Carbon Input and Maize Productivity as Influenced by Tillage, Crop Rotation, Residue Management and Biochar in a Semiarid Region in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.2. Treatments and Experimental Design
2.3. Management of Nonexperimental Variables
2.4. Measurements
2.4.1. Aboveground Biomass Yields
2.4.2. Soil Organic Carbon and Carbon Input
2.4.3. Maize Yield and Yield Components
2.5. Data Analysis
3. Results
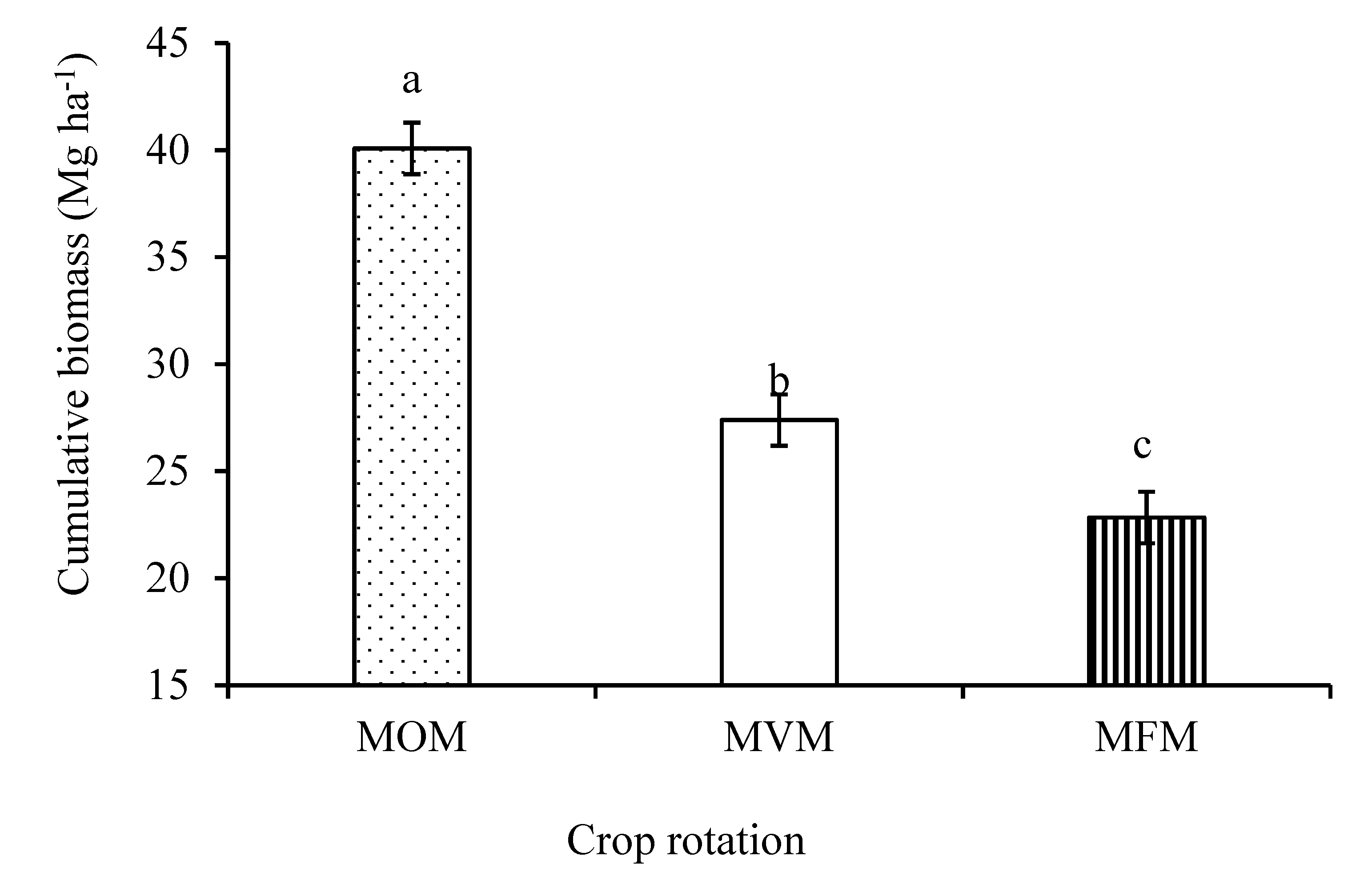
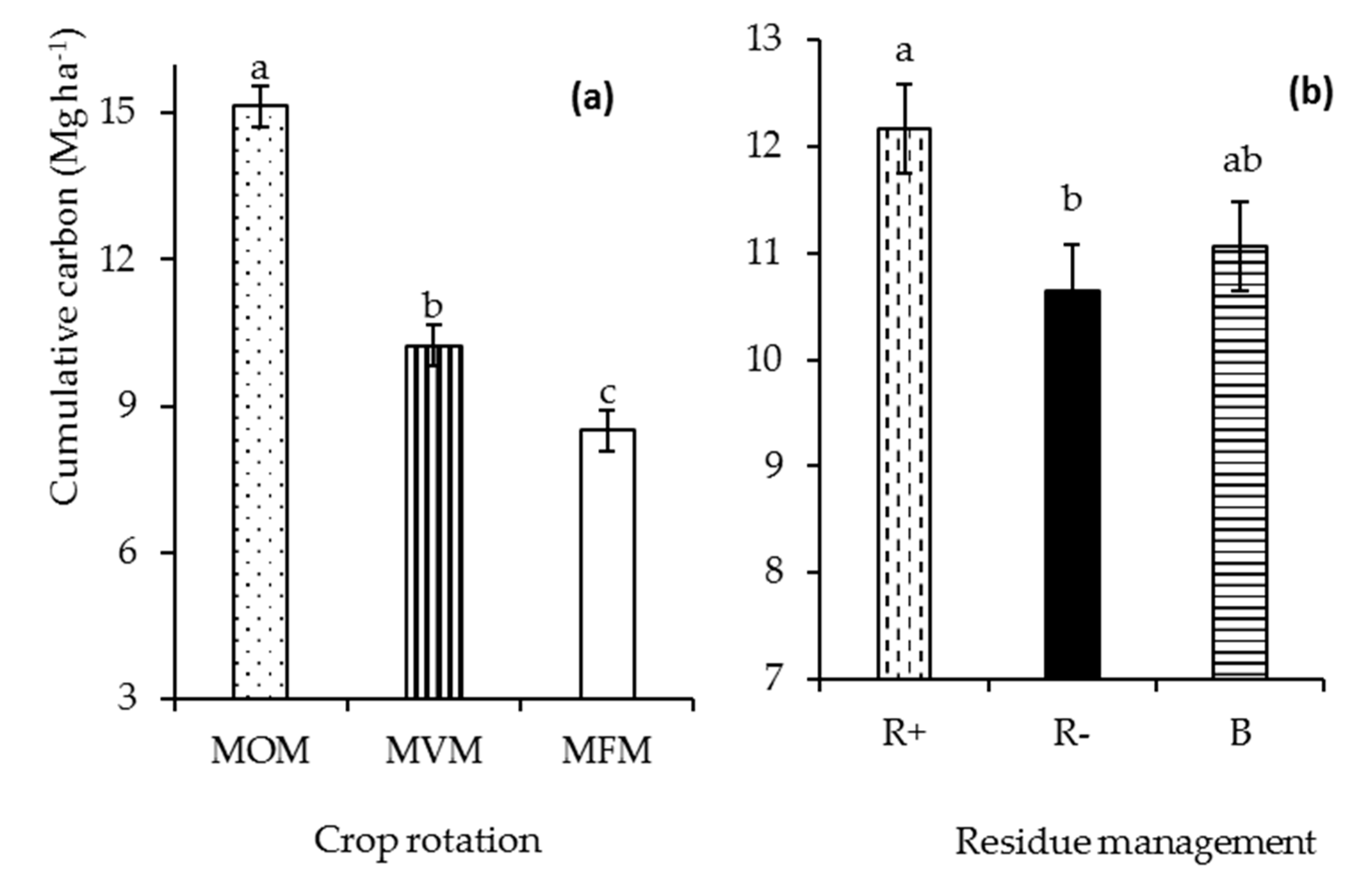
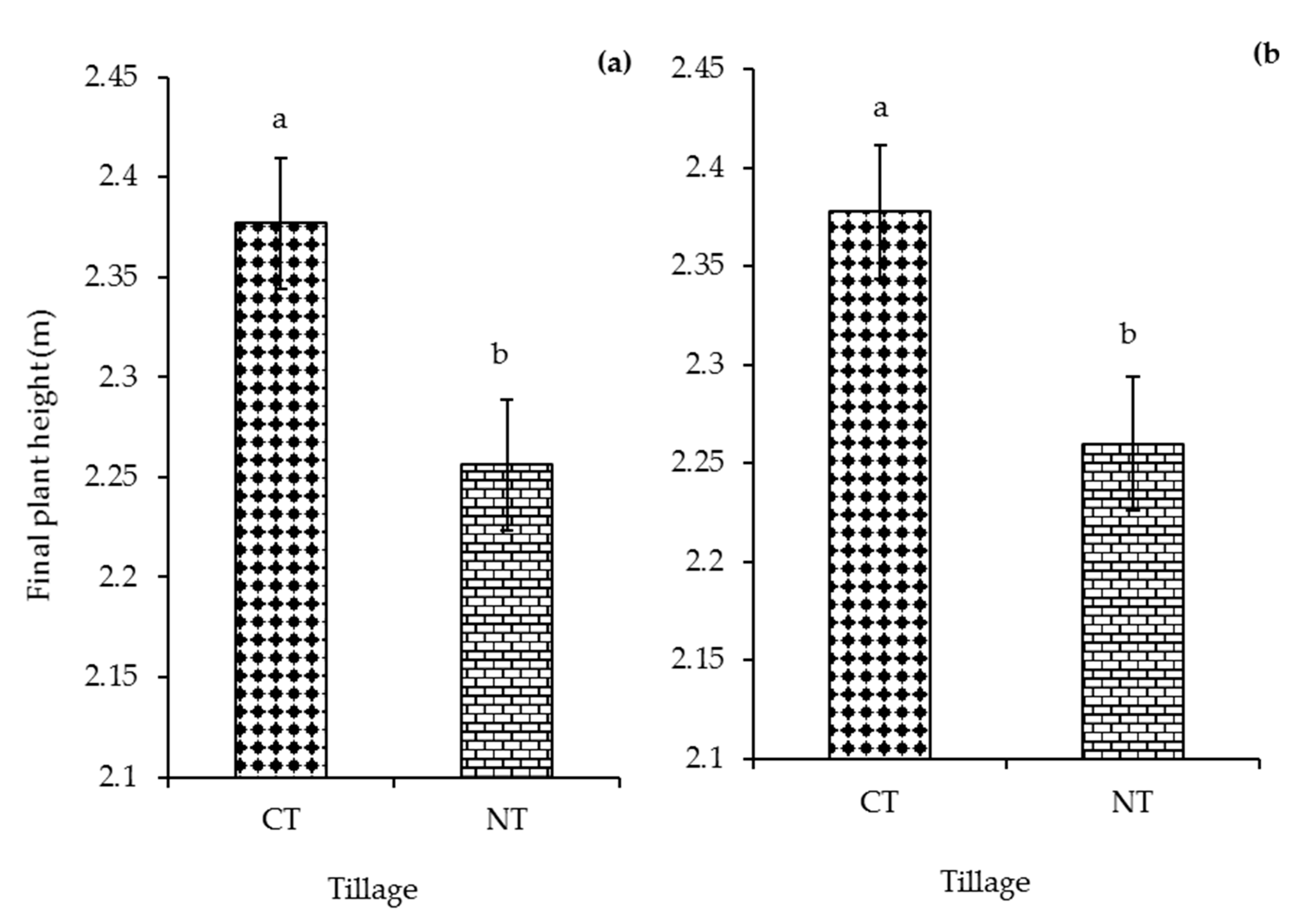
3.1. Aboveground Biomass Production and Carbon Input
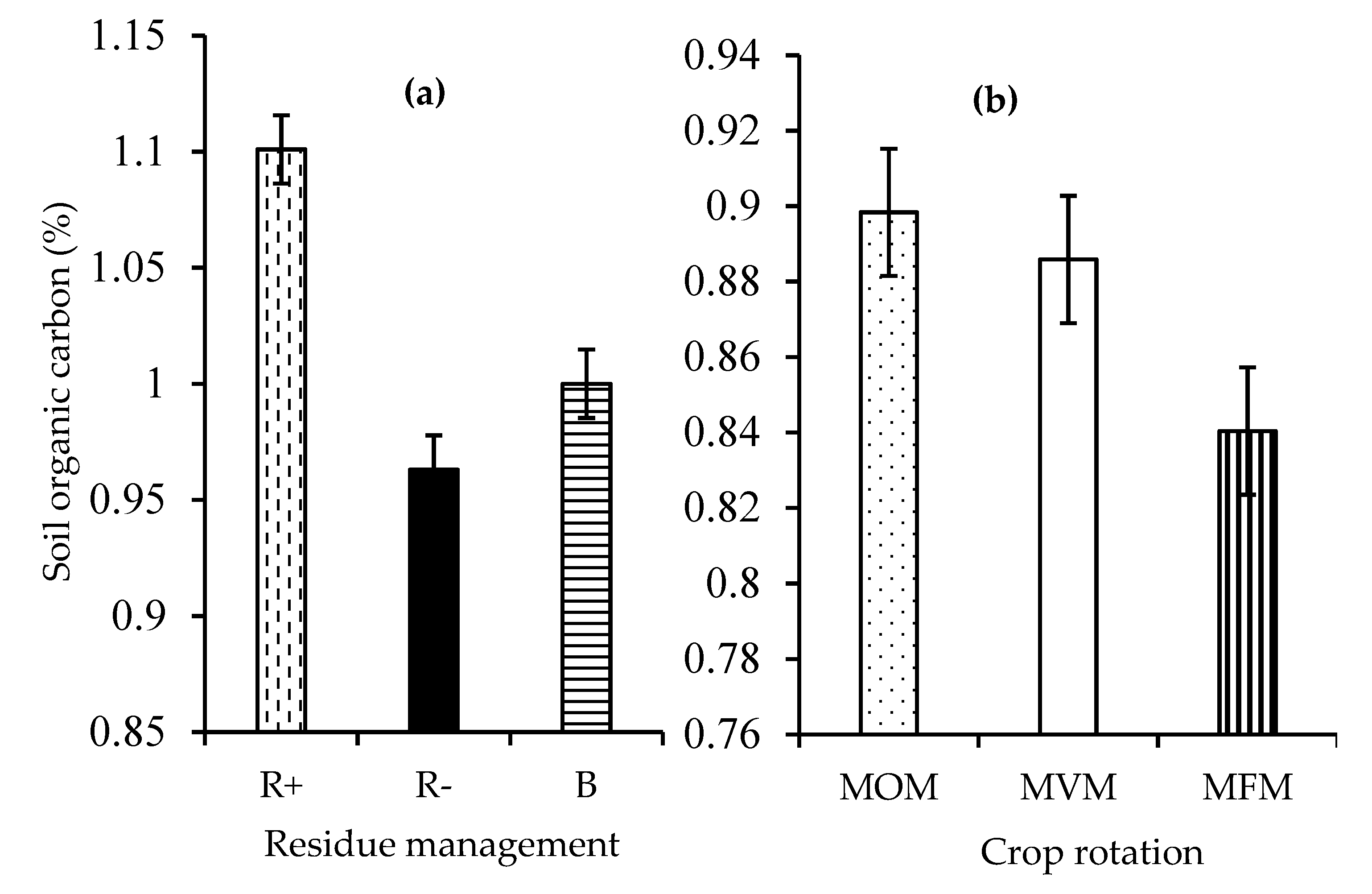
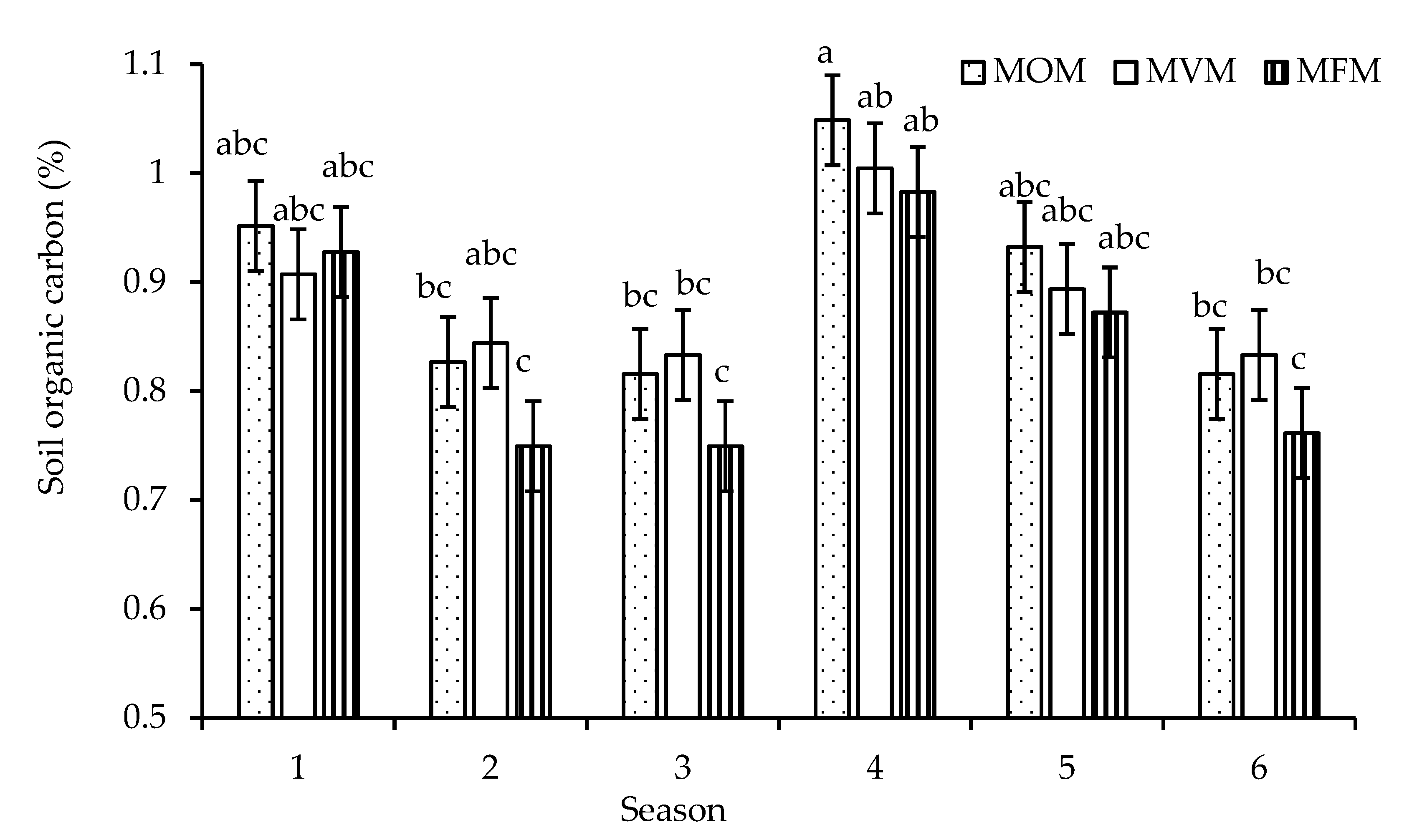
3.2. Soil Organic Carbon
3.3. Maize Yield Components and Grain Yield
4. Discussion
4.1. Aboveground Biomass Production and Carbon Input from Plant Residues
4.2. Soil Organic Carbon
4.3. Maize Grain Yield and Yield Components
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Muzangwa, L.; Mnkeni, P.N.S.; Chiduza, C. Assessment of Conservation Agriculture Practices by Smallholder Farmers in the Eastern Cape Province of South Africa. Agronomy 2017, 7, 46. [Google Scholar] [CrossRef]
- Pokharel, D.; Jha, R.K.; Tiwari, T.P.; Gathala, M.K.; Shrestha, H.K.; Panday, D. Is conservation agriculture a potential option for cereal-based sustainable farming system in the Eastern Indo-Gangetic Plains of Nepal? Cogent Food Agric. 2018, 4, 1557582. [Google Scholar] [CrossRef]
- Giller, K.E.; Andersson, J.A.; Corbeels, M.; Kirkegaard, J.; Mortensen, D.; Erenstein, O.; Vanlauwe, B. Beyond conservation agriculture. Front. Plant Sci. 2015, 6, 870. [Google Scholar] [CrossRef] [PubMed]
- Mnkeni, P.N.S.; Chiduza, C.; Modi, A.T.; Stevens, J.B.; Monde, N.; van der Stoep, I.; Dladla, R. Best management practices for smallholder farming on two irrigation schemes in the Eastern Cape and KwaZulu-Natal through participatory adaptive research. WRC Rep. 2010, 478, 359. [Google Scholar]
- Von Loeper, W.; Musango, J.; Brent, A.; Drimie, S. Analysing challenges facing smallholder farmers and conservation agriculture in South Africa: A system dynamics approach. S. Afr. J. Econ. Manag. Sci. 2016, 19, 747–773. [Google Scholar] [CrossRef]
- Mazvimavi, K.; Twomlow, S. Socio-economic and institutional factors influencing adoptionof conservation farming by vulnerable households in Zimbabwe. Agric. Syst. 2009, 101, 20–29. [Google Scholar] [CrossRef]
- Valbuena, D.; Erenstein, O.; Tui, S.H.-K.; Abdoulaye, T.; Claessens, L.; Duncan, A.J.; Gérard, B.; Rufino, M.C.; Teufel, N.; van Rooyen, A. Conservation Agriculture in mixed crop–livestock systems: Scoping crop residue trade-offs in Sub-Saharan Africa and South Asia. Field Crop. Res. 2012, 132, 175–184. [Google Scholar] [CrossRef]
- Thierfelder, C.; Mombeyarara, T.; Mango, N.; Rusinamhodzi, L. Integration of conservation agriculture in smallholder farming systems of southern Africa: Identification of key entry points. Int. J. Agric. Sustain. 2013, 11, 317–330. [Google Scholar] [CrossRef]
- Khorami, S.S.; Kazemeini, S.A.; Afzalinia, S.; Gathala, M.K. Changes in soil properties and productivity under different tillage practices and wheat genotypes: A short-term study in Iran. Sustainability 2018, 10, 3273. [Google Scholar] [CrossRef]
- Derpsch, R.; Friedrich, T.; Kassam, A.; Hongwen, L. Current status of adoption of no-till farming in the world and some of its main benefits. Int. J. Agric. Biol. Eng. 2010, 3, 1–25. [Google Scholar]
- Food and Agriculture Organization [FAO]. Conservation Agriculture. 2008. Available online: http://www.fao.org/ag/ca/1a.html (accessed on 4 August 2015).
- Kassam, A.; Friedrich, T.; Shaxson, F.; Pretty, J. The spread of conservation agriculture: Justification; sustainability and uptake. Int. J. Agric. Sustain. 2009, 7, 292–320. [Google Scholar] [CrossRef]
- Delgado, J.A.; Groffman, P.M.; Nearing, M.A.; Goddard, T.; Reicosky, D.; Lal, R.; Kitchen, N.R.; Rice, C.W.; Towery, D.; Salon, P. Conservation practices to mitigate and adapt to climate change. J. Soil Water Conserv. 2011, 66, 118–129. [Google Scholar] [CrossRef]
- Lal, R. Enhancing crop yields in the developing countries through restoration of the soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2005, 17, 197–209. [Google Scholar] [CrossRef]
- Sithole, N.; Magwaza, L.S.; Mafongoya, P.L. Conservation agriculture and its impact on soil quality and maize yield: A South African perspective. Soil Tillage Res. 2016, 162, 55–67. [Google Scholar] [CrossRef]
- Zuber, S.M.; Behnke, G.D.; Nafziger, E.D.; Villamil, M.B. Carbon and Nitrogen Content of Soil Organic Matter and Microbial Biomass under Long-Term Crop Rotation and Tillage in Illinois, USA. Agriculture 2018, 8, 37. [Google Scholar] [CrossRef]
- Nyamangara, J.; Nyengerai, K.; Masvaya, E.N.; Tirivavi, R.; Mashingaidze, N.; Mupangwa, W.; Dimes, J.; Hove, L.; Twomlow, S. Effect of conservation agriculture on maize yield in the semi-arid areas of Zimbabwe. Exp. Agric. 2014, 50, 159–177. [Google Scholar] [CrossRef]
- Ding, G.; Liu, X.; Herbert, S.; Novak, J.; Amarasiriwardena, D.; Xing, B. Effect of cover crop management on soil organic matter. Geoderma 2006, 130, 229–239. [Google Scholar] [CrossRef]
- Murungu, F.; Chiduza, C.; Muchaonyerwa, P. Mulch effects on soil moisture productivity in warm-temperate climate of South Africa. Soil Tillage Res. 2011, 112, 58–65. [Google Scholar] [CrossRef]
- Murungu, F.; Chiduza, C.; Muchaonyerwa, P.; Mnkeni, P. Decomposition, nitrogen and phosphorus mineralization from winter-grown cover crop residues and suitability for a smallholder farming system in South Africa. Nutr. Cycl. Agroecosyst. 2011, 89, 115–123. [Google Scholar] [CrossRef]
- Thierfelder, C.; Wall, P.C. Effects of conservation agriculture on soil quality and productivity in contrasting agro-ecological environments of Zimbabwe. Soil Use Manag. 2012, 28, 209–222. [Google Scholar] [CrossRef]
- Dube, E.; Chiduza, C.; Muchaonyerwa, P. Conservation agriculture effects on plant nutrients and maize grain yield after four years of maize–winter cover crop rotations. S. Afr. J. Plant Soil 2013, 30, 227–232. [Google Scholar] [CrossRef]
- Araya, T.; Cornelis, W.M.; Nyssen, J.; Govaerts, B.; Bauer, H.; Gebreegziabher, T.; Oicha, T.; Raes, D.; Sayre, K.D.; Haile, M.; et al. Effects of conservation agriculture on runoff, soil loss and crop yield under rainfed conditions in Tigray, Northern Ethiopia. Soil Use Manag. 2011, 27, 404–414. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, Y.; Chen, C.; Sun, Y.; Feng, J.; Deng, A.; Song, Z.; Zhang, W. The impacts of conservation agriculture on crop yield in China depend on specific practices, crops and cropping regions. Crop J. 2014, 2, 289–296. [Google Scholar] [CrossRef]
- Araya, T.; Cornelis, W.M.; Nyssen, J.; Govaerts, B.; Getnet, F.; Bauer, H.; Raes, D.; Amare, K.; Haile, M.; Deckers, J. Medium-term effects of conservation agriculture for in situ soil and water management and crop productivity in the northern Ethiopian highlands. Field Crops Res. 2012, 132, 53–62. [Google Scholar] [CrossRef]
- Mashingaidze, N.; Madakadze, C.; Twomlow, S.; Nyamangara, J.; Hove, L. Crop yield and weed growth under conservation agriculture in semi-arid Zimbabwe. Soil Tillage Res. 2012, 124, 102–110. [Google Scholar] [CrossRef]
- Paul, B.K.; Vanlauwe, B.; Ayuke, F.; Gassner, A.; Hoogmoed, M.; Hurisso, T.; Koala, S.; Lelei, D.; Ndabamenye, T.; Six, J. Medium-term impact of tillage and residue management on soil aggregate stability, soil carbon and crop productivity. Agric Ecosyst. Environ. 2013, 164, 14–22. [Google Scholar] [CrossRef]
- Paudel, B.; Radovich, T.J.K.; Chan-Halbrendt, C.; Crow, S.; Tamang, B.B.; Halbrendt, J.; Thapa, K. Effect of Conservation Agriculture on Maize-based Farming System in the Mid-hills of Nepal. Procedia Eng. 2014, 78, 327–336. [Google Scholar] [CrossRef]
- Hatfield, J.L. Managing soils to achieve greater water use efficiency: A review. Agron. J. 2001, 93, 271–280. [Google Scholar] [CrossRef]
- Erenstein, O. Crop residue mulching in tropical and semi-tropical countries. An evaluation of research availability and other technological implications. Soil Tillage Res. 2002, 67, 115–133. [Google Scholar] [CrossRef]
- Turmel, M.S.; Speratti, A.; Baudron, F.; Verhulst, N.; Govaerts, B. Crop residue management and soil health: A systems analysis. Agric. Syst. 2002, 134, 6–16. [Google Scholar] [CrossRef]
- Van Ittersum, M.K.; Van Bussel, L.G.; Wolf, J.; Grassini, P.; Van Wart, J.; Guilpart, N.; Claessens, L.; de Groot, H.; Wiebe, K.; Mason-D’Croz, D.; et al. Can sub-Saharan Africa feed itself? Proc. Natl. Acad. Sci. USA 2016, 113, 14964–14969. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.R. Soil erosion and agricultural sustainability. Proc. Natl. Acad. Sci. USA 2007, 104, 13268–13272. [Google Scholar] [CrossRef] [PubMed]
- Novotny, E.H.; Hayes, M.H.; Madari, B.E.; Bonagamba, T.J.; Azevedo, E.R.D.; Souza, A.A.D.; Song, G.; Nogueira, C.M.; Mangrich, A.S. Lessons from the Terra Preta de Índios of the Amazon region for the utilisation of charcoal for soil amendment. J. Braz. Chem. Soc. 2009, 20, 1003–1010. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyan, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Nyambo, P.; Taeni, T.; Chiduza, C.; Araya, T. Effects of Maize Residue Biochar Amendments on Soil Properties and Soil Loss on Acidic Hutton Soil. Agronomy 2018, 8, 256. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction, Biochar for Environmental Management. Sci. Technol. 2009, 1, 1–12. [Google Scholar]
- Novak, J.M.; Lima, I.M.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Charcaterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Lehmann, J.; Da Silva, J.P., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World reference base for soil resources. In World Soil Resources Report No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Mandiringana, O.T.; Mnkeni, P.N.S.; Mkile, Z.; Van Averbeke, W.; Van Ranst, E.; Verplancke, H. Mineralogy and fertility status of selected soils of the Eastern Cape Province, South Africa. Comms. Soil Sci. Plant Anal. 2005, 36, 2431–2446. [Google Scholar] [CrossRef]
- Dube, E.; Chiduza, C.; Muchaonyerwa, P. Conservation agriculture effects on soil organic matter on a Haplic Cambisol after four years of maize–oat and maize–grazing vetch rotations in South Africa. Soil Tillage Res. 2012, 123, 21–28. [Google Scholar] [CrossRef]
- Gura, I.; Mnkeni, P.N.S. Crop rotation and residue management effects under no till on the soil quality of a Haplic Cambisol in Alice, Eastern Cape, South Africa. Geoderma 2019, 337, 927–934. [Google Scholar] [CrossRef]
- Verhulst, N.; Cox, R.; Govaerts, B. Yield and Yield Components: A Practical Guide for Comparing Crop Management Practices; CIMMYT: Houston, TX, USA, 2013. [Google Scholar]
- Agri Laboratory Association of Southern Africa [AgriLASA]. Soil Handbook; Pretoria (South Africa): Agri Laboratory Association of Southern Africa: Pretoria, South Africa, 2004. [Google Scholar]
- LECO Truspec CN Carbon/Nitrogen Determinator Instructions Manual; LECO Corporation: St Joseph, MO, USA, 2003.
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; A Wiley Inter-Science Publication: Singapore, 1984; pp. 1–627. [Google Scholar]
- Alemu, B.; Melaku, S.; Prasad, N.K. Effects of varying seed proportions and harvesting stages on biological compatibility and forage yield of oats (Avena sativa L.) and vetch (Vicia villosa R.) mixtures. Livest. Res. Rural Dev. 2007, 19, 12. [Google Scholar]
- Muzangwa, L.; Chiduza, C.; Muchaonyerwa, P. Biomass production, weed suppression, nitrogen and phosphorus uptake in white oat (Avena sativa L.) and grazing vetch (Vicia dasycarpa L.) cover crop bicultures under an irrigated no-tillage system. South Afr. J. Plant Soil 2012, 29, 135–141. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, I.; Hontoria, C.; Gabriel, J.L.; Alonso-Ayuso, M.; Quemada, M. Cover crops to mitigate soil degradation and enhance soil functionality in irrigated land. Geoderma 2018, 322, 81–88. [Google Scholar] [CrossRef]
- Dube, E.; Chiduza, C.; Muchaonyerwa, P. High biomass yielding winter cover crops can improve phosphorus availability in soil. S. Afr. J. Sci. 2014, 110, 1–4. [Google Scholar] [CrossRef]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwat Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops-A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Lichter, K.; Dendooven, L.; Deckers, J. Influence of permanent raised bed planting and residue management on physical and chemical soil quality in rain fed maize/wheat systems. Plant Soil 2007, 291, 39–54. [Google Scholar] [CrossRef]
- Ella, V.B.; Reyes, M.R.; Mercado, A.J.; Ares, A.; Padre, R. Conservation agriculture increases soil organic carbon and residual water content in upland crop production systems. Eurasian J. Soil Sci. 2016, 5, 24–29. [Google Scholar] [CrossRef]
- Moussadek, R.; Mrabet, R.; Dahan, R.; Zouahri, A.; El Mourid, M.; Van Rans, E. Tillage System Affects Soil Organic Carbon Storage and Quality in Central Morocco. Appl. Environ. Soil Sci. 2014, 654796. [Google Scholar] [CrossRef]
- Luca, E.F.; Chaplot, V.; Mutema, M.; Feller, C.; Ferreira, M.L.; Cerri, C.C.; Couto, H.T.Z. Effect of conversion from sugarcane preharvest burning to residues green-trashing on SOC stocks and soil fertility status: Results from different soil conditions in Brazil. Geoderma 2018, 310, 238–248. [Google Scholar] [CrossRef]
- Dalal, R.C.; Allen, D.E.; Wang, W.J.; Reeves, S.; Gibson, I. Organic carbon and total nitrogen stocks in a Vertisol following 40 years of no-tillage, crop residue retention and nitrogen fertilisation. Soil Tillage Res. 2011, 112, 133–139. [Google Scholar] [CrossRef]
- Li, L.-l.; Huang, G.-b.; Zhang, R.; Bill, B.; Guangdi, L.; Kwong, Y.C. Benefits of Conservation Agriculture on Soil and Water Conservation and Its Progress in China. Agric. Sci. China 2011, 10, 850–859. [Google Scholar] [CrossRef]
- Rusinamhodzi, L.; Corbeels, M.; van Wijk, M.T.; Rufino, M.C.; Nyamangara, J.; Giller, K.E. A meta-analysis of long-term effects of conservation agriculture on maize grain yield under rain-fed conditions. Agron. Sustain. Dev. 2011, 31, 657–673. [Google Scholar] [CrossRef]
- Monnie, F. Effect of Biochar on Soil Physical Properties, Water Use Efficiency, and Growth of Maize in a Sandy Loam Soil. Ph.D. Thesis, University of Ghana, Accra, Ghana, 2016. [Google Scholar]
- Katterer, T.; Roobroeck, D.; Andrén, O.; Kimutai, G.; Karltun, E.; Kirchmann, H.; Nyberg, G.; Vanlauwe, B.; de Nowina, K.R. Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crop. Res. 2019, 235, 18–26. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, B.; Corbeels, M.; Tittonell, P. Conservation agriculture and smallholder farming in Africa: The heretics’ view. Field Crop. Res. 2009, 114, 23–34. [Google Scholar] [CrossRef]
- Thierfelder, C.; Rusinamhodzi, L.; Ngwira, A.; Mupangwa, W.; Nyagumbo, I.; Kassie, G.; Cairns, J. Conservation agriculture in Southern Africa: Advances in knowledge. Renew. Agric. Food Syst. 2015, 30, 328–348. [Google Scholar] [CrossRef]
- Thierfelder, C.; Mutenje, M.; Mujeyi, A.; Mupangwa, W. Where is the limit? Lessons learned from long-term conservation agriculture research in Zimuto Communal Area, Zimbabwe. Food Secur. 2014, 7, 15. [Google Scholar] [CrossRef]
- Wolfe, A.M.; Eckert, D.J. Crop sequence and surface residue effects on the performance of no-till corn grown on a poorly drained soil. Agron. J. 1999, 91, 363–367. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Kwaw-Mensah, D. Effect of tillage and nitrogen rate on corn yield and nitrogen and phosphorus uptake in a corn–soybean rotation. Agron. J. 2007, 99, 1548–1558. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]


| Crop Rotation | Winter 2015 (Season 1) | Summer 2015/2016 (Season 2) | Winter 2016 (Season 3) | Summer 2016/2017 (Season 4) | Winter 2017 (Season 5) | Summer 2017/2018 (Season 6) |
|---|---|---|---|---|---|---|
| MFM | Fallow | Maize | Fallow | Maize | Fallow | Maize |
| MOM | Oat | Maize | Oat | Maize | Oat | Maize |
| MVM | Vetch | Maize | Vetch | Maize | Vetch | Maize |
| MFM | MVM | MOM | |
|---|---|---|---|
| Mg ha−1 | |||
| Conventional tillage | 11.4 | 12.4 | 16.1 |
| No-tillage | 9.4 | 13.0 | 16.7 |
| Month | Rainfall | Irrigation | Temperature | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (mm) | (°C) | ||||||||
| 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |
| Jan | 75.7 | 25.2 | 89.9 | 0 | 40 | 25 | 22.7 | 23.9 | 21.5 |
| Feb | 124 | 85.3 | 73.2 | 0 | 20 | 30 | 22.2 | 22.6 | 22.04 |
| Mar | 83.5 | 75 | 34.3 | 0 | 20 | 20 | 20.3 | 20.8 | 21.21 |
| Apr | 65.5 | 43.3 | 22.1 | 0 | 0 | 0 | 16.1 | 18.5 | 18.24 |
| May | 7.2 | 7.6 | 18.7 | 0 | 0 | 0 | 15.6 | 15.5 | 16.22 |
| Jun | 33 | 13.2 | 2.8 | 0 | 30 | 0 | 12.1 | 13.6 | 13.35 |
| Jul | 92.5 | 43.9 | 4.3 | 0 | 0 | 30 | 11.2 | 12.5 | 12.35 |
| Aug | 20.8 | 10.9 | 66.0 | 0 | 30 | 0 | 14.4 | 15.4 | 13.03 |
| Sep | 60.7 | 32.8 | 23.1 | 0 | 25 | 0 | 15.3 | 15.6 | 15.92 |
| Oct | 17.5 | 18.3 | 84.8 | 50 | 0 | 0 | 18.5 | 17.1 | 16.16 |
| Nov | 72.6 | 40.3 | 91.7 | 30 | 25 | 20 | 19.2 | 18.9 | 17.8 |
| Dec | 1.8 | 14 | 48.3 | 40 | 55 | 20 | 22.7 | 22.7 | 19.02 |
| Winter 2015 (Season 1) | Summer 2015/16 (Season 2) | Winter 2016 (Season 3) | Summer 2016/2017 (Season 4) | Winter 2017 (Season 5) | Summer 2017/2018 (Season 6) | |
|---|---|---|---|---|---|---|
| NT | 1.00bcdef | 1.1ab | 1.22a | 1.11ab | 1.10ab | 1.08bc |
| CT | 0.92def | 1.00bcde | 0.86f | 1.04bcd | 0.95cdef | 0.87ef |
| 2015/2016 Season | 2016/2017 Season | 2017/2018 Season | |
|---|---|---|---|
| Mg ha−1 | |||
| Tillage | |||
| CT | 4.85 | 3.68 | 4.92 |
| NT | 4.39 | 3.83 | 4.70 |
| LSD0.05 | ns | ns | ns |
| Crop rotation | |||
| MFM | 4.52 | 3.69 | 4.85 |
| MOM | 4.48 | 3.91 | 4.54 |
| MVM | 4.87 | 3.66 | 5.04 |
| LSD0.05 | ns | ns | ns |
| Residue management | |||
| Residue removed | 5.18a | 3.20b | 5.15 |
| Residue retained | 4.26b | 3.38b | 4.59 |
| Biochar | 4.42ab | 4.53a | 4.68 |
| LSD0.05 | 0.76 | 0.60 | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyambo, P.; Chiduza, C.; Araya, T. Carbon Input and Maize Productivity as Influenced by Tillage, Crop Rotation, Residue Management and Biochar in a Semiarid Region in South Africa. Agronomy 2020, 10, 705. https://doi.org/10.3390/agronomy10050705
Nyambo P, Chiduza C, Araya T. Carbon Input and Maize Productivity as Influenced by Tillage, Crop Rotation, Residue Management and Biochar in a Semiarid Region in South Africa. Agronomy. 2020; 10(5):705. https://doi.org/10.3390/agronomy10050705
Chicago/Turabian StyleNyambo, Patrick, Cornelius Chiduza, and Tesfay Araya. 2020. "Carbon Input and Maize Productivity as Influenced by Tillage, Crop Rotation, Residue Management and Biochar in a Semiarid Region in South Africa" Agronomy 10, no. 5: 705. https://doi.org/10.3390/agronomy10050705
APA StyleNyambo, P., Chiduza, C., & Araya, T. (2020). Carbon Input and Maize Productivity as Influenced by Tillage, Crop Rotation, Residue Management and Biochar in a Semiarid Region in South Africa. Agronomy, 10(5), 705. https://doi.org/10.3390/agronomy10050705





