Fate of a 15N-labeled Urea Pulse in Heavily Fertilized Banana Crops
Abstract
1. Introduction
2. Materials and Methods
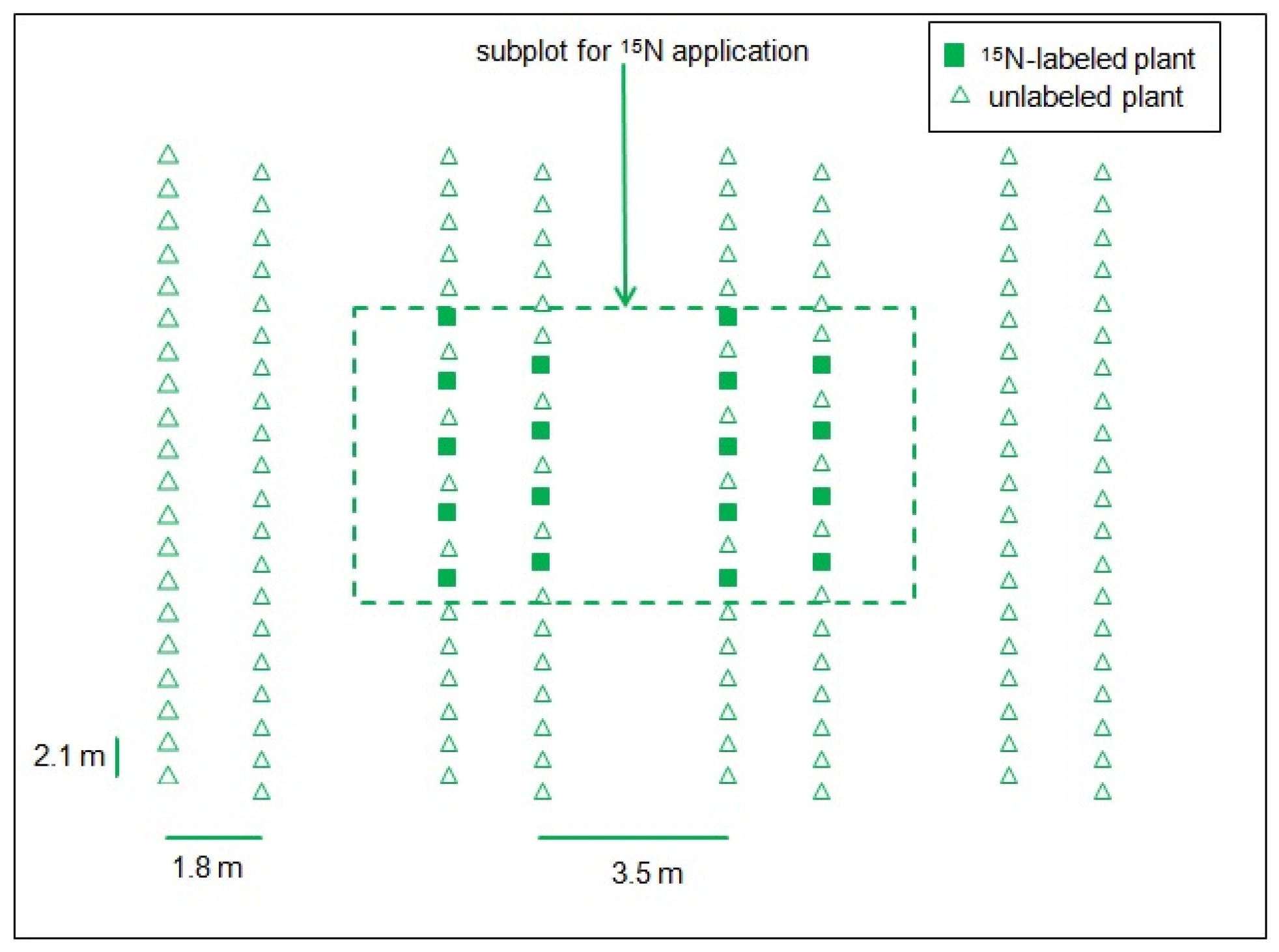
2.1. Experimental Site and Design of the Field Experiment
2.2. Plant and Soil Sampling
2.3. Calculations and Statistical Analysis
3. Results
3.1. Weather Conditions
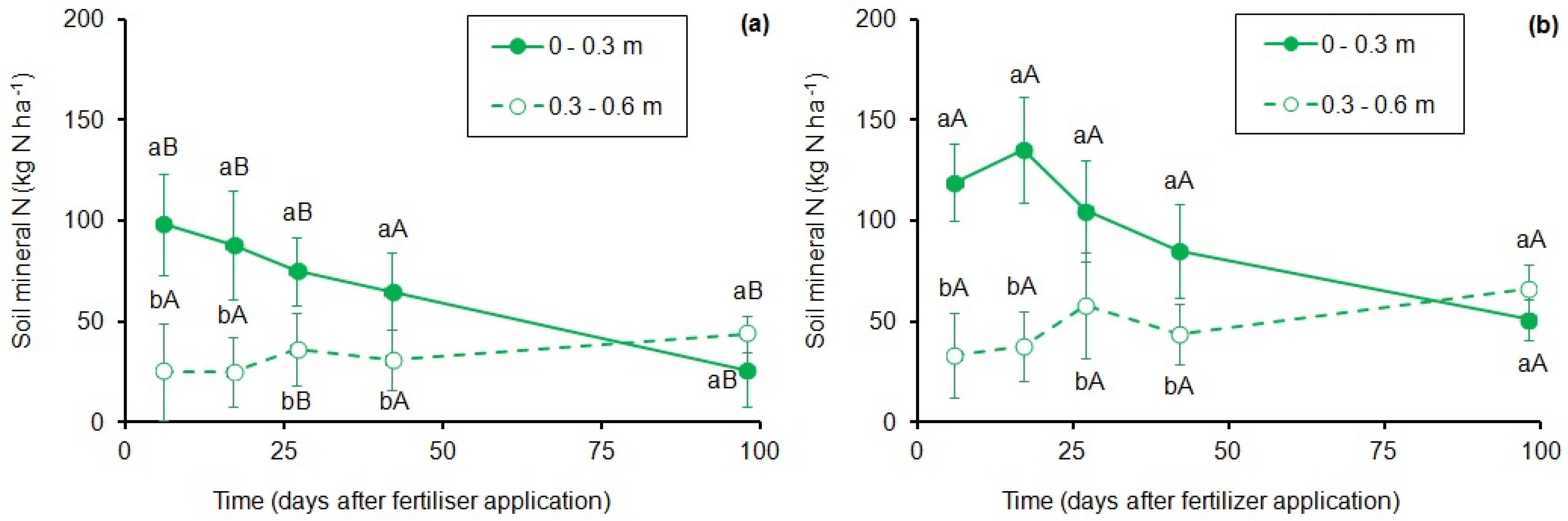
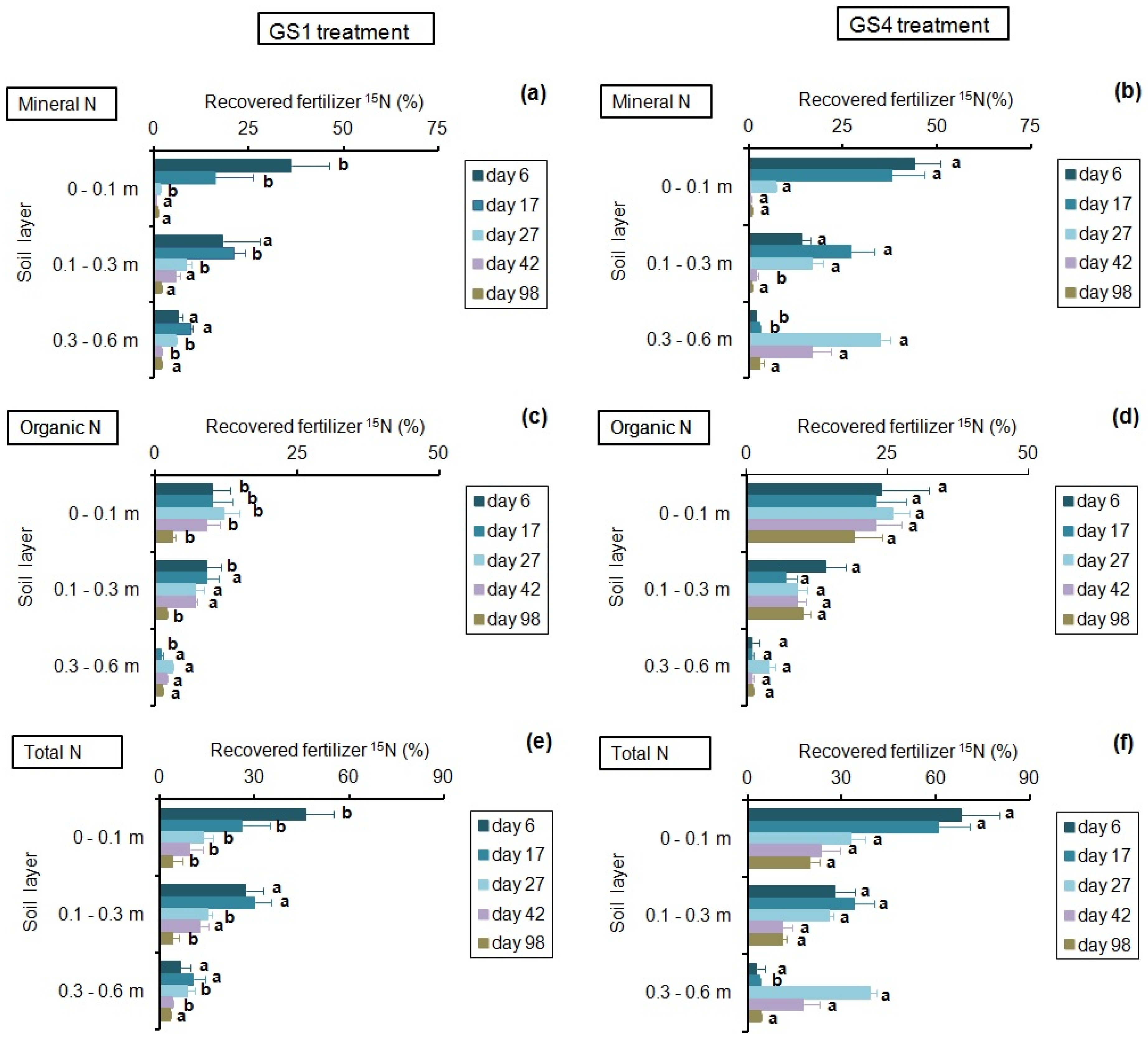
3.2. Dynamics of Soil N Fractions
3.3. Nitrogen Biomass and Fertilizer N Recovery in Plants at Harvest
4. Discussion
4.1. Fate of Fertilizer N in Plants
4.2. Factors Affecting NUE and Fertilizer N Recovery
4.2.1. Fertilizer N Dilution in the Soil
4.2.2. N Leaching
4.2.3. N Immobilization
4.3. Overview of Fertilizer N Dynamics in Banana Crops
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aryal, D.R.; Geissen, V.; Ponce-Mendoza, A.; Ramos-Reyes, R.R.; Becker, M. Water quality under intensive banana production and extensive pastureland in tropical Mexico. J. Plant Nutr. Soil Sci. 2012, 175, 553–559. [Google Scholar] [CrossRef]
- Agreste Guadeloupe La Culture de la Banane. Premiers Résultats de L’enquête Statistique Réalisée en 2016 Auprès de 128 Bananeraies en Guadeloupe. Available online: http://daaf.guadeloupe.agriculture.gouv.fr/IMG/pdf/Agreste_Banane_24052018_cle8fa1f4.pdf (accessed on 14 December 2019). (In French).
- World Banana Forum. FAO Good Agricultural Practices for Bananas. Available online: http://www.fao.org/3/a-i6917e.pdf (accessed on 31 January 2020).
- Raphael, L. Biodisponibilité de L’azote en Cultures Bananières sur Nitisol. Ph.D. Thesis, Université des Antilles, Guadeloupe, France, 2006. (In French). [Google Scholar]
- Dorel, M.; Achard, R.; Tixier, P. SIMBA-N: Modeling nitrogen dynamics in banana populations in wet tropical climate. Application to fertilization management in the Caribbean. Eur. J. Agron. 2008, 29, 38–45. [Google Scholar] [CrossRef]
- Chopin, P.; Tirolien, J.; Blazy, J.M. Ex-ante sustainability assessment of cleaner banana production systems. J. Clean. Prod. 2016, 139, 15–24. [Google Scholar] [CrossRef]
- Lassoudière, A. Le Bananier et sa Culture; Editions Quæ: Versailles, France, 2007. (In French) [Google Scholar]
- Raphael, L.; Sierra, J.; Recous, S.; Ozier-Lafontaine, H.; Desfontaines, L. Soil turnover of crop residues from the banana (Musa AAA cv. Petite-Naine) mother plant and simultaneous uptake by the daughter plant of released nitrogen. Eur. J. Agron. 2012, 38, 117–123. [Google Scholar] [CrossRef]
- Kashaija, I.N.; McIntyre, B.D.; Ssali, H.; Kizito, F. Spatial distribution of roots, nematode populations and root necrosis in highland banana in Uganda. Nematology 2004, 6, 7–12. [Google Scholar]
- Dorel, M.; Lakhia, S.; Pététin, C.; Bouamer, S.; Risède, J.M. No-till banana planting on crop residue mulch: Effect on soil quality and crop functioning. Fruits 2010, 65, 55–68. [Google Scholar] [CrossRef]
- Sansoulet, J.; Cabidoche, Y.M.; Cattan, P. Adsorption and transport of nitrate and potassium in an Andosol under banana (Guadeloupe, French West Indies). Eur. J. Soil Sci. 2007, 58, 478–489. [Google Scholar] [CrossRef]
- Perin, A.; Guerra, J.G.M.; Espindola, J.A.A.; Teixeira, M.G.; Busquet, R.N.B. Banana plant performance intercropping with perennial herbaceous legumes. Cienc. Agrotecnologia 2009, 33, 1511–1517. [Google Scholar] [CrossRef][Green Version]
- Castillo González, A.M.; Hernández Maruri, J.A.; Avitia García, E.; Pineda Pineda, J.; Valdéz Aguilar, L.A.; Corona Torres, T. Macronutrient extraction in banana “Dominico” (Musa spp.). Phyton 2011, 80, 65–72. [Google Scholar]
- de Melo, A.S.; da Silva, C.D.; Fernandes, P.D.; Sobral, L.F.; Brito, M.E.B.; Dantas, J.D.M. Alteration of the physiologic characteristics in banana under fertirrigation conditions. Cienc. Rural. 2009, 39, 733–741. [Google Scholar]
- Ripoche, A.; Achard, R.; Laurens, A.; Tixier, P. Modeling spatial partitioning of light and nitrogen resources in banana cover-cropping systems. Eur. J. Agron. 2012, 41, 81–91. [Google Scholar] [CrossRef]
- FAO FAO/Unesco Soil Map of the World. World Soil Resources Report 60; FAO: Rome, Italy, 1988. [Google Scholar]
- Fillery, I.R.P.; Recous, S. Use of enriched 15N sources to study soil N transformations. In Stable Isotope Techniques in the Study of Biological Processes and Functioning of Ecosystems; Unkovich, M., Pate, J., McNeill, A., Gibbs, D.J., Eds.; Kluwer Acadmic Publisher: Dordrecht, The Netherlands, 2001; Volume 40, pp. 167–194. [Google Scholar]
- Recous, S.; Machet, J.M. Short term immobilization and crop uptake of fertilizer N applied to winter wheat: Effect of date of application in spring. Plant Soil 1999, 206, 137–149. [Google Scholar] [CrossRef]
- Peng, W.; Zeng, Y.; Shi, Q.; Huang, S. Responses of rice yield and the fate of fertilizer nitrogen to soil organic carbon. Plant Soil Environ. 2017, 63, 416–421. [Google Scholar]
- Jiang, C.; Lu, D.; Zu, C.; Shen, J.; Wang, S.; Guo, Z.; Zhou, J.; Wang, H. One-time root-zone N fertilization increases maize yield, NUE and reduces soil N losses in lime concretion black soil. Sci. Rep. 2018, 8, 10258. [Google Scholar] [CrossRef]
- Rowe, E.C.; Van Noordwijk, M.; Suprayogo, D.; Cadisch, G. Nitrogen use efficiency of monoculture and hedgerow intercropping in the humid tropics. Plant Soil 2005, 268, 61–74. [Google Scholar] [CrossRef]
- Hgaza, V.K.; Diby, L.N.; Oberson, A.; Tschannen, A.; Tié, B.T.; Sangakkara, U.R.; Aké, S.; Frossard, E. Nitrogen use by yam as affected by mineral fertilizer application. Agron. J. 2012, 104, 1558–1568. [Google Scholar] [CrossRef]
- McSwiney, C.P.; Snapp, S.S.; Gentry, L.E. Use of N immobilization to tighten the N cycle in conventional agroecosystems. Ecol. Appl. 2010, 20, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Couto-Vázquez, A.; González-Prieto, S.J. Fate of 15N-fertilizers in the soil-plant system of a forage rotation under conservation and plough tillage. Soil Till. Res. 2016, 161, 10–18. [Google Scholar] [CrossRef]
- Cabidoche, Y.M.; Achard, R.; Cattan, P.; Clermont-Dauphin, C.; Massat, F.; Sansoulet, J. Long-term pollution by chlordecone of tropical volcanic soils in the French West Indies: A simple leaching model accounts for current residue. Environ. Pollut. 2009, 157, 1697–1705. [Google Scholar] [CrossRef]
- Sierra, J.; Fontaine, S.; Desfontaines, L. Factors controlling N mineralization, nitrification, and nitrogen losses in an Oxisol amended with sewage sludge. Soil Res. 2001, 39, 519–534. [Google Scholar] [CrossRef]
- Sierra, J.; Renault, P. Temporal pattern of oxygen concentration in a hydromorphic soil. Soil Sci. Soc. Am. J. 1998, 62, 1398–1405. [Google Scholar] [CrossRef]
- Clermont-Dauphin, C.; Cabidoche, Y.M.; Meynard, J.M. Effects of intensive mono-cropping of bananas on properties of volcanic soils in the uplands of the French West Indies. Soil Use Manag. 2004, 20, 105–113. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Quiñones, A.; Legaz, F.; Primo-Millo, E. Nitrogen-use efficiency of young citrus trees as influenced by the timing of fertilizer application. J. Plant Nutr. Soil Sci. 2012, 175, 282–292. [Google Scholar] [CrossRef]

| Treatment | Plant Organ | Dry Biomass | N Content | N Biomass |
|---|---|---|---|---|
| (Mg ha−1) | (% DM) | (kg N ha−1) | ||
| GS1 | Leaves | 2.9 cA | 1.5 aA | 43 aA |
| Stem | 4.6 bA | 0.9 bA | 39 aA | |
| Roots | 1.4 cA | 0.5 cA | 7 cA | |
| Fruit | 7.0 aB | 0.7 bA | 46 aB | |
| Sucker | 0.9 eA | 1.8 aA | 16 bA | |
| Whole plant | 16.8 B | 1.2 A | 151 B | |
| GS4 | Leaves | 3.1 cA | 1.6 aA | 49 bA |
| Stem | 4.6 bA | 0.9 bA | 43 bA | |
| Roots | 1.5 dA | 0.6 cA | 9 dA | |
| Fruit | 9.9 aA | 0.7 bA | 73 aA | |
| Sucker | 0.9 eA | 1.8 aA | 16 cA | |
| Whole plant | 20.0 A | 1.2 A | 190 A |
| Compartment | Treatment | |
|---|---|---|
| GS1 | GS4 | |
| Fertilizer 15N Recovery (%) | ||
| Plant | ||
| Leaves | 4.6 bA | 4.5 bA |
| Stem | 3.5 cA | 3.2 cA |
| Roots | 0.8 eA | 0.8 eA |
| Fruit | 14.2 aA | 13.0 aA |
| Sucker | 2.5 dA | 2.0 dA |
| Total (NUE) | 25.6 A | 23.5 A |
| Soil | ||
| 0–0.3 m | 8.0 aB | 31.1 aA |
| 0.3–1.2 m | 6.2 aA | 7.1 bA |
| Total | 14.2 B | 38.2 A |
| Plant + Soil | 39.8 B | 61.7 A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raphael, L.; Recous, S.; Ozier-Lafontaine, H.; Sierra, J. Fate of a 15N-labeled Urea Pulse in Heavily Fertilized Banana Crops. Agronomy 2020, 10, 666. https://doi.org/10.3390/agronomy10050666
Raphael L, Recous S, Ozier-Lafontaine H, Sierra J. Fate of a 15N-labeled Urea Pulse in Heavily Fertilized Banana Crops. Agronomy. 2020; 10(5):666. https://doi.org/10.3390/agronomy10050666
Chicago/Turabian StyleRaphael, Line, Sylvie Recous, Harry Ozier-Lafontaine, and Jorge Sierra. 2020. "Fate of a 15N-labeled Urea Pulse in Heavily Fertilized Banana Crops" Agronomy 10, no. 5: 666. https://doi.org/10.3390/agronomy10050666
APA StyleRaphael, L., Recous, S., Ozier-Lafontaine, H., & Sierra, J. (2020). Fate of a 15N-labeled Urea Pulse in Heavily Fertilized Banana Crops. Agronomy, 10(5), 666. https://doi.org/10.3390/agronomy10050666





