The Effect of Soil Volume Availability on Opuntia ficus-indica Canopy and Root Growth
Abstract
1. Introduction
2. Materials and Methods
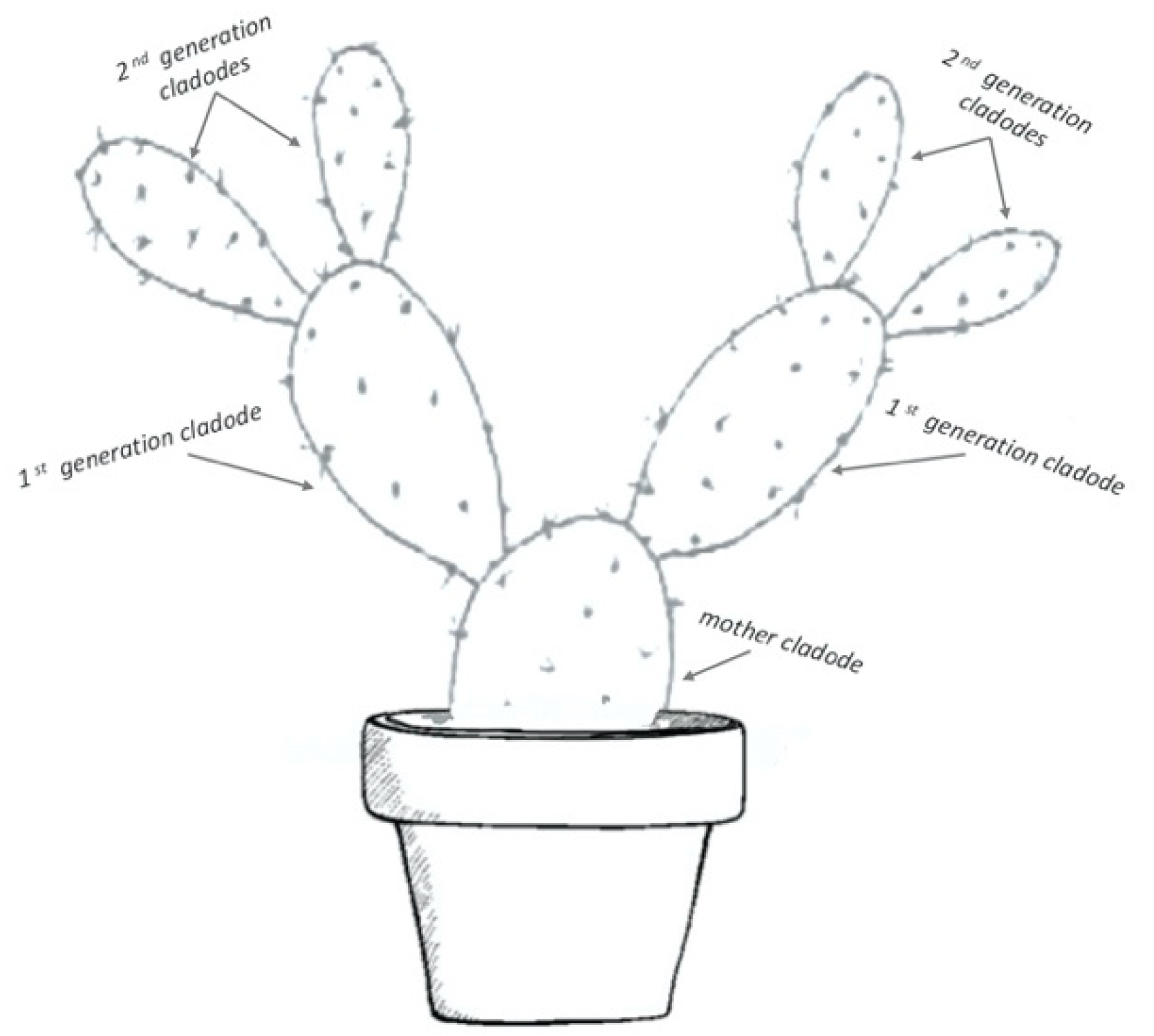
2.1. Cultivation of Opuntia ficus-indica Cladodes and Experimental Design
2.2. Canopy Measurements
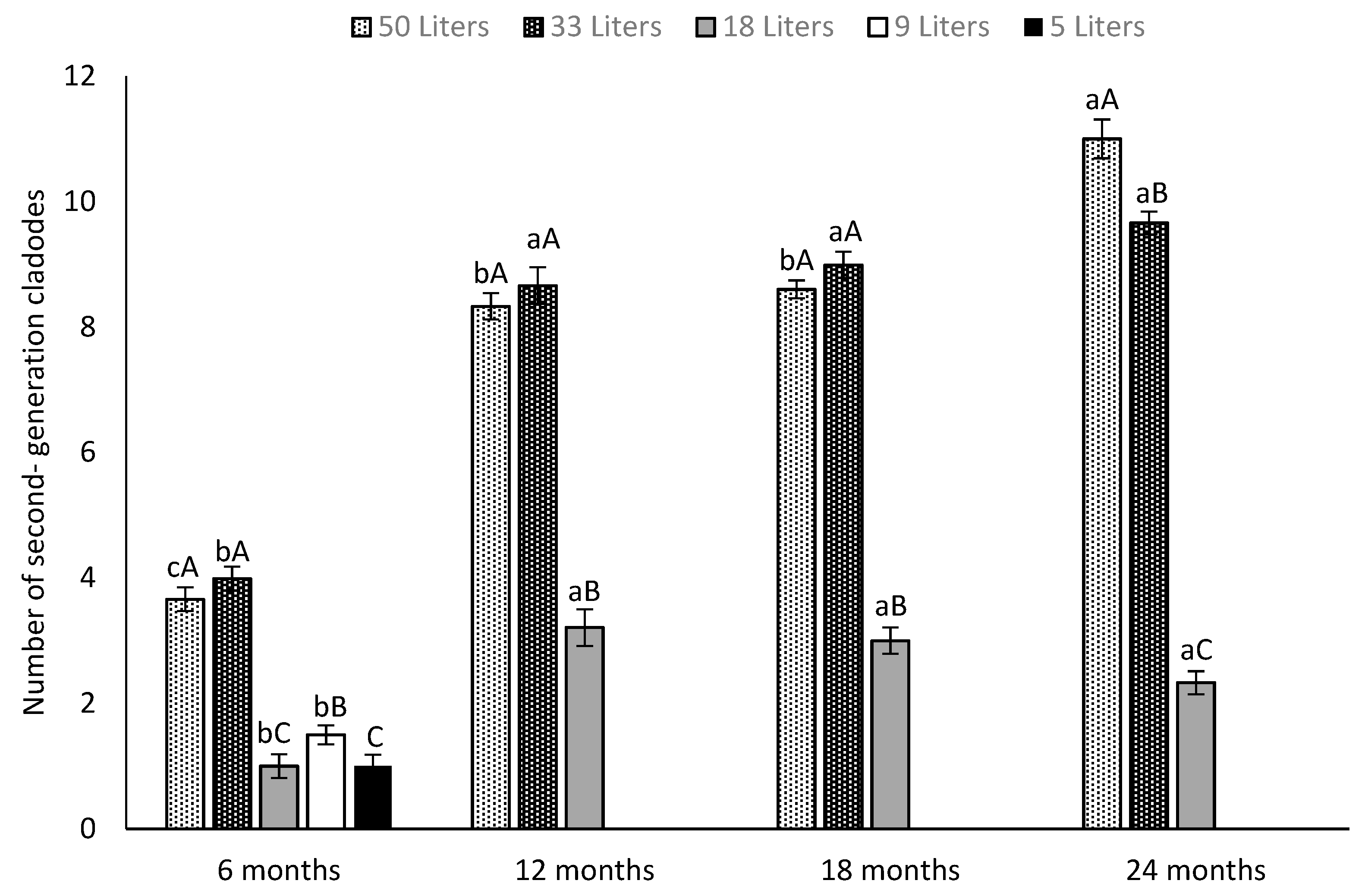
2.2.1. Number of Cladodes Per Plant
2.2.2. Cladodes’ Surface Area (cm2)
2.2.3. Cladode Measurements: Fresh and Dry Weight, Thickness
2.2.4. Starch Content Analysis
2.3. Root Measurements
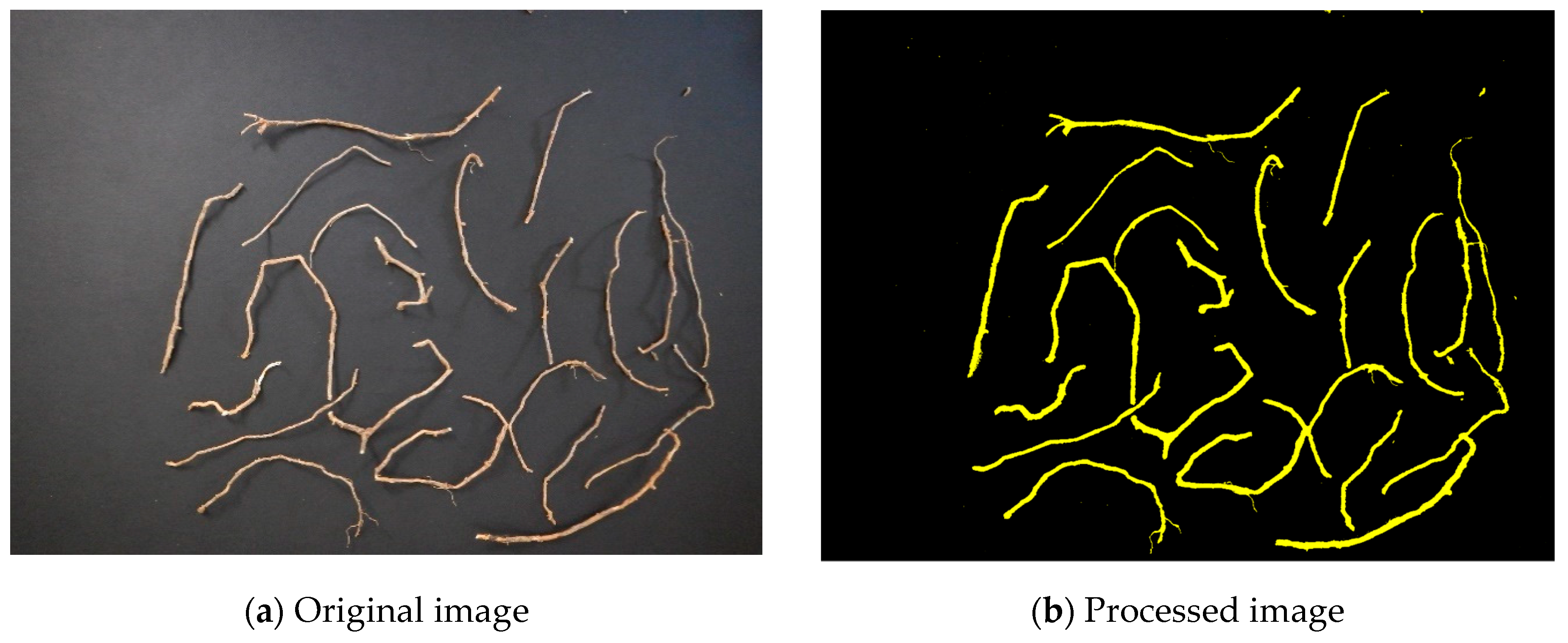
2.3.1. Root Surface Area
2.3.2. Root Volume
2.4. Statistical Analysis
3. Results
3.1. Canopy Growth and Surface Area
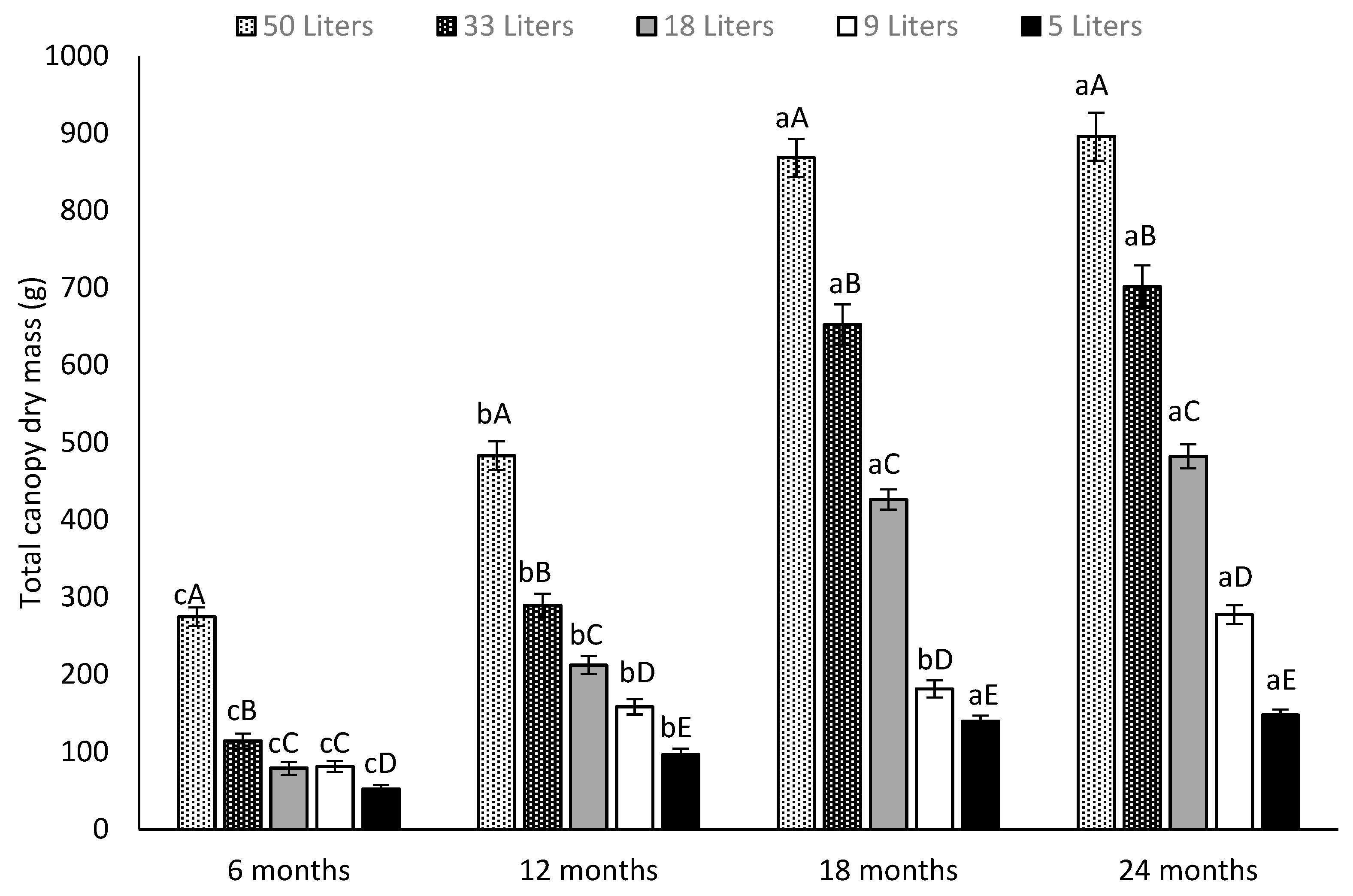
3.2. Canopy Dry Mass
3.3. Roots Measurements
3.3.1. Root Surface Area
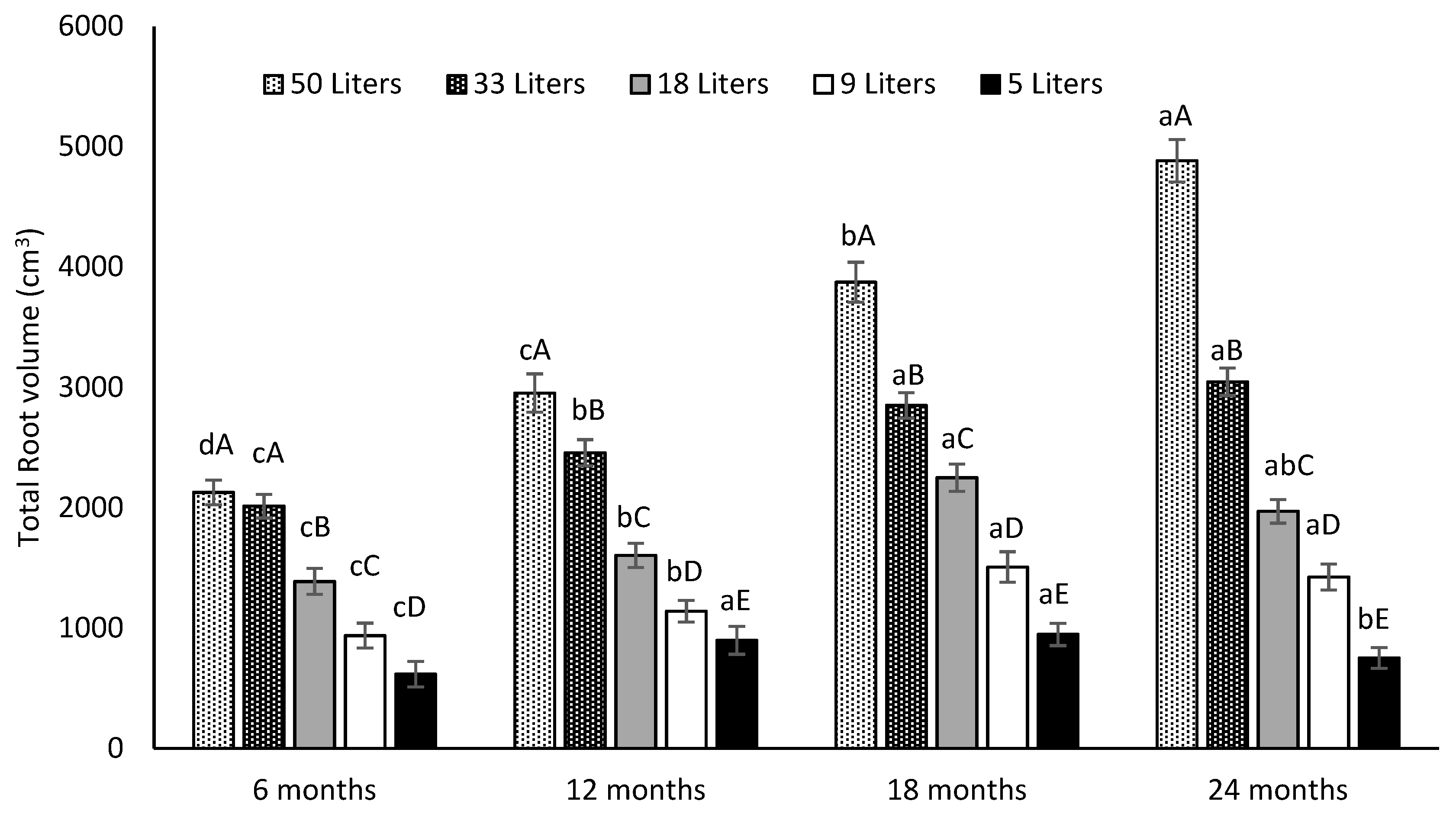
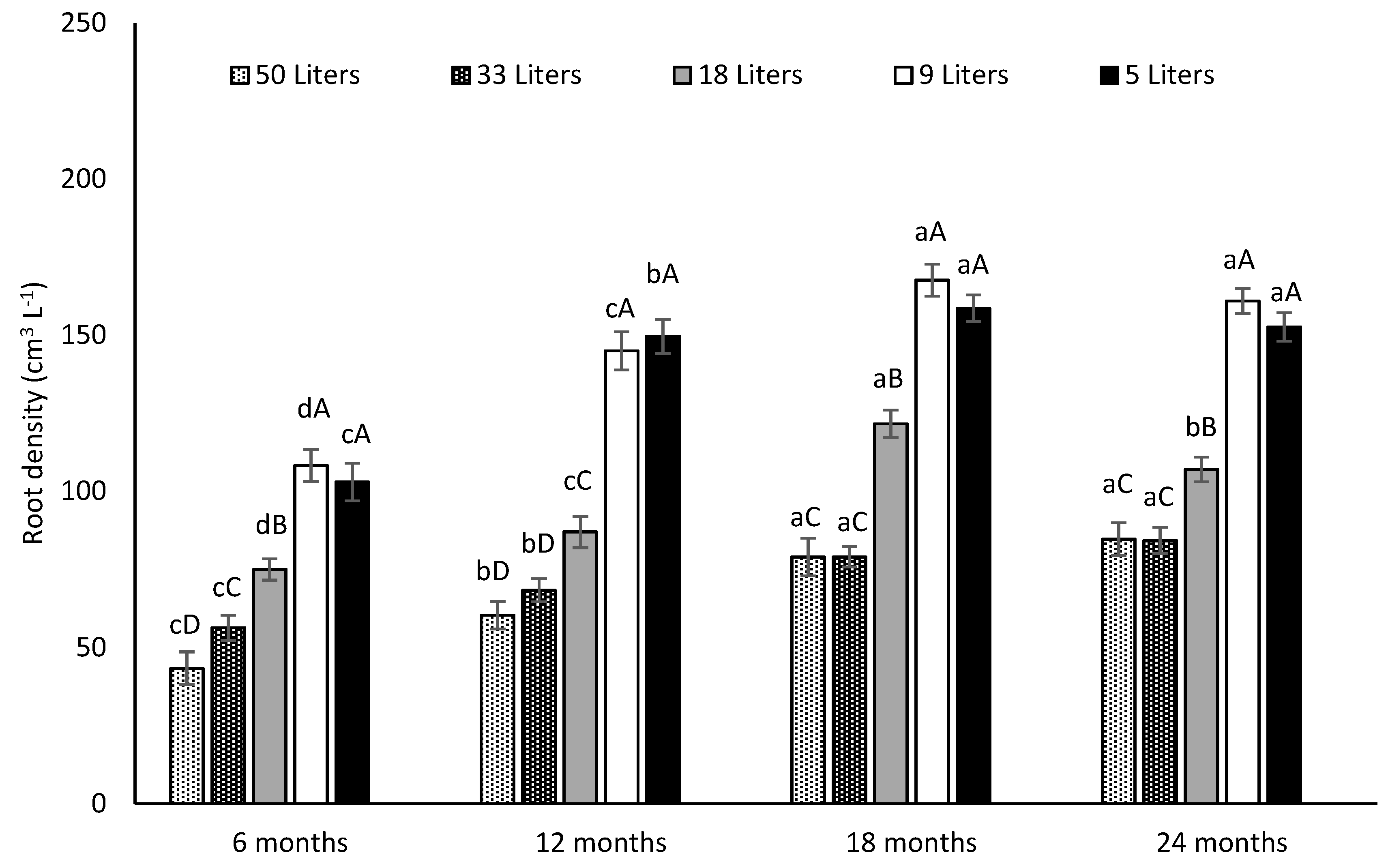
3.3.2. Total Root Volume and Root Density
3.4. Root/Canopy Ratios
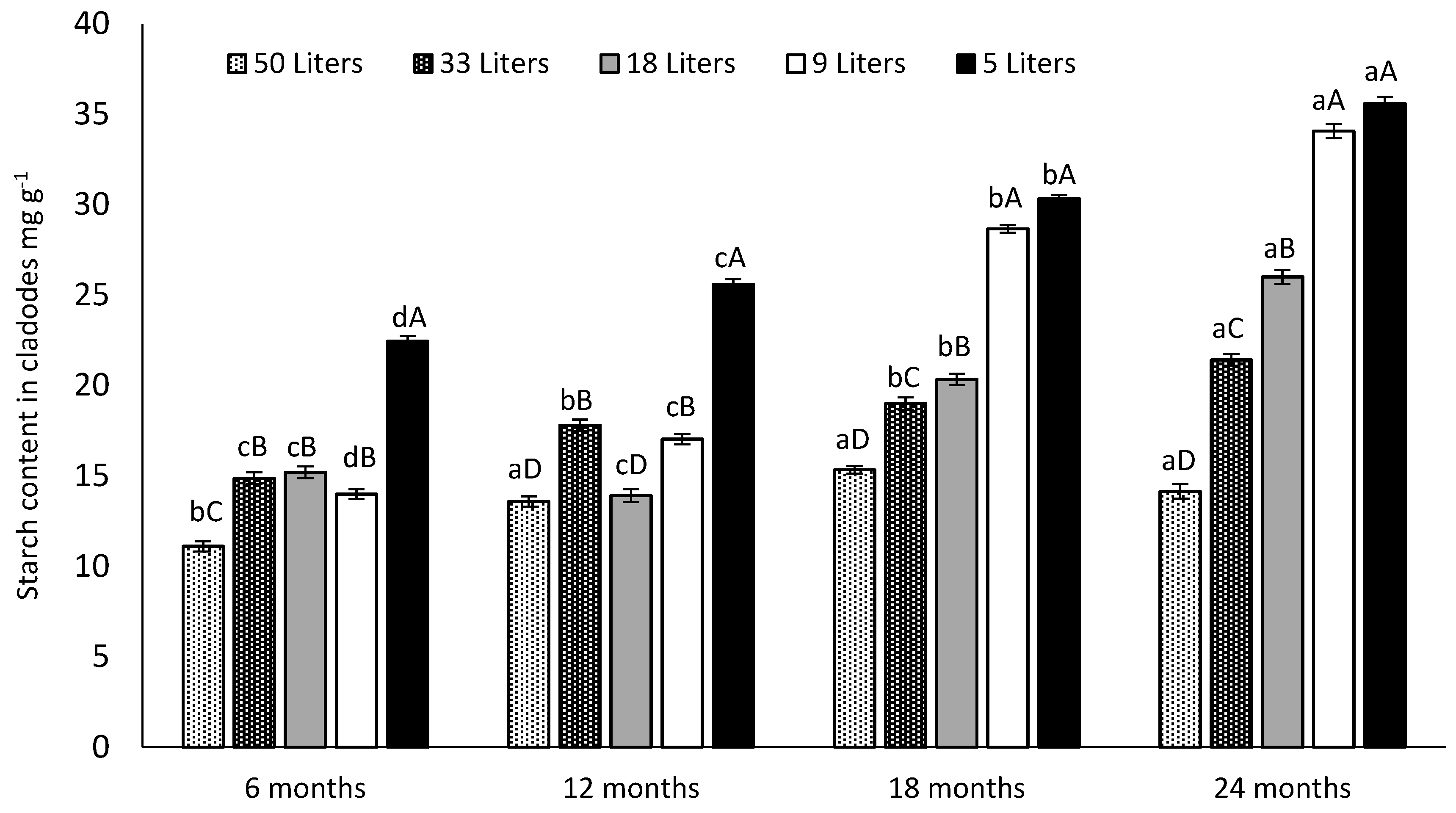
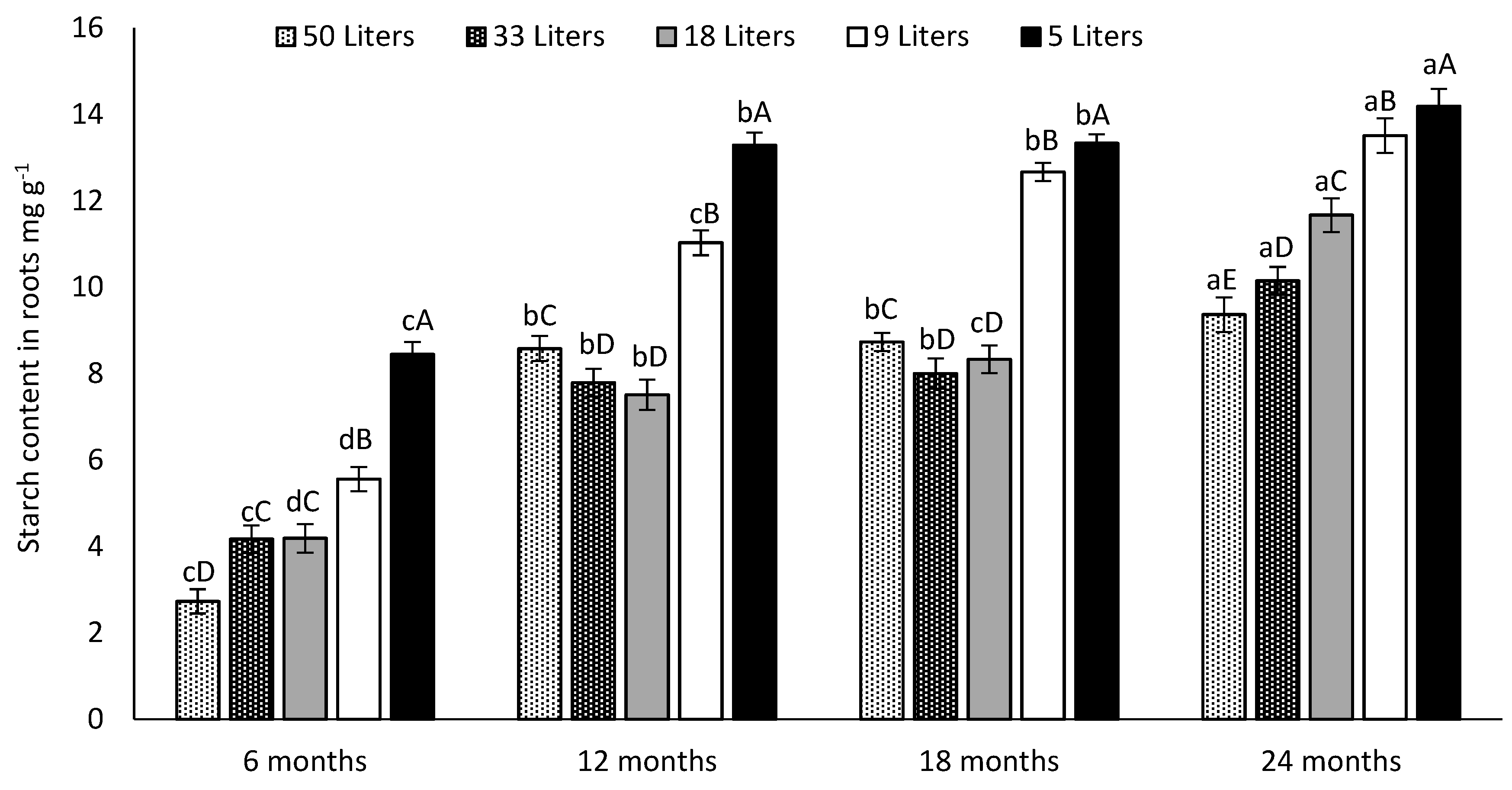
3.5. Canopy and Cladodes Starch Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faust, M. Physiology of Temperate Zone Fruit Trees; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Brouwer, R. Functional equilibrium: Sense or nonsense? Neth. J. Agri. Sci. 1983, 31, 335–348. [Google Scholar]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource limitation in plants–An economic analogy. Annu. Rev. Ecol. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Maib, K.M.; Andrews, P.K.; Lang, G.A.; Mullinix, K. (Eds.) Tree Fruit Physiology: Growth and Development; Good Fruit Grower: Yakima, WA, USA, 1996; pp. 69–80. [Google Scholar]
- Fernandez, J.E.; Moreno, F.; Martin-Aranda, J.; Fereres, E. Olive-tree root dynamics under different soil water regimes. Agric. Mediterr. 1992, 122, 225–235. [Google Scholar]
- Bravdo, B.A.; Levin, I.; Assaf, R. Control of root size and root environment of fruit trees for optimal fruit production. J. Plant Nutr. 1992, 15, 699–712. [Google Scholar] [CrossRef]
- Myers, S.C. Root restriction of apple and peach with in-ground fabric containers. Acta Hort. 1992, 322, 215–219. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature (IUCN). The Nature of Drylands: Diverse Ecosystems, Diverse Solutions; Eastern and Southern Africa Regional Office: Nairobi, Kenya, 2008. [Google Scholar]
- Ferrol, N.; Calvente, R.; Cano, C.; Barea, T.M.; Azcon-Aguilar, C. Analysing arbuscular mycorrhizal fungal diversity in shrub-associated resource islands from a desertification threatened semiarid Mediterranean ecosystem. Appl. Soil Ecol. 2004, 25, 123–133. [Google Scholar] [CrossRef]
- Le Houérou, H.N. The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. J. Arid Environ. 1996, 33, 135–159. [Google Scholar] [CrossRef]
- Pimienta-Barrios, E.; Muñoz-Urias, A.; González del Castillo-Aranda, M.E.; Nobel, P.S. Effects of benomyl and drought on the mycorrhizal development and daily net CO2 uptake of a wild plats Opuntia in a rocky semiarid environment. Ann. Bot. 2003, 92, 1–7. [Google Scholar] [CrossRef]
- Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; Hernandez, M.H. Systematic notes and detailed description of Opuntia ficus-indica (L.) MILL. (Cactacea). Agrociencia 2005, 39, 395–408. [Google Scholar]
- Hegwood, D.A. Human health discoveries with Opuntia sp. (Prickly pear). Sci. Hortic. 1990, 25, 1515–1516. [Google Scholar] [CrossRef]
- Snyman, H.A. Effect of various water applications on root development of Opuntia ficus-indica and O. robusta under greenhouse growth conditions. J. Prof. Assoc. Cactus Dev. 2004, 6, 35–61. [Google Scholar]
- Inglese, P.; Inglese, G.; Liguori, G. Fruit productivity and carbon gain of Opuntia ficus-indica (L.) Mill. trees. Isr. J. Plant Sci. 2012, 60, 283–290. [Google Scholar]
- Nobel, P.S.; Huang, B. Hydraulic and structural changes for lateral roots of two desert succulents in response to soil drying and rewetting. Int. J. Plant Sci. 1992, 153, S163–S170. [Google Scholar] [CrossRef]
- Inglese, P.; Pace, L.S. Root confinement affects canopy growth, dry matter partitioning, carbon assimilation and field behavior of Opuntia ficus-indica potted plants. Acta Hort. 2000, 516, 97–105. [Google Scholar] [CrossRef]
- Liguori, G.; Inglese, G.; Pernice, F.; Sortino, G.; Inglese, P. CO2 uptake of Opuntia ficus-indica (L.) Mill. whole trees and single cladodes, in relation to plant water status and cladode age. Ital. J. Agron. 2013, 8, e3. [Google Scholar] [CrossRef]
- Hassan, S.; Inglese, P.; Gristina, L.; Liguori, G.; Novara, A.; Louhaichi, M.; Sortino, G. Root growth and soil carbon turnover in Opuntia ficus-indica as affected by soil volume availability. Eur. J. Agron. 2019, 105, 104–110. [Google Scholar] [CrossRef]
- Hassid, W.; Neufeld, E. Quantitative determination of starch in plant tissue. In Methods in Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1964; Volume 4, pp. 33–36. [Google Scholar]
- Hoffpauir, C. Report on starch in plants. J. Assoc. Off. Anal. Chem. 1959, 39, 423–442. [Google Scholar]
- McCready, R.; Guggolz, J.; Silviera, V.; Owens, H. Determination of starch and amylose in vegetables, application to peas. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Viles, F.; Silverman, L. Determination of starch and cellulose with anthrone. Anal. Chem. 1949, 21, 950–953. [Google Scholar] [CrossRef]
- Louhaichi, M.; Johnson, M.D.; Woerz, A.L.; Jasra, A.W.; Johnson, D.E. Digital charting technique for monitoring rangeland vegetation cover at local scale. Int. J. Agric. Biol. 2010, 12, 406–410. [Google Scholar]
- Louhaichi, M.; Clifton, K.; Hassan, S.; Johnson, D.E. A reliable and non-destructive method for estimating forage shrub cover and biomass in arid environments using digital vegetation charting technique. Agrofor. Syst. 2018, 92, 1341–1352. [Google Scholar] [CrossRef]
- Louhaichi, M.; Hassan, S.; Johnson, D.E. VegMeasure. Volume 2: Image Processing Manual; ICARDA: Amman, Jordan, 2018; p. 28. [Google Scholar]
- Congalton, R.G. A review of assessing the accuracy of classification of remotely sensed data. Remote. Sens Environ. 1991, 37, 35–46. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839. [Google Scholar] [CrossRef]
- Nobel, P.S. Environmental Biology of Agaves and Cacti; Cambridge University Press: Cambidge, NY, USA, 1988; p. 270. [Google Scholar]
- Potgieter, J.; D’Aquino, S. Fruit production and psot-harvest management. In Crop Ecology, Cultivation and Uses of Cactus Pear; Inglese, P., Ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; pp. 51–72. [Google Scholar]
- Inglese, P.; Barbera, G.; La Mantia, T. Seasonal reproductive and vegetative growth patterns and resource al-location during cactus pear Opuntia ficus-indica (L.) Mill. Fruit growth. HortScience 1999, 34, 69–72. [Google Scholar] [CrossRef]
- Hess, L.; De Kroon, H. Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self-discrimination. J. Ecol. 2007, 95, 241–251. [Google Scholar] [CrossRef]
- Ronchi, C.P.; DaMatta, F.M.; Batista, K.D.; Moraes, G.; Loureiro, M.E.; Ducatti, C. Growth and photosynthetic down-regulation in Coffea arabica in response to restricted root volume. Funct. Plant Biol. 2006, 33, 1013–1023. [Google Scholar] [CrossRef]
- Zhu, L.N.; Wang, S.P.; Yang, T.Y.; Zhang, C.X.; Xu, W.P. Vine growth and nitrogen metabolism of ‘Fujiminori’ grapevines in response to root restriction. Sci. Hortic. 2006, 107, 143–149. [Google Scholar] [CrossRef]
- Dichio, B.; Romano, M.; Nuzzo, V.; Xiloyannis, C. Soil water availability and relationship between canopy and roots in young olive trees (cv Coratina). Acta Hort. 2002, 586, 255–258. [Google Scholar] [CrossRef]
- Endean, F.; Carlson, L. The effect of rooting volume on the early growth of lodgepole pine seedlings. Can. J. For. Res. 1975, 5, 55–60. [Google Scholar] [CrossRef]
- Majdi, H. Changes in fine root production and longevity in relation to water and nutrient availability in a Norway spruce stand in northern Sweden. Tree Physiol. 2001, 21, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, K.; Truu, J.; Truu, M.; Kaar, E.; Ostonen, I.; Alama, S. Black alder as a perspective deciduous species for reclaiming of oil shale mining areas. In Brownfields III Prevention, Assessment, Rehabilitation and Development of Brownfield Sites; Brebbia, C.A., Mander, U., Eds.; WIT Press: Southampton, UK, 2006; pp. 87–97. [Google Scholar]
- Snyman, H.A. Effect of water stress on root growth of Opuntia ficus-indica and O. robusta. Proc. S. Afr. J. Anim. Sci. 2004, 34, 101–103. [Google Scholar]
- Snyman, H.A. A case study on in situ rooting profiles and water-use efficiency of cactus pears, Opuntia ficus-indica and O. robusta. J. Prof. Assoc. Cactus Dev. 2005, 7, 1–21. [Google Scholar]
- Wang, C.Q. Growth rule of year-on and year-off apple trees and recommended cultivation practices. J. Shanxi Agric. Sci. 2005, 33, 35–39. [Google Scholar]
- Wang, L.Q.; Wei, Q.P.; Tang, F.; Shu, H.R. Annual dynamic pattern of new roots of apple trees. J. Shandong Agric. Univ. 1997, 28, 8–14. [Google Scholar]
- Nobel, P.S.; Cui, M.Y.; Miller, P.M.; Luo, Y.Q. Influences of soil volume and an elevated CO2 level on growth and CO2 exchange for exchange for the crassulacean acid metabolism plant Opuntia ficus indica. Physiol. Plant. 1994, 90, 173–180. [Google Scholar] [CrossRef]
- Bauhus, J.; Messier, C. Soil exploitation strategies of fine roots in different tree species of the southern boreal forest of eastern Canada. Can. J. For. Res. 1999, 29, 260–273. [Google Scholar] [CrossRef]
- Rewald, B.; Ephrath, J.E.; Rachmilevitch, S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell Environ. 2011, 34, 33–42. [Google Scholar] [CrossRef]

| 6 Months | 12 Months | 18 Months | 24 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| fine root surface area (<2 mm) | ||||||||||||
| 50 L | 2043.95 cA | ± | 27.80 | 2766.48 bA | ± | 27.41 | 2942.97 aA | ± | 43.02 | 2995.08 aA | ± | 51.89 |
| 33 L | 2049.02 bA | ± | 35.80 | 2587.38 aB | ± | 33-30 | 1991.56 bB | ± | 39.21 | 2082.59 bB | ± | 43.03 |
| 18 L | 1354.66 bB | ± | 47.89 | 1706.53 bC | ± | 50.59 | 1899.62 aC | ± | 21.17 | 1285.41 cC | ± | 31.06 |
| 9 L | 1263.04 bC | ± | 36.90 | 1495.34 aD | ± | 47.42 | 1081.74 bD | ± | 40.07 | 876.25 cD | ± | 21.09 |
| 5 L | 522.17 cD | ± | 41.92 | 1024.29 aE | ± | 41.96 | 856.02 bE | ± | 18.01 | 524.11 cE | ± | 15.32 |
| medium root surface area (2–5 mm) | ||||||||||||
| 50 L | 528.34 dA | ± | 34.54 | 696.74 cA | ± | 35.60 | 1377.36 bA | ± | 20.43 | 1730.44 aA | ± | 25.01 |
| 33 L | 516.74 cA | ± | 21.35 | 545.94 cB | ± | 30.22 | 1072.1 aB | ± | 18.87 | 957.86 bB | ± | 18.03 |
| 18 L | 380.65 bB | ± | 40.06 | 371.34 bC | ± | 39.67 | 768.67 aC | ± | 31.08 | 747.14 aC | ± | 21.05 |
| 9 L | 325.21 dB | ± | 15.71 | 370.32 cC | ± | 18.21 | 679.49 aD | ± | 25.01 | 543.87 bD | ± | 32.08 |
| 5 L | 271.24 cC | ± | 14.40 | 237.01 dD | ± | 8.43 | 401.81 aE | ± | 40.13 | 316.31 bE | ± | 17.22 |
| large root surface area (>5 mm) | ||||||||||||
| 50 L | 98.38 dA | ± | 10.73 | 141.60 cA | ± | 4.01 | 311.71 bA | ± | 10.23 | 913.03 aA | ± | 31.08 |
| 33 L | 61.70 dB | ± | 5.80 | 117.69 cB | ± | 7.23 | 317.22 bA | ± | 24.01 | 718.10 aB | ± | 20.01 |
| 18 L | 52.50 cB | ± | 6.21 | 62.52 cC | ± | 5.07 | 184.81 bB | ± | 18.05 | 388.93 aC | ± | 11.04 |
| 9 L | 33.97 dC | ± | 4.72 | 49.65 cD | ± | 6.12 | 83.67 bC | ± | 4.21 | 238.14 aD | ± | 19.01 |
| 5 L | 29.90 cC | ± | 3.51 | 25.69 cE | ± | 2.89 | 54.25 bD | ± | 6.04 | 131.92 aE | ± | 7.02 |
| total root surface area | ||||||||||||
| 50 L | 2670.67 dA | ± | 32.09 | 3604.82 cA | ± | 34.21 | 4632.03 bA | ± | 42.04 | 5638.55 aA | ± | 61.03 |
| 33 L | 2627.46 cA | ± | 44.62 | 3251.01 bB | ± | 43.26 | 3380.88 bB | ± | 50.23 | 3758.55 aB | ± | 48.07 |
| 18 L | 1787.81 cB | ± | 47.21 | 2140.39 cC | ± | 51.03 | 2853.12 aB | ± | 28.71 | 2421.48 bC | ± | 33.01 |
| 9 L | 1622.22 bC | ± | 38.21 | 1915.31 aD | ± | 35.16 | 1844.90 aC | ± | 42.05 | 1658.26 bD | ± | 26.05 |
| 5 L | 823.31 cD | ± | 25.04 | 1286.99 aE | ± | 28.08 | 1312.08 aD | ± | 35.01 | 972.32 bE | ± | 35.02 |
| 6 Months | 12 Months | 18 Months | 24 Months | |
|---|---|---|---|---|
| 50 Liters | 0.28 ± 0.03 aD | 0.22 ± 0.03 aC | 0.18 ± 0.02 bA | 0.19 ± 0.02 abA |
| 33 Liters | 0.74 ± 0.05 aA | 0.32 ± 0.05 bA | 0.18 ± 0.02 cA | 0.16 ± 0.02 cA |
| 18 Liters | 0.53 ± 0.06 aB | 0.28 ± 0.03 bAb | 0.17 ± 0.06 cA | 0.15 ± 0.03 cA |
| 9 Liters | 0.42 ± 0.04 aC | 0.26 ± 0.02 bB | 0.19 ± 0.05 cA | 0.17 ± 0.02 cA |
| 5 Liters | 0.45 ± 0.03 aC | 0.27 ± 0.03 bB | 0.17 ± 0.03 cA | 0.17 ± 0.05 cA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, S.; Liguori, G.; Inglese, P.; Louhaichi, M.; Sortino, G. The Effect of Soil Volume Availability on Opuntia ficus-indica Canopy and Root Growth. Agronomy 2020, 10, 635. https://doi.org/10.3390/agronomy10050635
Hassan S, Liguori G, Inglese P, Louhaichi M, Sortino G. The Effect of Soil Volume Availability on Opuntia ficus-indica Canopy and Root Growth. Agronomy. 2020; 10(5):635. https://doi.org/10.3390/agronomy10050635
Chicago/Turabian StyleHassan, Sawsan, Giorgia Liguori, Paolo Inglese, Mounir Louhaichi, and Giuseppe Sortino. 2020. "The Effect of Soil Volume Availability on Opuntia ficus-indica Canopy and Root Growth" Agronomy 10, no. 5: 635. https://doi.org/10.3390/agronomy10050635
APA StyleHassan, S., Liguori, G., Inglese, P., Louhaichi, M., & Sortino, G. (2020). The Effect of Soil Volume Availability on Opuntia ficus-indica Canopy and Root Growth. Agronomy, 10(5), 635. https://doi.org/10.3390/agronomy10050635









