Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Winemaking Conditions
2.2. Commercial Multiflora Bee Pollen, Yeast (Saccharomyces cerevisiae) and Lactic Acid Bacteria (Oenococcus oeni)
2.3. Analytical Measurements
2.4. Statistics Analysis
3. Results and Discussion
3.1. Effects of Bee Pollen on the Physicochemical Properties of Tintilla de Rota Red Grape Must
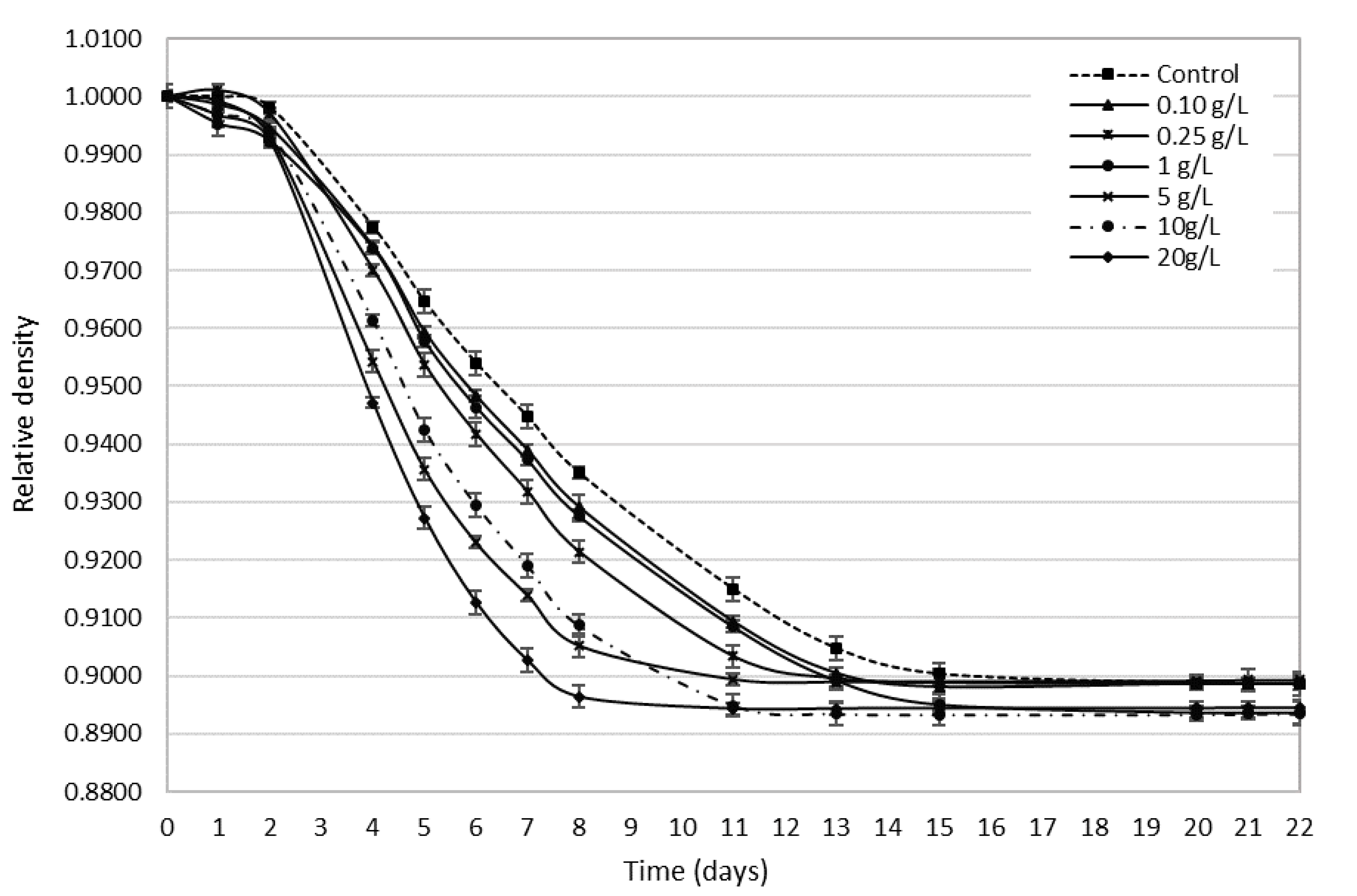
3.2. Bee Pollen’s Influence on Alcohol Fermentation Kinetics
3.3. Bee Pollen Influence on Yeast Growth Kinetics of Alcoholic Fermentation
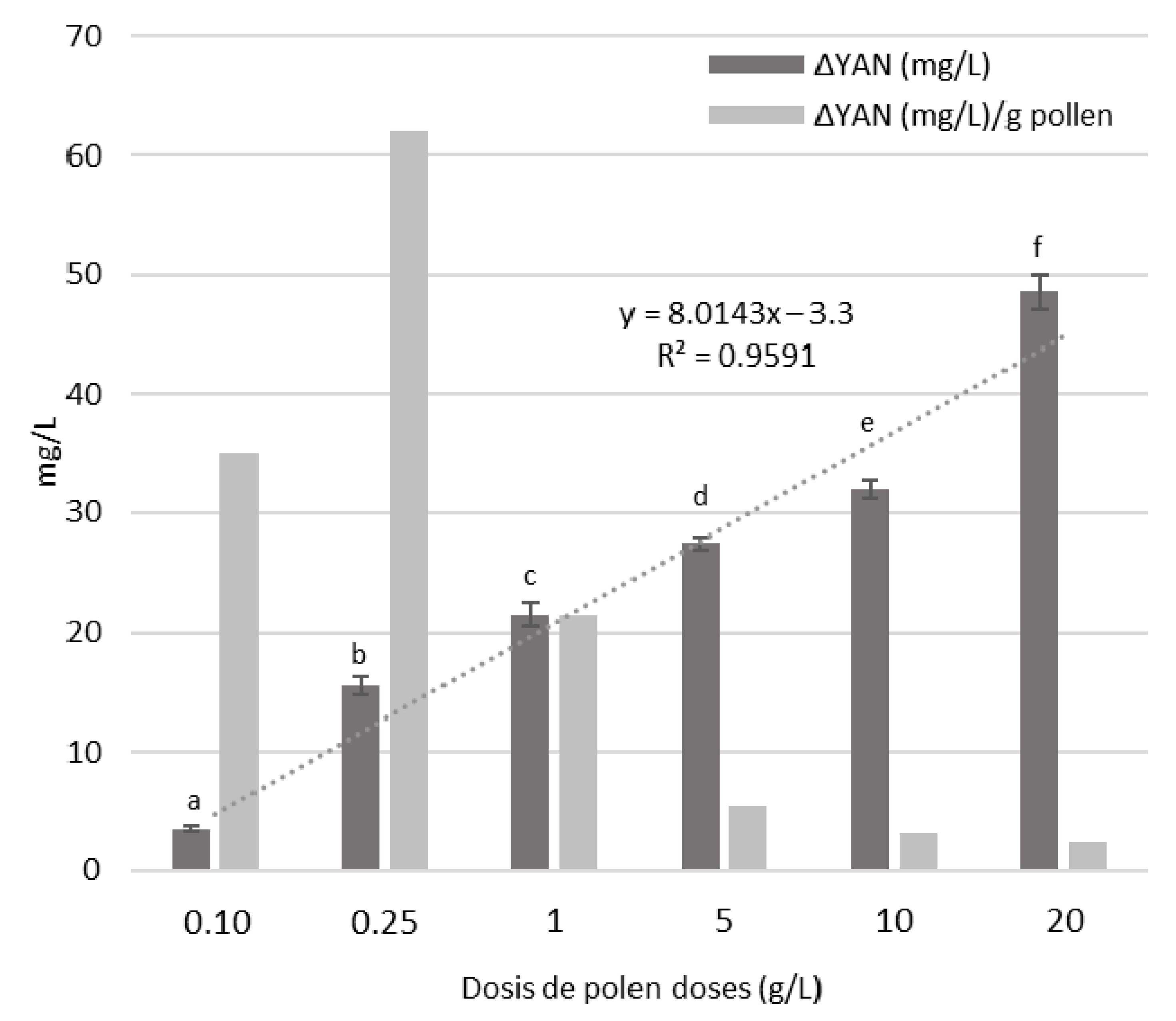
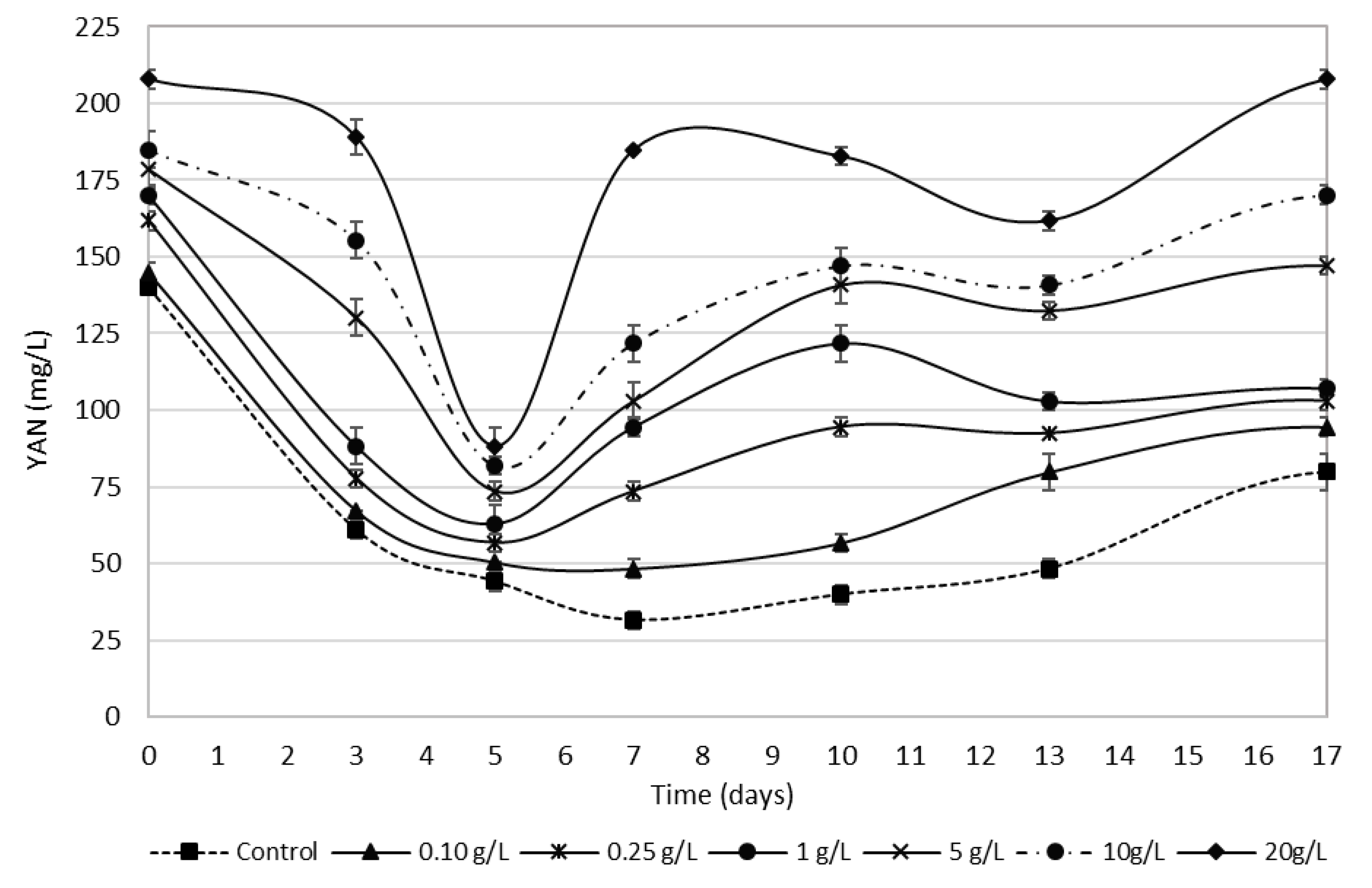
3.4. Bee Pollen Influence on YAN Evolution during Alcoholic Fermentation
3.5. Influence of Bee Pollen Dose on Malic Acid Content during Malolactic Fermentation
3.6. Evaluation of the Pollen Dose on the Physicochemical Properties and Color Parameters of the Final Wines.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suárez-Lepe, J.A. Levaduras Vínicas. Funcionalidad y Uso en Bodega; Madrid Mundi-Prensa: Madrid, Spain, 1997. [Google Scholar]
- Fleet, G.H. Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Webb, L.B.; Whetton, P.H.; Barlow, E.W.R. Modelled impact of future climate change on the phenology of winegrapes in Australia. Aust. J. Grape Wine Res. 2007, 13, 165–175. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Briche, E.; Beltrando, G.; Somot, S.; Quénol, H. Critical analysis of simulated daily temperature data from the ARPEGE-climate model: Application to climate change in the Champagne wine-producing region. Clim. Chang. 2014, 123, 241–254. [Google Scholar] [CrossRef]
- Sacchelli, S.; Fabbrizzi, S.; Menghini, S. Climate change effects and adaptation strategies in the wine sector: A quantitative literature review. Wine Econ. Policy 2016, 5, 114–126. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Ough, C.; Davenport, M.; Joseph, K. Effects of Certain Vitamins on Growth and Fermentation Rate of Several Commercial Active Dry Wine Yeasts. Am. J. Enol. Vitic. 1989, 40, 208–213. [Google Scholar]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of alcoholic fermentation kinetics: Its variability and significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Bataillon, M.; Rico, A.; Sablayrolles, J.M.; Salmon, J.M.; Barre, P. Early thiamin assimilation by yeasts under enological conditions: Impact on alcoholic fermentation kinetics. J. Ferment. Bioeng. 1996, 82, 145–150. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effects of Sugar Concentration Processes in Grapes and Wine Aging on Aroma Compounds of Sweet Wines—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1051–1071. [Google Scholar] [CrossRef]
- Mira de Orduña Heidinger, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 7, 1844–1855. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Henschke, P.A.; Jiranek, V. Yeast: Metabolism of nitrogen compounds. In Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993; pp. 77–164. [Google Scholar]
- Bely, M.; Sablayrolles, J.-M.; Barre, P. Automatic detection of assimilable nitogen deficiencies during alcoholic fermentation in enological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Barre, P.; Blondin, P.; Dequin, S.; Feuillat, M.; Sablayrolles, J.-M.; Salmon, J.-M. La levure de fermentation alcoolique. In Oenologie: Fondements Scientifiques et Technologiques; TEC and DOC Lavoisier: Paris, France, 1998; pp. 414–495. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A.; Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A. Tratado de Enologia: Microbiología del Vino. Vinificaciones; Mundi Prensa: Madrid, Spain, 2003. [Google Scholar]
- Bell, S.-J.J.; Henschke, P. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G.; Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. Dev. Food Sci. 1995, 37, 1659–1694. [Google Scholar]
- Coldea, T.E.; Mudura, E.; Rotar, A.M.; Rodica, C.; Salanță, L.; Cornea, A. Chemical composition, total phenolic content and colour of red wine obtained from grapes autochthonous to Romania. J. Agroaliment. Process. Technol. 2015, 21, 181–185. [Google Scholar]
- Paul Schreiner, R.; Osborne, J.; Skinkis, P.A. Nitrogen requirements of pinot noir based on growth parameters, must composition, and fermentation behavior. Am. J. Enol. Vitic. 2018, 69, 45–58. [Google Scholar] [CrossRef]
- Stines, A.P.; Grubb, J.; Gockowiak, H.; Henschke, P.A.; Høj, P.B.; Heeswijck, R. Van Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: Influence of vine cultivar, berry maturity and tissue type. Aust. J. Grape Wine Res. 2000, 6, 150–158. [Google Scholar] [CrossRef]
- Spayd, S.E.; Wample, R.L.; Evans, R.G.; Stevens, R.G.; Seymour, B.J.; Nagel, C.W. Nitrogen fertilization of white Riesling grapes in Washington. Must and wine composition. Am. J. Enol. Vitic. 1994, 45, 34–42. [Google Scholar]
- Wample, R.L.; Spayd, S.E.; Evans, R.G.; Stevens, R.G. Nitrogen fertilization of White Riesling grapes in Washington: Nitrogen seasonal effects on bud cold hardiness and carbohydrate reserves. Am. J. Enol. Vitic. 1993, 44, 159–167. [Google Scholar]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Chiva, R.; Guillamón, J.M. Arginine addition in the stationary phase influences the fermentation rate and synthesis of aroma compounds in a synthetic must fermented by three commercial wine strains. Lwt Food Sci. Technol. 2015, 60, 1009–1016. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-López, F.N.; Guillamon, J.M. Nitrogen requirements of commercial wine yeast strains during fermentation of a synthetic grape must. Food Microbiol. 2012, 31, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, P.; Roldán, A.; Caro, I.; Pérez, L. Kinetic study of the velum formation by Saccharomyces cerevisiae (beticus ssp.) during the biological aging of wines. Process. Biochem. 2010, 45, 493–499. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Env. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. Lwt Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Ibarz, M.J.; Cacho, J.; Ferreira, V. Effect of the addition of ammonium and amino acids to musts of Airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005, 89, 163–174. [Google Scholar] [CrossRef]
- Arias-Gil, M.; Garde-Cerdán, T.; Ancín-Azpilicueta, C. Influence of addition of ammonium and different amino acid concentrations on nitrogen metabolism in spontaneous must fermentation. Food Chem. 2007, 103, 1312–1318. [Google Scholar] [CrossRef]
- Kotarska, K.; Czupryński, B.; Kłosowski, G. Effect of various activators on the course of alcoholic fermentation. J. Food Eng. 2006, 77, 965–971. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Effect on white grape must of multiflora bee pollen addition during the alcoholic fermentation process. Molecules 2018, 23, 1321. [Google Scholar] [CrossRef] [PubMed]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Use of multiflora bee pollen as a flor velum yeast growth activator in biological aging wines. Molecules 2019, 24, 1763. [Google Scholar] [CrossRef]
- Human, H.; Nicolson, S.W. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry 2006, 67, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Barth, O.M.; Almeida-Muradian, L.B.; Pamplona, L.C.; Coimbra, S.; Barth, O.M. Chemical composition and botanical evaluation of dried bee pollen pellets. J. Food Compos. Anal. 2005, 18, 105–111. [Google Scholar]
- Paramás, A.M.G.G.; Bárez, J.A.G.; Marcos, C.C.; García-Villanova, R.J.; Sánchez, J.S.; Gonzalez-Paramas, A.M.; Garcia-Villanova, R.J.; Gonza, A.M.; Paramás, A.M.G.G.; Bárez, J.A.G.; et al. HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem. 2006, 95, 148–156. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Dong, J.; Zhang, H. Breaking the cells of rape bee pollen and consecutive extraction of functional oil with supercritical carbon dioxide. Innov. Food Sci. Emerg. Technol. 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Campos, M.G.R.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczesna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem, N.; Yusuf, B.; Gerçek, C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1–15. [Google Scholar] [CrossRef]
- Salanţă, L.; Borza, N.E.; Tofană, M.; Pop, C.R.; Mudura, E.; Mihai, M. Comparison of Ferments in the Process of Functional Beverage Making. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca Food Sci. Technol. 2018, 75, 90–92. [Google Scholar] [CrossRef]
- Roldán, A.; van Muiswinkel, G.C.J.; Lasanta, C.; Caro, I. Effect of the Addition of Fermentative Activators on Honey-Wine (Mead) Fermentation. In Proceedings of the 18th International Congress of Chemical and Process Engineering, Praha, Czech Republic, 24–28 August 2008; pp. 1875–2108. [Google Scholar]
- Roxas Clemente y Rubio, S. Ensayo Sobre las Variedades de la Vid Comun que Vegetan en Andalucía: Con un Índice Etimológico y Tres Listas de Plantas en Que se Caracterizan Varias Especies Nuevas; Imprenta de Villapando: Madrid, Spain, 1807. [Google Scholar]
- OIV. Office International de la Vigne et du Vin. Recl. Des. Méthodes Int. D’analyse Des. Vins Des. Moûts Édition Off. 2014, 1, 1–16. [Google Scholar]
- Aerny, J. Composés azotés des moûts et des vins. Rev. Suisse Vitic. Arboric. Hortic. 1997, 28, 161–168. [Google Scholar]
- Almela, L.; Javaloy, S.; Fernández-López, J.A.; López-Roca, J.M. Varietal classification of young red wines in terms of chemical and colour parameters. J. Sci. Food Agric. 1996, 70, 173–180. [Google Scholar] [CrossRef]
- Heredia, F.J.; Troncoso, A.M.; Guzmán-Chozas, M. Multivariate characterization of aging status in red wines based on chromatic parameters. Food Chem. 1997, 60, 103–108. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Campos, M.G.; Cunha, A.; Markham, K.R. Bee-pollen: Composition, properties, and applications. In Bee products (Properties, Applications, and Apitherapy); Mizrahi, A., Lensky, Y., Eds.; Springer: Boston, MA, USA, 1997; pp. 93–100. ISBN 0-306-45502-1. [Google Scholar]
- Da Silva, G.R.; da Natividade, T.B.; Camara, C.A.; da Silva, E.M.S.; dos Santos, F.D.; Silva, T.M.S. Identification of Sugar, Amino Acids and Minerals from the Pollen of Jandaíra Stingless Bees (Melipona subnitida). Food Nutr. Sci. 2014, 5, 1015–1021. [Google Scholar]
- Roldán, A.; Van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2011, 126, 574–582. [Google Scholar] [CrossRef]
- Stanley, G.A.; Douglas, N.G.; Every, E.J.; Tzanatos, T.; Pamment, N.B. Inhibition and stimulation of yeast growth by acetaldehyde. Biotechnol. Lett. 1993, 15, 1199–1204. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; Freitas, A.D.S.D.; Barth, O.M.; Almeida-Muradian, L.B.D. Phenolic profile by HPLC-MS, biological potential, and nutritional value of a promising food: Monofloral bee pollen. J. Food Biochem. 2018, 42, e12536. [Google Scholar] [CrossRef]
- Matei, F.; Toma, A.; Filofteia, C.; Moraru, A.; Constantinescu-aruxandei, D. Bee Collected Pollen with Enhanced Health Benefits, Produced by Fermentation with a Kombucha Consortium. Nutrients 2018, 10, 1365. [Google Scholar]
- Togores, J.H. Tratado de Enología I; Ediciones Mundi-Prensa Libros: Madrid, Spain, 2011. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Donéche, B.; Lonvaud, A.; Glories, Y.; Maugean, A. Traité D’oenologie: Microbiologie du Vin. Vinifications, 3rd ed.; Dunod Editions: Paris, France, 2017. [Google Scholar]
- Zeeman, W.; Snyman, J.P.; van Wyk, C.J. The influence of yeast strain and malolactic fermentation on some volatile bouquet substances and on quality of table wines. Proc. Univ. Calif. Davis Grape Wine Centen. Symp. 1982, 79–90. [Google Scholar]
- Davis, C.R.; Wibowo, D.; Eschenbruch, R.; Lee, T.H.; Fleet, G.H. Practical implications of malolactic fermentation: A review. Am. J. Enol. Vitic. 1985, 36, 290–301. [Google Scholar]
- Conrad, R. Bee Pollen—An Overview. The Magazine of the Beekeper. 2017. Available online: https://www.beeculture.com/bee-pollen-overview-2/ (accessed on 17 May 2018).
- Barbosa, C.; Falco, V.; Mendes-Faia, A.; Mendes-Ferreira, A. Nitrogen addition influences formation of aroma compounds, volatile acidity and ethanol in nitrogen deficient media fermented by Saccharomyces cerevisiae wine strains. J. Biosci. Bioeng. 2009, 108, 99–104. [Google Scholar] [CrossRef]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The impact of single amino acids on growth and volatile aroma production by Saccharomyces cerevisiae strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A.; Joyeux, A.; Desens, C. Inhibition of malolactic fermentation of wines by products of yeast metabolism. J. Sci. Food Agric. 1988, 44, 183–191. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; da Silva Lins, A.C.; Maria Barbosa-Filho, J.; da Silva, E.M.S.; Freitas, B.M.; de Assis Ribeiro dos Santos, F. Chemical composition and free radical scavenging activity of pollen loads from stingless bee Melipona subnitida Ducke. J. Food Compos. Anal. 2006, 19, 507–511. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Agra, M.d.F.; Silva, E.M.S.; Reis, I.T.; Freitas, B.M. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). Acad. Bras. Cienc. 2009, 81, 173–178. [Google Scholar] [CrossRef]
- Bogdanov, S. Pollen: Production, Nutrition and Health: A Review. 2017. Available online: https://www.bee-hexagon.net/pollen/ (accessed on 4 May 2018).




| Parameters | Tintilla de Rota Grape Must Bee Pollen Doses | ||||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |
| Sugar (g/L) | 240.7 ± 0.8 a | 240.7 ± 0.8 a | 244.3 ± 0.6 a | 243.3 ± 0.6 a | 241.2 ± 0.8 a | 257.6 ± 0.5 b | 256.4 ± 0.0 b |
| Total acidity (g/L TH2) | 5.28 ± 0.03 a | 5.28 ± 0.03 a | 5.28 ± 0.03 a | 5.28 ± 0.03 a | 5.22 ± 0.03 a | 5.34 ± 0.03 a | 5.99 ± 0.03 b |
| pH | 3.67 ± 0.01 a | 3.68 ± 0.01 a | 3.71 ± 0.01 a,b,c | 3.72 ± 0.01 a,b,c | 3.73 ± 0.01 a,b,c | 3.78 ± 0.01 b,c | 3.80 ± 0.01 c |
| YAN (mg/L) | 140 ± 0 a | 145 ± 4 a | 162 ± 4 b | 170 ± 1 c | 179 ± 4 d | 185 ± 8 e | 208 ± 4 f |
| Parameters | Tintilla de Rota Grape Wines Bee Pollen Doses | ||||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 g/L | 0.25 g/L | 1 g/L | 5 g/L | 10 g/L | 20 g/L | |
| % Alcohol v/v | 13.97 ± 0.06 a,b | 13.97 ± 0.14 a,b | 14.22 ± 0.03 a | 14.15 ± 0.03 a | 13.65 ± 0.07 b | 14.14 ± 0.03 a | 13.89 ± 0.08 a,b |
| pH | 3.79 ± 0.01 a,b | 3.73 ± 0.02 b | 3.84 ± 0.01 a,b | 3.84 ± 0.01 a,b | 3.81 ± 0.01 a,b | 3.89 ± 0.01 a | 3.90 ± 0.01 a |
| Total Acidity (g/L) | 4.83 ± 0.04 a | 4.88 ± 0.01 a | 4.83 ± 0.04 a | 4.93 ± 0.04 a | 4.88 ± 0.01 a | 4.93 ± 0.04 a | 4.78 ± 0.04 a |
| Volatile Acidity (g/L) | 0.63 ± 0.01 a | 0.56 ± 0.01 b | 0.58 ± 0.02 b | 0.44 ± 0.01 c | 0.44 ± 0.01 c | 0.50 ± 0.01 d | 0.53 ± 0.01 e |
| Glucose (g/L) | 0.378 ± 0.002 a | 0.282 ± 0.002 b,c,d,e | 0.291 ± 0.000 c,d,e | 0.384 ± 0.004 a | 0.375 ± 0.005 a | 0.282 ± 0.001 d | 0.294 ± 0.004 e |
| Fructose (g/L) | 0.293 ± 0.008 a | 0.371 ± 0.007 b | 0.297 ± 0.001 a | 0.324 ± 0.003 c | 0.270 ± 0.002 d,f | 0.254 ± 0.004 e | 0.273 ± 0.000 f |
| YAN* (mg/L) | 92 ± 4 a | 110 ± 4 b | 119 ± 6 c | 127 ± 4 d | 166 ± 8 e | 179 ± 4 f | 226 ± 8 g |
| Anthocyanin (mg/L) | 777.13 ± 1.24 a | 833.13 ± 1.24 b | 725.50 ± 0.01 c,d,e | 772.31 ± 0.62 a | 709.31 ± 0.62 d | 743.44 ± 0.62 e | 795.94 ± 0.62 a |
| Tannins (g/L) | 2.65 ± 0.01 a | 2.67 ± 0.01 a | 2.61 ± 0.01 a | 2.74 ± 0.01 b | 2.59 ± 0.01 a | 2.51 ± 0.01 c | 2.40 ± 0.01 d |
| TPI | 60.48 ± 1.81 a | 66.38 ± 0.89 b | 74.36 ± 0.41 c | 75.48 ± 0.47 c | 79.53 ± 0.84 d | 83.56 ± 1.66 e | 88.72 ± 0.98 f |
| L* | 78.092 ± 0.030 a | 75.999 ± 0.048 a | 77.810 ± 0.010 a | 75.593 ± 0.035 a | 75.593 ± 0.035 a | 77.721 ± 0.023 a | 77.984 ± 0.172 a |
| a* | 19.959 ± 0.066 a | 16.297 ± 0.060 b | 18.332 ± 0.025 c,e | 14.429 ± 0.025 d | 18.625 ± 0.023 e | 18.642 ± 0.014 e | 18.745 ± 0.079 e |
| b* | 5.256 ± 0.095 a | 6.446 ± 0.160 b | 7.006 ± 0.041 c | 5.890 ± 0.066 d,e | 6.092 ± 0.391 e,f | 5.434 ± 0.005 a | 6.158 ± 0.483 f |
| C* | 20.64 ± 0.06 a | 17.53 ± 0.01 b | 19.63 ± 0.03 c,f | 15.58 ± 0.05 d,e | 15.58 ± 0.05 e | 19.42 ± 0.01 f | 19.73 ± 0.22 f |
| Tone (N) | 0.631 ± 0.001 a | 0.609 ± 0.001 a | 0.742 ± 0.001 b,f | 0.665 ± 0.002 c,d | 0.682 ± 0.002 d,e | 0.689 ± 0.001 e | 0.733 ± 0.002 f |
| Colorant Intensity (IC) | 0.795 ± 0.001 a | 1.226 ± 0.008 b | 0.935 ± 0.001 c,f,g | 1.150 ± 0.001 d | 0.949 ± 0.001 e,f | 0.922 ± 0.001 f,g | 0.897 ± 0.001 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions. Agronomy 2020, 10, 634. https://doi.org/10.3390/agronomy10050634
Amores-Arrocha A, Sancho-Galán P, Jiménez-Cantizano A, Palacios V. Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions. Agronomy. 2020; 10(5):634. https://doi.org/10.3390/agronomy10050634
Chicago/Turabian StyleAmores-Arrocha, Antonio, Pau Sancho-Galán, Ana Jiménez-Cantizano, and Víctor Palacios. 2020. "Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions" Agronomy 10, no. 5: 634. https://doi.org/10.3390/agronomy10050634
APA StyleAmores-Arrocha, A., Sancho-Galán, P., Jiménez-Cantizano, A., & Palacios, V. (2020). Bee Pollen as Oenological Tool to Carry out Red Winemaking in Warm Climate Conditions. Agronomy, 10(5), 634. https://doi.org/10.3390/agronomy10050634








