [Google Scholar]
- Bayendi Loudit, S.M.; Poligui, R.N.; Verheggen, F.; Francis, F. Occurrence of Aphids and their predators within vegetable crops in peri-urban areas in Libreville (Gabon). Commun. Agric. Appl. Biol. Sci. 2017, 82/2, 199–205. [Google Scholar]
- Achigan-Dako, E.G.; Pasquini, M.W.; Assogba-Komlan, F.; N’danikou, S.; Dansi, A.; Ambrose-Oji, B. Traditional Vegetables in Benin; Institut National des Recherches Agricoles du Bénin. Impr. du CENAP: Cotonou, Benin, 2010. [Google Scholar]
- Stefanazzi, N.; Stadler, T.; Ferrero, A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2011, 69, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage publications: Los Angeles, CA, USA, 2018; ISBN 13: 9781412975148. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2020. Available online: https://www.researchgate.net/publication/303256192_Agricolae_Statistical_Procedures_for_Agricultural_Research (accessed on 15 May 2020).
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 March 2020).
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Ogayo, K.O.; Ogweno, J.O.; Nyaanga, J.G.; Ogendo, J.O.; Wagara, I.N.; Ochola, S.O. Biofficacy of lion’s ear and African basil extracts in management of adult two-spotted spider mite on french beans. In Proceedings of the RUFORUM Fourth Biennial Conference, Maputo, Mozambique, 19–25 July 2014; Volume 21, pp. 199–203. [Google Scholar]
- Asawalam, E.; Emosairue, S.; Hassanali, A. Essential oil of Ocimum grattissimum (Labiatae) as Sitophilus zeamais (Coleoptera: Curculionidae) protectant. Afr. J. Biotechnol. 2008, 7, 3771–3776. [Google Scholar]
- Del Fabbro, S.; Nazzi, F. Repellent effect of sweet basil compounds on Ixodes ricinus ticks. Exp. Appl. Acarol. 2008, 45, 219–228. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Dahlin, I.; Vucetic, A.; Ninkovic, V.; Dahlin, I.; Vucetic, A.N.V. Changed host plant volatile emissions induced by chemical interaction between unattacked plants reduce aphid plant acceptance with intermorph variation. J. Pest Sci. 2015, 88, 249–257. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, Z.; Gao, Y.; Bian, L.; Sun, X.; Chen, Z. Volatiles from non-host aromatic plants repel tea green leafhopper Empoasca vitis. Entomol. Exp. Appl. 2014, 153, 156–169. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.G.; Wright, C.J. Variation in the essential oils in different leaves of basil (Ocimum basilicum L.) at day time. Open Hortic. J. 2009, 2, 13–16. [Google Scholar] [CrossRef]
- Chapman, R.F.; Bernays, E.A.; Simpson, S.J. Attraction and repulsion of the aphid, Cavariella aegopodii, by plant odors. J. Chem. Ecol. 1981, 7, 881–888. [Google Scholar] [CrossRef]
- Gbrays, B.; Dancewicz, K.; Halarewiez-Pacan, A.; Janusz, E. Effect of natural monoterpenes on the behaviour of the peach potato aphid Myzus persicae (Sulz.). IOBC Wprs Bull. 2005, 28, 29–34. [Google Scholar]
| Factors | Df | Chisq | p-Value |
|---|---|---|---|
| Plants | 1 | 0.17 | 0.680 |
| Aphids | 2 | 0.99 | 0.608 |
| Treatments | 2 | 12.74 | 0.001 |
| Distance | 2 | 35.63 | <0.001 |
| Aphids: Treatments | 4 | 2.74 | 0.603 |
| Aphis: Distance | 4 | 1.63 | 0.803 |
| Treatments: Distance | 4 | 627.82 | <0.001 |
| Aphids: Treatments: Distance | 8 | 23.43 | 0.002 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Control | Ah Versus Ah + Og | Ah Versus Ah + Ob | ||||
| Aphids | Responding aphids (%) a | p-value | Responding aphids (%) a | p-value | Responding aphids (%) a | p-value |
| A. craccivora | 166 (83) | <0.001 | 182 (91) | <0.001 | 167 (84) | <0.001 |
| A. fabae | 155 (78) | <0.001 | 158 (79) | <0.001 | 163 (82) | <0.001 |
| M. persicae | 167 (84) | <0.001 | 166 (83) | <0.001 | 178 (89) | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarou, B.B.; Bokonon-Ganta, A.H.; Verheggen, F.J.; Lognay, G.C.; Francis, F. Aphid Behavior on Amaranthus hybridus L. (Amaranthaceae) Associated with Ocimum spp. (Lamiaceae) as Repellent Plants. Agronomy 2020, 10, 736. https://doi.org/10.3390/agronomy10050736
Yarou BB, Bokonon-Ganta AH, Verheggen FJ, Lognay GC, Francis F. Aphid Behavior on Amaranthus hybridus L. (Amaranthaceae) Associated with Ocimum spp. (Lamiaceae) as Repellent Plants. Agronomy. 2020; 10(5):736. https://doi.org/10.3390/agronomy10050736
Chicago/Turabian StyleYarou, Boni Barthélémy, Aimé H. Bokonon-Ganta, François J. Verheggen, Georges C. Lognay, and Frédéric Francis. 2020. "Aphid Behavior on Amaranthus hybridus L. (Amaranthaceae) Associated with Ocimum spp. (Lamiaceae) as Repellent Plants" Agronomy 10, no. 5: 736. https://doi.org/10.3390/agronomy10050736
APA StyleYarou, B. B., Bokonon-Ganta, A. H., Verheggen, F. J., Lognay, G. C., & Francis, F. (2020). Aphid Behavior on Amaranthus hybridus L. (Amaranthaceae) Associated with Ocimum spp. (Lamiaceae) as Repellent Plants. Agronomy, 10(5), 736. https://doi.org/10.3390/agronomy10050736