The Role of Deep Roots in Sorghum Yield Production under Drought Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of Photosynthetic Parameters, Fluorescence Parameters and SPAD Values.
2.3. Collection of Root Bleeding Sap
2.4. Determination of Osmotic Adjustment Substance and Hormone Content in Root Bleeding Sap
2.5. Determination of Dry Matter Weight and Yield
2.6. Contribution of Roots of Different Depths to Yield
2.7. Statistical Analysis
3. Results
3.1. Effects of Root Removal on Photosynthetic Performance
3.2. Effects of Root Removal on Root Bleeding
3.3. Effects of Root Removal on Dry Matter Accumulation and Yield
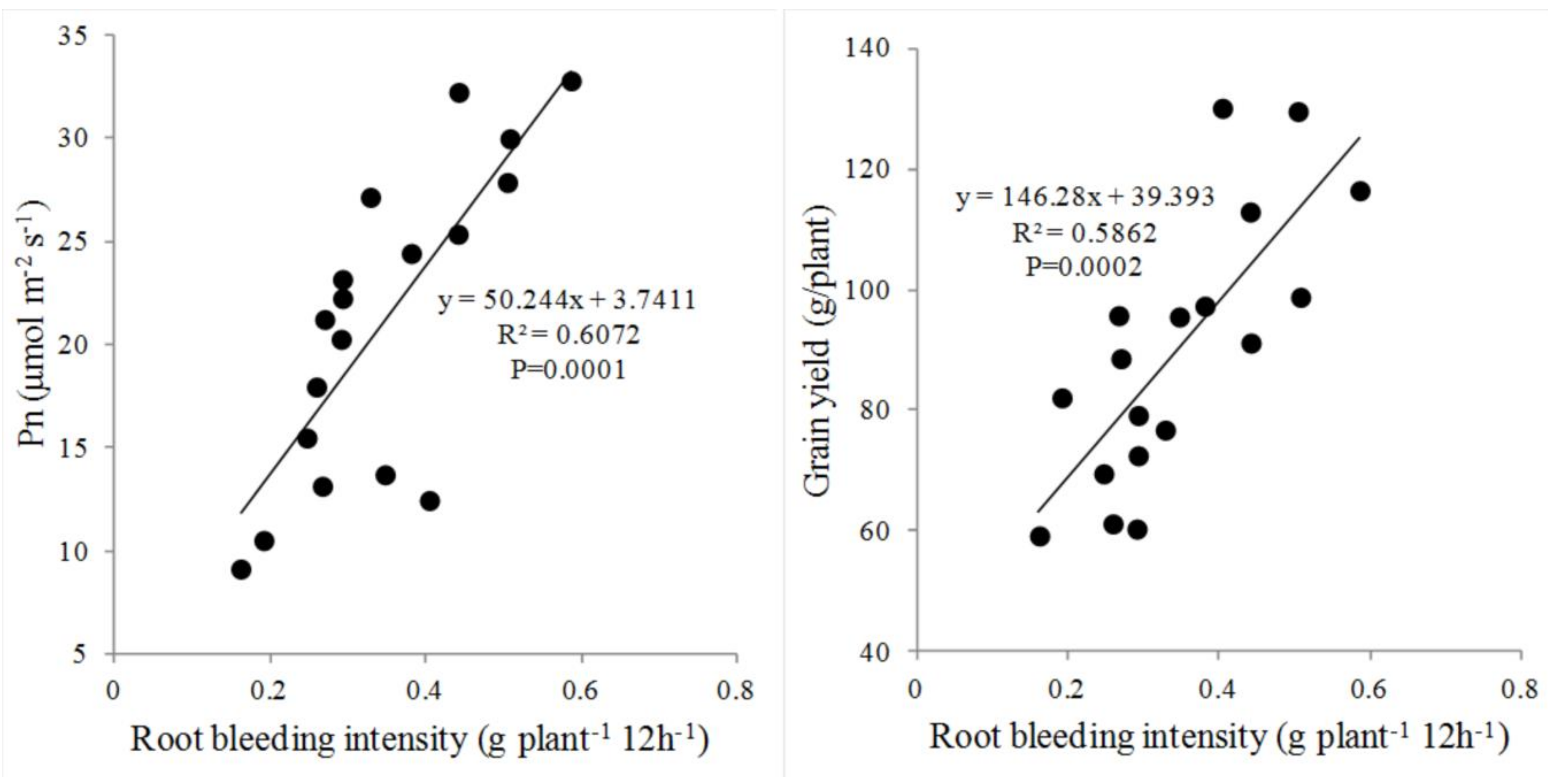
3.4. Correlation of Root Bleeding with Grain Yield and Pn
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arai-Sanoh, Y.; Takai, T.; Yoshinaga, S.; Nakano, H.; Kojima, M.; Sakakibara, H.; Kondo, M.; Uga, Y. Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 2014, 4, 5563. [Google Scholar] [CrossRef] [PubMed]
- Rachmilevitch, S.; Lambers, H.; Huang, B. Root respiratory characteristics associated with plant adaptation to high soil temperature for geothermal and turf-type Agrostis species. J. Exp. Bot. 2006, 57, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.V.D.; Bouillet, J.P.; Gonçalves, J.L.D.M.; Junior, C.H.A.; Trivelin, P.C.O.; Hinsinger, P.; Jourdan, C.; Nouvellon, Y.; Stape, J.L.; Laclau, J.P. Functional specialization of Eucalyptus fine roots: Contrasting potential uptake rates for nitrogen, potassium and calcium tracers at varying soil depths. Funct. Ecol. 2011, 25, 996–1006. [Google Scholar] [CrossRef]
- Guo, H.; York, L.M. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. J. Exp. Bot. 2019, 70, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, J.; Krishnamurthy, L.; Crouch, J.H.; Serraj, R. Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crop. Res. 2006, 95, 171–181. [Google Scholar] [CrossRef]
- Liedgens, M.; Richner, W. Relation between maize (Zea mays L.) leaf area and root density observed with minirhizotrons. Eur. J. Agron. 2001, 15, 131–141. [Google Scholar] [CrossRef]
- Craine, J.M.; Wedin, D.A.; Chapin, F.S.; Reich, P.B. Relationship between the structure of root systems and resource use for 11 North American grassland plants. Plant Ecol. 2003, 165, 85–100. [Google Scholar] [CrossRef]
- Vamerali, T.; Saccomani, M.; Bona, S.; Mosca, G.; Guarise, M.; Ganis, A. A comparison of root characteristics in relation to nutrient and water stress in two maize hybrids. Plant Soil 2003, 255, 157–167. [Google Scholar]
- Bakhshandeh, S.; Kertesz, M.A.; Corneo, P.E.; Dijkstra, F.A. Dual-labeling with15N and H218O to investigate water and N uptake of wheat under different water regimes. Plant Soil 2016, 408, 429–441. [Google Scholar] [CrossRef]
- Sebastian, J.; Yee, M.; Viana, W.G.; Rellan-Alvarez, R.; Feldman, M.; Priest, H.D.; Trontin, C.; Lee, T.; Jiang, H.; Baxter, I.; et al. Grasses suppress shoot-borne roots to conserve water during drought. Proc. Natl. Acad. Sci. USA 2016, 113, 8861–8866. [Google Scholar] [CrossRef]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can Changes in Canopy and/or Root System Architecture Explain Historical Maize Yield Trends in the U.S. Corn Belt? Crop Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Gewin, V. Food: An underground revolution. Nature 2010, 466, 552–553. [Google Scholar] [CrossRef] [PubMed]
- Thorup-Kristensen, K.; Rasmussen, C.R. Identifying new deep-rooted plant species suitable as undersown nitrogen catch crops. J. Soil Water Conserv. 2015, 70, 399–409. [Google Scholar] [CrossRef]
- Giambelluca, T.W.; Mudd, R.G.; Liu, W.; Ziegler, A.D.; Kobayashi, N.; Kumagai, T.; Miyazawa, Y.; Lim, T.K.; Huang, M.Y.; Fox, J.; et al. Evapotranspiration of rubber (Hevea brasiliensis) cultivated at two plantation sites in Southeast Asia. Water Resour. Res. 2016, 52, 660–679. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Christopher, J.; de Voil, P.; Hammer, G.L. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct. Plant Biol. 2006, 33, 823. [Google Scholar] [CrossRef]
- Palta, J.A.; Chen, X.; Milroy, S.P.; Rebetzke, G.J.; Dreccer, M.F.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Cortasa, M.S.; Loges, R. Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 2009, 322, 101–114. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Dresbøll, D.B.; Kristensen, H.L. Crop yield, root growth, and nutrient dynamics in a conventional and three organic cropping systems with different levels of external inputs and N re-cycling through fertility building crops. Eur. J. Agron. 2012, 37, 66–82. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Funct. Plant Biol. 2010, 37, 147–156. [Google Scholar] [CrossRef]
- Kell, D.B. Breeding crop plants with deep roots: Their role in sustainable carbon, nutrient and water sequestration. Ann. Bot. 2011, 108, 407–418. [Google Scholar] [CrossRef]
- Wasson, A.P.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Richards, R.A.; Watt, M. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J. Exp. Bot. 2014, 65, 6231–6249. [Google Scholar] [CrossRef] [PubMed]
- Thorup-Kristensen, K.; Kirkegaard, J. Root system-based limits to agricultural productivity and efficiency: The farming systems context. Ann. Bot. 2016, 118, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Ong, C.K. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Warren, J.M.; Meinzer, F.C.; Brooks, J.R.; Coulombe, R. Native root xylem embolism and stomatal closure in stands of Douglas-fir and ponderosa pine: Mitigation by hydraulic redistribution. Oecologia 2004, 141, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Bleby, T.M.; Mcelrone, A.J.; Jackson, R.B. Water uptake and hydraulic redistribution across large woody root systems to 20 m depth. Plant Cell Environ. 2010, 33, 2132–2148. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. 2018. Available online: http://faostat3.fao.org/download/Q/QC/E (accessed on 24 April 2020).
- Wang, N.; Wang, Y.T.; Yu, J.L.; Zhou, Y.F.; Wu, Q.; Gao, Y.; Xu, W.J.; Huang, R.D. Prioritization of feasible physiological parameters in drought tolerance evaluation in sorghum: A grey relational analysis. Zemdirbyste 2015, 102, 457–464. [Google Scholar] [CrossRef][Green Version]
- Haussmann, B.; Mahalakshmi, V.; Reddy, B.; Seetharama, N.; Hash, C.; Geiger, H. QTL mapping of stay-green in two sorghum recombinant inbred populations. Theor. Appl. Genet. 2002, 106, 133–142. [Google Scholar] [CrossRef]
- Mace, E.S.; Tai, S.S.; Gilding, E.K.; Li, Y.H. Whole-genome sequencing reveals untapped genetic potential in Africa’s indigenous cereal crop sorghum. Nat. Commun. 2013, 4, 2320. [Google Scholar] [CrossRef]
- Paterson, A.H. Genomics of Sorghum. Int. J. Plant Genomics 2008, 2008, 362451. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zeng, T.R.; Liu, X.Y.; Gao, C.X.; Li, Y.X.; Li, C.H.; Song, Y.C.; Shi, Y.S.; Wang, T.Y.; Li, Y. Transcriptomic profiling of sorghum leaves and roots responsive to drought stress at the seedling stage. J. Integ. Agric. 2019, 18, 1980–1995. [Google Scholar] [CrossRef]
- Wang, D.Q.; Zhou, Y.F.; Lu, Z.B.; Xiao, M.J.; Xu, W.J.; Huang, R.D. Root morphology and activity of stay green sorghum under water stress. Agric. Res. Arid Area. 2012, 30, 73–76. [Google Scholar]
- Smit, A.L.; Bengough, A.G.; Engels, C.; De Noordwijk, V. Root Methods: A Handbook. J. Agron. Crop Sci. 2002, 188, 64. [Google Scholar]
- Maeght, J.L.; Rewald, B.; Pierret, A. How to study deep roots—And why it matters. Front. Plant Sci. 2013, 4, 299. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Yang, L.; Cong, W.; Zu, Y.; Tang, Z. The improved resistance to high salinity induced by trehalose is associated with ionic regulation and osmotic adjustment in Catharanthus roseus. Plant Physiol. Biochem. 2014, 77, 140–148. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebetaine synthesis in maize. Plant Sci. 2004, 166, 141–149. [Google Scholar] [CrossRef]
- Guzel, S.; Terzi, R. Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot. Stud. 2013, 54, 26. [Google Scholar] [CrossRef]
- Sun, S.W.; Lin, Y.C.; Weng, Y.M.; Chen, M.J. Efficiency improvements on ninhydrin method for amino acid quantification. J. Food Compos. Anal. 2006, 19, 112–117. [Google Scholar] [CrossRef]
- Chu, G.; Chen, T.T.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Morphological and physiological traits of roots and their relationships with water productivity in water-saving and drought-resistant rice. Field Crop. Res. 2014, 162, 108–119. [Google Scholar] [CrossRef]
- Guan, D.H.; Al-Kaisi, M.M.; Zhang, Y.S.; Duan, L.S.; Tan, W.M.; Zhang, M.C.; Li, Z.H. Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crop. Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Munns, R.; Passioura, J.; Colmer, T.; Byrt, C. Osmotic adjustment and energy limitations to plant growth in saline soil. New phytol. 2019, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Fan, S.; Du, L.; Shen, Y.; Xing, L.; Li, Y.; Ma, J.; Han, M. Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2016, 107, 178–186. [Google Scholar] [CrossRef]
- Abid, M.; Shao, Y.H.; Liu, S.X.; Wang, F.; Gao, J.W.; Jang, D.; Tian, Z.W.; Dai, T.B. Pre-drought priming sustains grain development under post-anthesis drought stress by regulating the growth hormones in winter wheat (Triticum aestivum L.). Planta 2017, 246, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Wang, D.Q.; Lu, Z.B.; Wang, N.; Wang, Y.T.; Li, F.X.; Xu, W.J.; Huang, R.D. Effects of Drought Stress on Photosynthetic Characteristics and Endogenous Hormone ABA and CTK Contents in Green-Stayed Sorghum. Sci. Agric. Sin. 2014, 47, 655–663. [Google Scholar]
- Song, R.; Wu, C.S.; Ma, L.Y. Effect of application of combined fertilizers on the root system of maize. Acta Agron. Sin. 2002, 28, 393–396. [Google Scholar]
- Qi, W.Z.; Liu, H.H.; Liu, P.; Dong, S.T.; Zhao, B.Q.; So, H.B.; Li, G.; Liu, H.D.; Zhang, J.W.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Ge, J.L.; Shi, L.; Gu, W.B.; Tang, Y.D.; Zhang, J.Z.; Jiang, C.D.; Ren, D.M. Photosynthetic Characteristics and the Regulation of PhotosystemⅡ Function in Salt-Stressed Sweet Sorghum Seedlings. Acta Agron. Sin. 2007, 33, 1272–1278. [Google Scholar]
- Liang, Y.; He, W.S.; Dai, X.H.; Ma, K.; Hou, X.Q. Effects of Planting Density and Row Spacing on Root-shoot Spatial Distribution and Grain Yield of Spring Maize. J. Maize Sci. 2016, 24, 97–102. [Google Scholar]
- Li, Y.S.; Feng, L.P.; Guo, M.L.; Han, X.X. Studies on the growth characteristics of root system and its relation with cultural practices and yield in cotton (G. hirsutum L.) the effects of cultural practices on the growth of root system and its relation with above ground parts and yield. Cotton Sci. 1992, 4, 59–66. [Google Scholar]
- Zhao, Q.Z.; Qiao, J.F.; Liu, H.; Tian, Z.Q. Relationship Between Root and Leaf Photosynthetic Characteristic in Rice. Sci. Agric. Sin. 2007, 40, 1064–1068. [Google Scholar]
- Schittenhelm, S.; Schroetter, S. Comparison of Drought Tolerance of Maize, Sweet Sorghum and Sorghum-Sudangrass Hybrids. J. Agron. Crop Sci. 2014, 200, 46–53. [Google Scholar] [CrossRef]
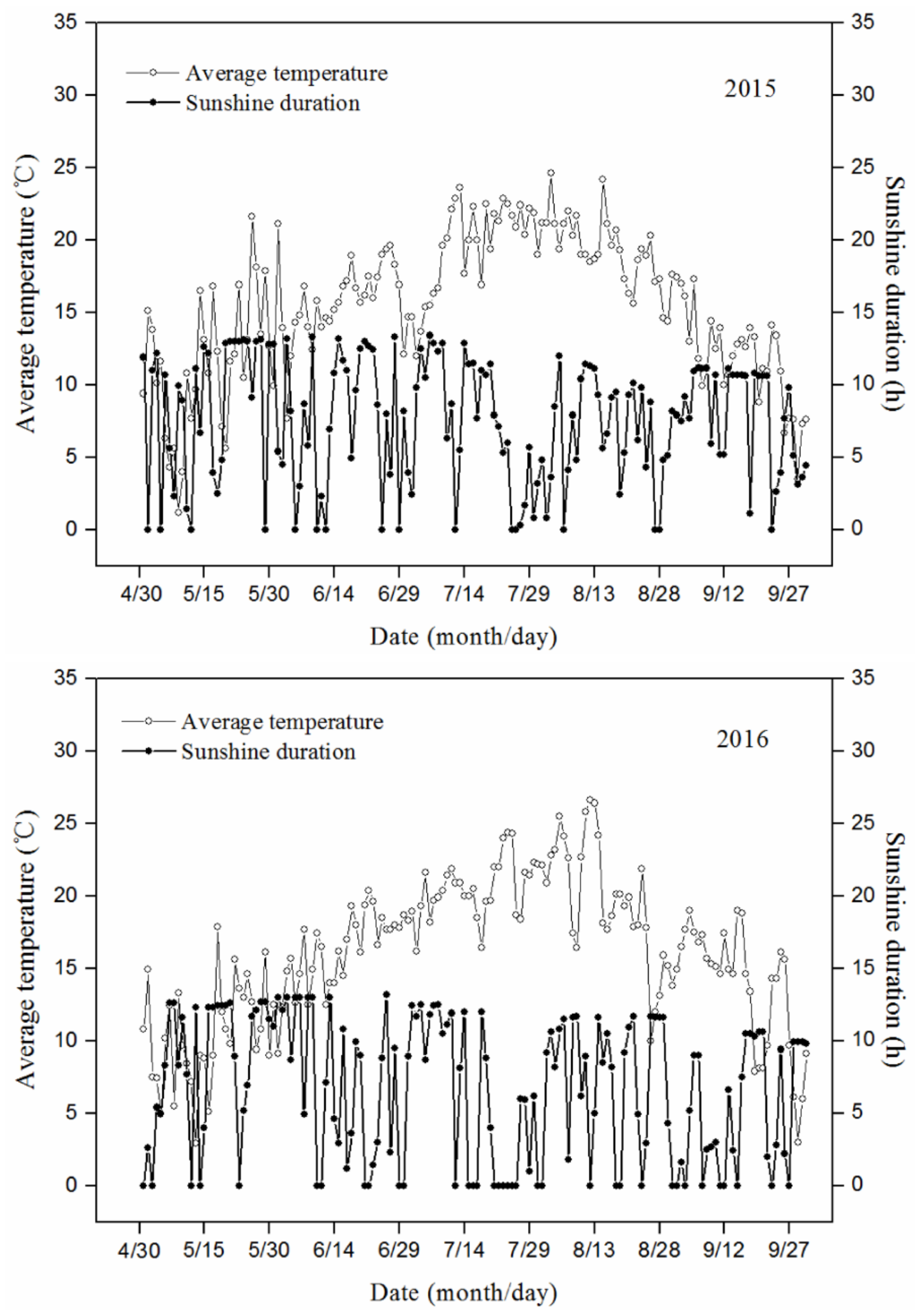

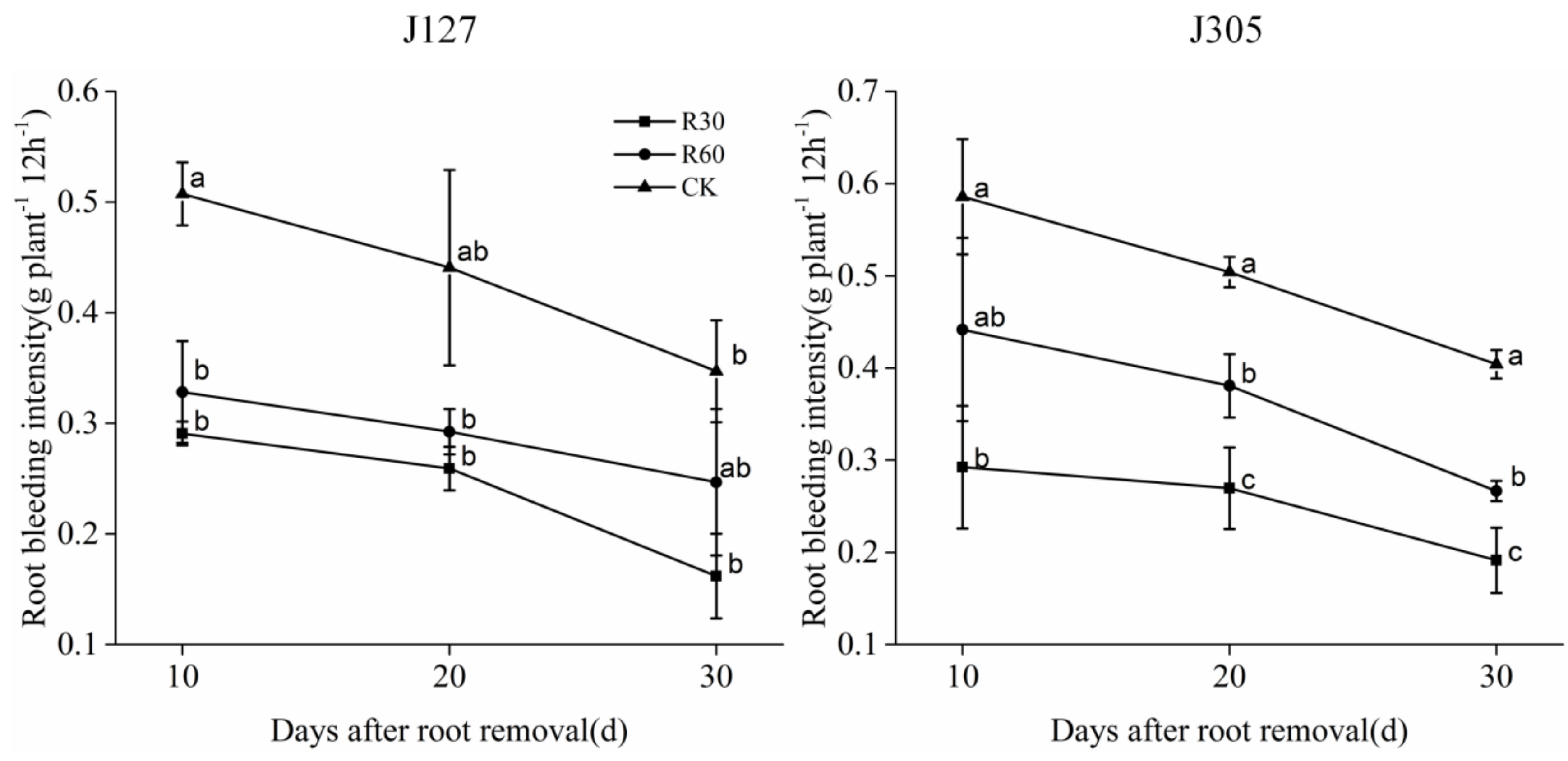
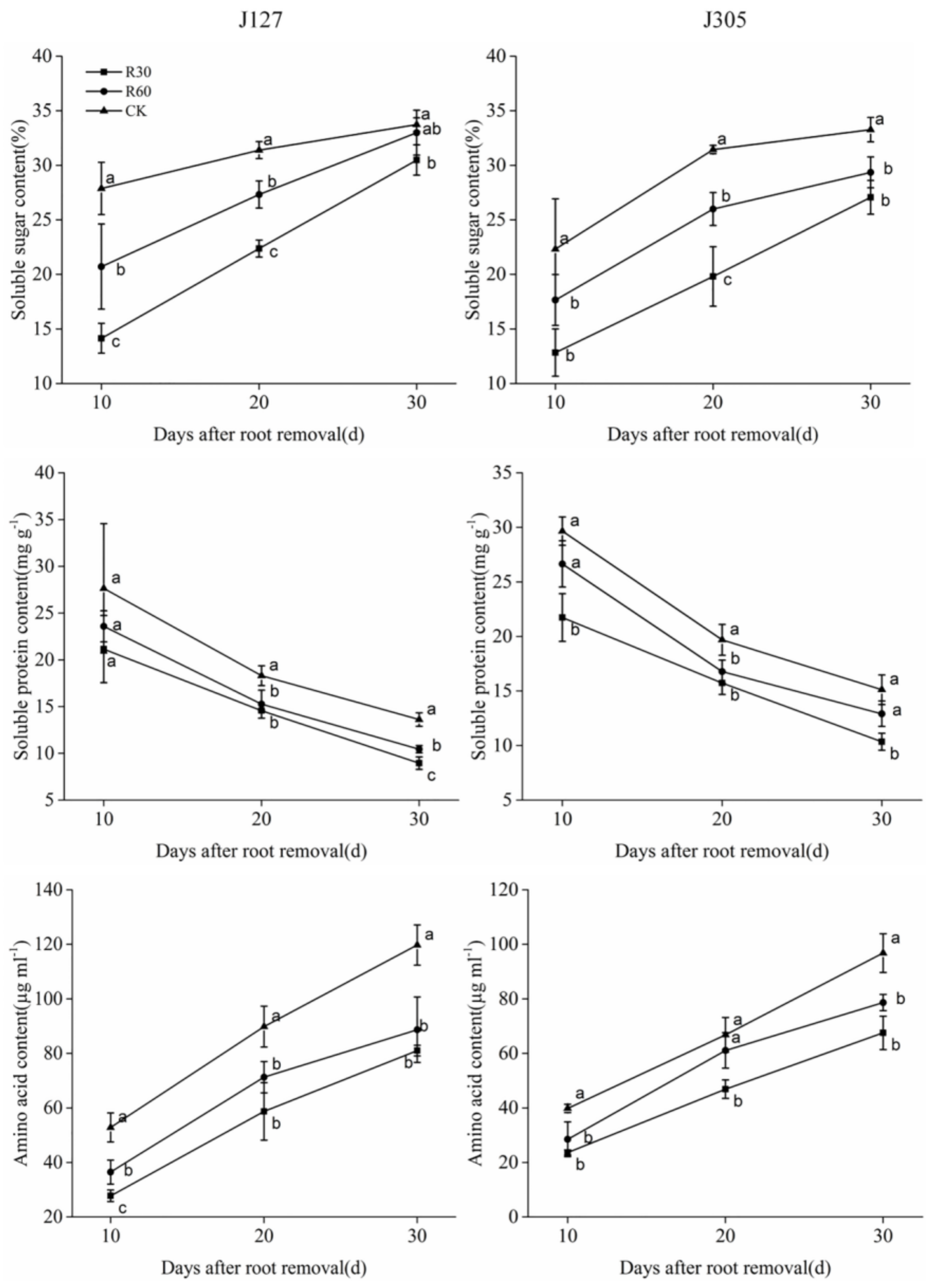
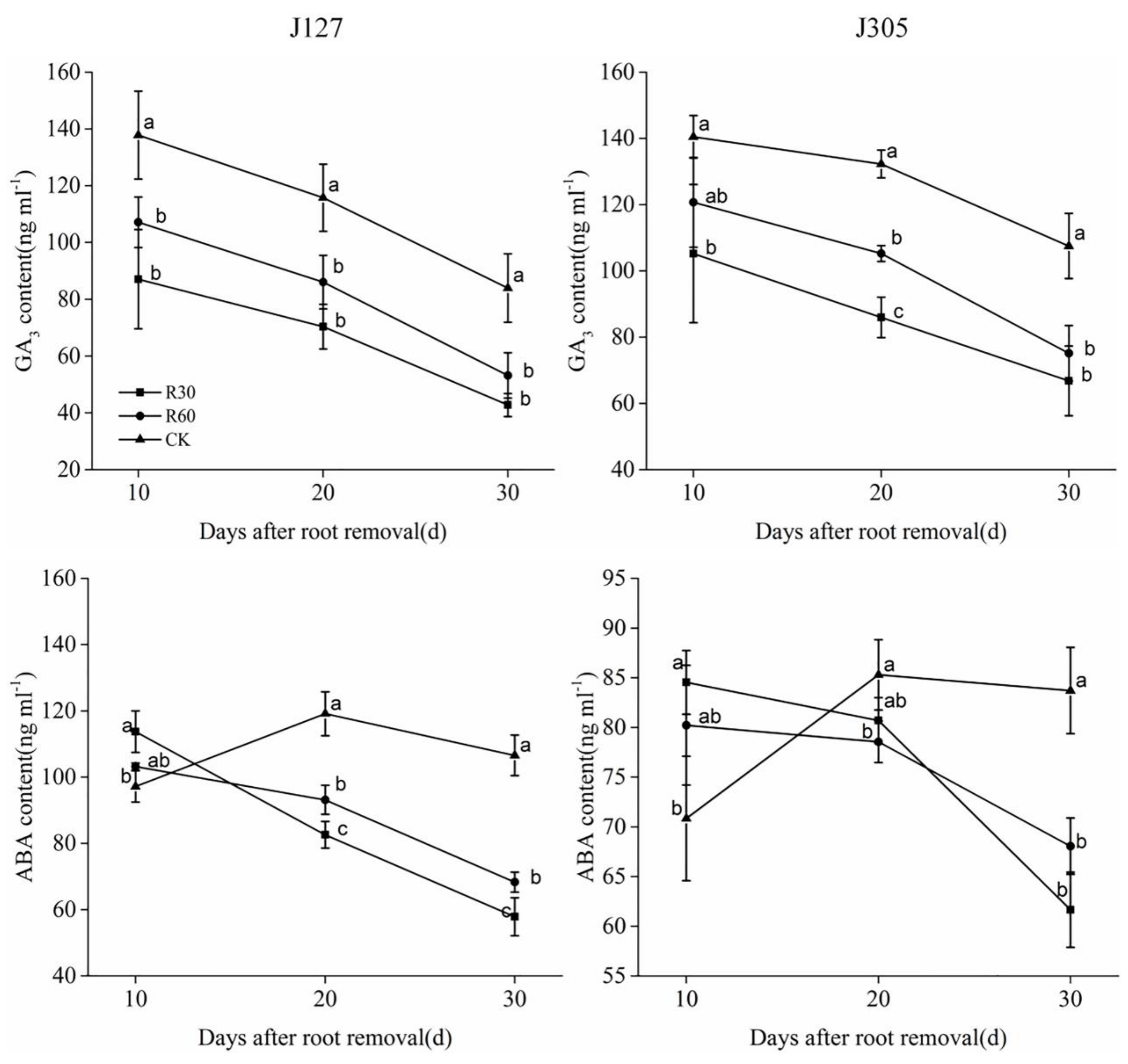
| Cultivar | Days after Root Removal | Treatment | Pn (μmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Tr (mmol·m−2·s−1) | SPAD Value |
|---|---|---|---|---|---|---|
| J127 | 10 d | R30 | 20.29 ± 2.51b | 0.20 ± 0.024b | 3.77 ± 0.79b | 45.07 ± 2.02b |
| R60 | 27.16 ± 0.20a | 0.22 ± 0.014ab | 5.90 ± 0.41a | 49.40 ± 3.58a | ||
| CK | 30.00 ± 1.85a | 0.25 ± 0.015a | 6.73 ± 0.48a | 55.65 ± 4.26a | ||
| 20 d | R30 | 17.97 ± 0.31b | 0.11 ± 0.004b | 3.05 ± 0.49c | 35.13 ± 2.86b | |
| R60 | 23.18 ± 2.55a | 0.13 ± 0.027a | 4.10 ± 0.20b | 37.93 ± 1.16b | ||
| CK | 25.37 ± 2.40a | 0.24 ± 0.025a | 4.96 ± 0.37a | 47.30 ± 2.61a | ||
| 30 d | R30 | 9.16 ± 2.40b | 0.06 ± 0.016b | 1.35 ± 0.37b | 29.03 ± 2.78b | |
| R60 | 11.39 ± 2.13ab | 0.09 ± 0.014ab | 1.64 ± 0.28a | 31.60 ± 4.92ab | ||
| CK | 13.50 ± 2.92a | 0.13 ± 0.014a | 1.65 ± 0.28a | 39.00 ± 4.40a | ||
| J305 | 10 d | R30 | 22.26 ± 1.81b | 0.20 ± 0.031b | 4.51 ± 0.51b | 48.63 ± 1.70b |
| R60 | 32.25 ± 2.79a | 0.25 ± 0.016ab | 5.38 ± 0.25ab | 54.50 ± 3.95ab | ||
| CK | 32.81 ± 2.44a | 0.30 ± 0.050a | 6.12 ± 0.99a | 57.66 ± 6.39a | ||
| 20 d | R30 | 21.24 ± 1.98b | 0.18 ± 0.017b | 3.52 ± 0.85a | 43.33 ± 6.64b | |
| R60 | 24.45 ± 2.25ab | 0.23 ± 0.045a | 4.35 ± 0.83a | 49.37 ± 1.30ab | ||
| CK | 27.88 ± 3.98a | 0.27 ± 0.011a | 5.09 ± 0.72a | 54.36 ± 6.21a | ||
| 30 d | R30 | 10.54 ± 2.52b | 0.10 ± 0.012b | 1.59 ± 0.25b | 32.00 ± 2.98b | |
| R60 | 12.47 ± 1.77a | 0.16 ± 0.008a | 1.76 ± 0.15a | 38.03 ± 3.07ab | ||
| CK | 13.17 ± 1.39a | 0.17 ± 0.010a | 1.81 ± 0.20a | 42.18 ± 3.76a |
| Cultivar | Days after Root Removal | Treatment | Fo | Fv/Fm | qL | ETR |
|---|---|---|---|---|---|---|
| J127 | 10 d | R30 | 255.36 ± 5.66a | 0.75 ± 0.050a | 0.22 ± 0.009a | 24.49 ± 0.90b |
| R60 | 230.67 ± 6.66b | 0.77 ± 0.003a | 0.24 ± 0.019a | 28.48 ± 0.89a | ||
| CK | 219.66 ± 5.64b | 0.79 ± 0.002a | 0.25 ± 0.029a | 31.05 ± 2.32a | ||
| 20 d | R30 | 278.35±9.10a | 0.69 ± 0.006b | 0.18 ± 0.029a | 21.53 ± 1.24c | |
| R60 | 250.41±4.87b | 0.75 ± 0.026a | 0.20 ± 0.034a | 25.61 ± 0.40b | ||
| CK | 236.31±5.00c | 0.77 ± 0.001a | 0.22±0.022a | 28.24 ± 0.94a | ||
| 30 d | R30 | 319.00±11.37a | 0.63 ± 0.039b | 0.17±0.015b | 16.05 ± 2.42b | |
| R60 | 293.463±16.57ab | 0.65 ± 0.019ab | 0.20 ± 0.034a | 21.80 ± 1.48a | ||
| CK | 258.33±15.51b | 0.70 ± 29a | 0.21 ± 25a | 24.24 ± 1.95a | ||
| J305 | 10 d | R30 | 236.14 ± 16.97a | 0.77 ± 0.004a | 0.22 ± 0.033b | 28.32 ± 1.00b |
| R60 | 227.20 ± 7.14ab | 0.78 ± 0.001a | 0.31 ± 0.054a | 31.14 ± 2.92ab | ||
| CK | 216.46 ± 6.85b | 0.79 ± 0.028a | 0.32 ± 0.025a | 34.66 ± 2.08a | ||
| 20 d | R30 | 261.96 ± 4.88a | 0.71 ± 0.022b | 0.17 ± 0.005c | 24.49 ± 0.53b | |
| R60 | 238.42 ± 7.23b | 0.75 ± 0.020ab | 0.23 ± 0.016b | 27.48 ± 0.39ab | ||
| CK | 228.67 ± 6.03c | 0.76 ± 0.025a | 0.27 ± 0.020a | 30.03 ± 2.80a | ||
| 30 d | R30 | 292.00 ± 19.67a | 0.62 ± 0.079b | 0.11 ± 0.100b | 20.06 ± 0.79b | |
| R60 | 262.67 ± 11.50ab | 0.67 ± 0.023ab | 0.14 ± 0.149a | 23.56 ± 2.02ab | ||
| CK | 246.67 ± 11.59b | 0.70 ± 0.012a | 0.16 ± 0.156a | 24.42 ± 0.74a |
| Cultivar | Days after Root Removal | Treatment | Leaf (g·plant−1) | Stem (g·plant−1) | Sheath (g·plant−1) | Panicle (g·plant−1) |
|---|---|---|---|---|---|---|
| J127 | 10 d | R30 | 21.67 ± 3.94a | 40.18 ± 4.76b | 14.95 ± 1.72a | 75.25 ± 3.53b |
| R60 | 24.41 ± 2.22a | 57.73 ± 9.56a | 16.92 ± 2.22a | 87.37 ± 6.85a | ||
| CK | 26.22 ± 1.94a | 61.14 ± 5.57a | 19.08 ± 3.65a | 98.31 ± 6.30a | ||
| 20 d | R30 | 18.53 ± 0.71b | 35.35 ± 1.32b | 12.05 ± 0.78c | 70.39 ± 5.39b | |
| R60 | 22.59 ± 1.85a | 48.85 ± 7.88a | 14.87 ± 0.70b | 78.71 ± 9.31ab | ||
| CK | 24.32 ± 1.54a | 56.94 ± 6.93a | 17.51 ± 0.66a | 87.72 ± 8.78a | ||
| 30 d | R30 | 14.15 ± 1.97b | 27.31 ± 13.34b | 8.43 ± 0.34c | 63.98 ± 2.61c | |
| R60 | 21.07 ± 0.74a | 40.87 ± 4.19ab | 10.35 ± 0.67b | 73.73 ± 3.13b | ||
| CK | 21.15 ± 1.41a | 51.41 ± 3.79a | 14.81 ± 0.77a | 84.89 ± 2.88a | ||
| J305 | 10 d | R30 | 25.70 ± 2.98a | 63.77 ± 7.72b | 19.12 ± 6.13a | 89.17 ± 8.81b |
| R60 | 28.76 ± 1.24a | 83.02 ± 4.04a | 19.73 ± 2.50a | 106.48 ± 1.73a | ||
| CK | 29.23 ± 1.68a | 85.83 ± 10.71a | 21.61 ± 3.31a | 115.24 ± 11.30a | ||
| 20 d | R30 | 21.98 ± 2.05b | 53.44 ± 10.77b | 14.52 ± 2.67a | 84.18 ± 2.93b | |
| R60 | 26.46 ± 2.51a | 79.43 ± 2.91a | 17.44 ± 2.53a | 102.95 ± 7.12a | ||
| CK | 28.27 ± 1.27a | 80.87 ± 6.90a | 20.59 ± 1.13a | 109.80 ± 5.87a | ||
| 30 d | R30 | 19.76 ± 1.84b | 50.41 ± 5.23b | 12.81 ± 2.45b | 70.06 ± 1.58c | |
| R60 | 25.22 ± 2.13a | 67.64 ± 8.78a | 16.77 ± 3.87a | 81.56 ± 0.23b | ||
| CK | 25.49 ± 2.95a | 73.15 ± 5.33a | 19.60 ± 1.60a | 95.14 ± 2.82a |
| Year | Cultivar | Treatment | Grain Yield (g/plant) | Biological Yield (g/plant) |
|---|---|---|---|---|
| 2015 | J127 | R30 | 60.34 ± 1.00c | 161.18 ± 8.30c |
| R60 | 72.99 ± 3.64b | 175.39 ± 0.71b | ||
| CK | 102.43 ± 9.24a | 197.71 ± 11.47a | ||
| J305 | R30 | 83.34 ± 4.82b | 193.44 ± 3.83b | |
| R60 | 94.77 ± 4.21b | 213.13 ± 5.56b | ||
| CK | 125.37 ± 7.72a | 231.10 ± 6.36a | ||
| 2016 | J127 | R30 | 54.48 ± 0.45c | 184.39 ± 25.07c |
| R60 | 84.82 ± 2.41b | 243.04 ± 9.08b | ||
| CK | 144.30 ± 2.28a | 295.32 ± 36.83a | ||
| J305 | R30 | 42.72 ± 10.88b | 164.21 ± 9.34b | |
| R60 | 66.37 ± 6.97b | 204.78 ± 20.35b | ||
| CK | 117.82 ± 13.33a | 290.40 ± 49.96a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wu, Q.; Gao, Y.; Zhang, J.; Wang, Y.; Zhang, R.; Zhou, Y.; Xiao, M.; Xu, W.; Huang, R. The Role of Deep Roots in Sorghum Yield Production under Drought Conditions. Agronomy 2020, 10, 611. https://doi.org/10.3390/agronomy10040611
Chen X, Wu Q, Gao Y, Zhang J, Wang Y, Zhang R, Zhou Y, Xiao M, Xu W, Huang R. The Role of Deep Roots in Sorghum Yield Production under Drought Conditions. Agronomy. 2020; 10(4):611. https://doi.org/10.3390/agronomy10040611
Chicago/Turabian StyleChen, Xiaofei, Qi Wu, Yue Gao, Jiao Zhang, Yitao Wang, Ruidong Zhang, Yufei Zhou, Muji Xiao, Wenjuan Xu, and Ruidong Huang. 2020. "The Role of Deep Roots in Sorghum Yield Production under Drought Conditions" Agronomy 10, no. 4: 611. https://doi.org/10.3390/agronomy10040611
APA StyleChen, X., Wu, Q., Gao, Y., Zhang, J., Wang, Y., Zhang, R., Zhou, Y., Xiao, M., Xu, W., & Huang, R. (2020). The Role of Deep Roots in Sorghum Yield Production under Drought Conditions. Agronomy, 10(4), 611. https://doi.org/10.3390/agronomy10040611




