Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Substrate and Biochar Properties
2.3. Substrate, Biochar, and Plant Analyses
2.4. Statistical Analysis
3. Results
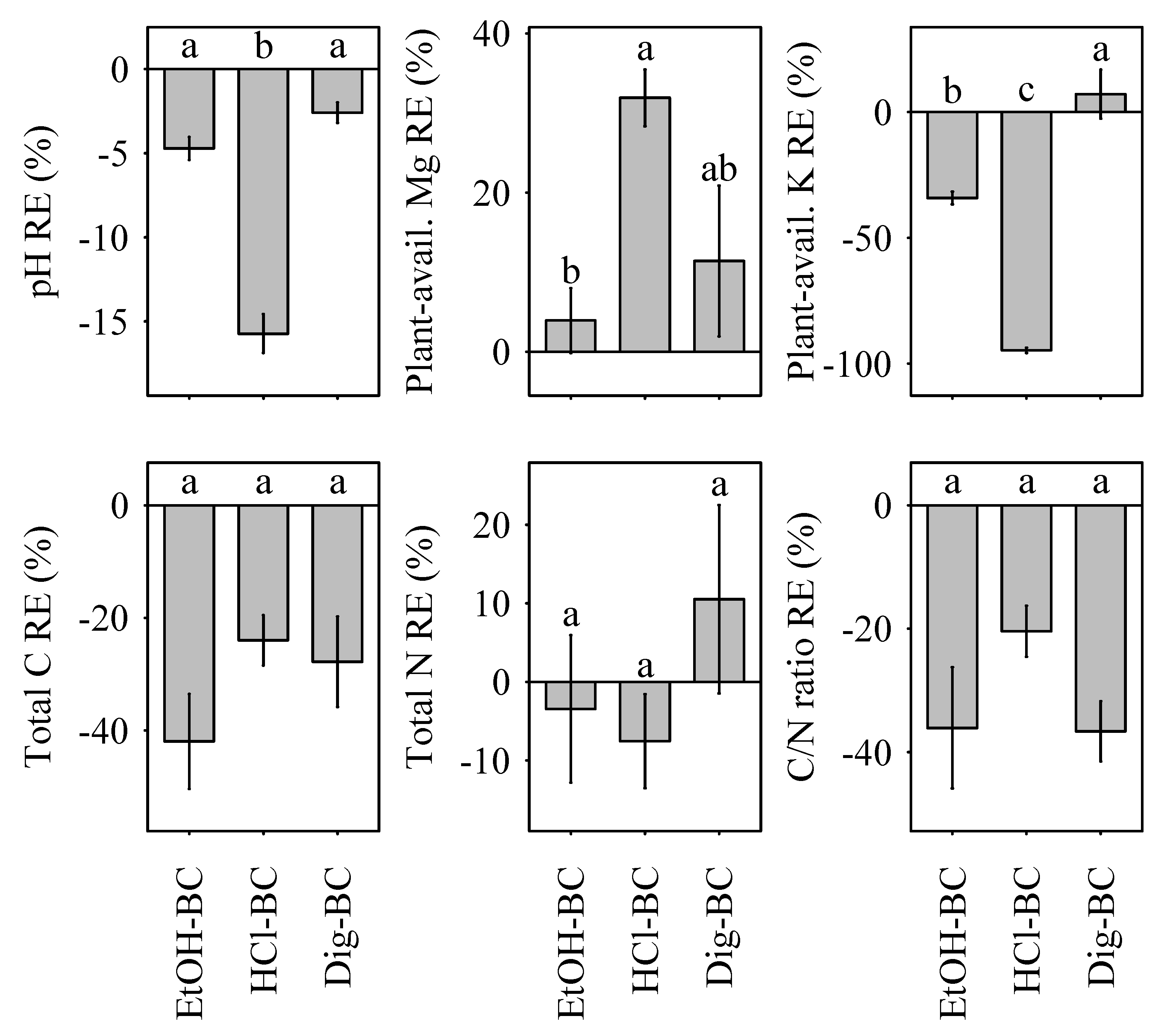
3.1. pH, C/N Ratio, and Nutrient Content in the Substrate
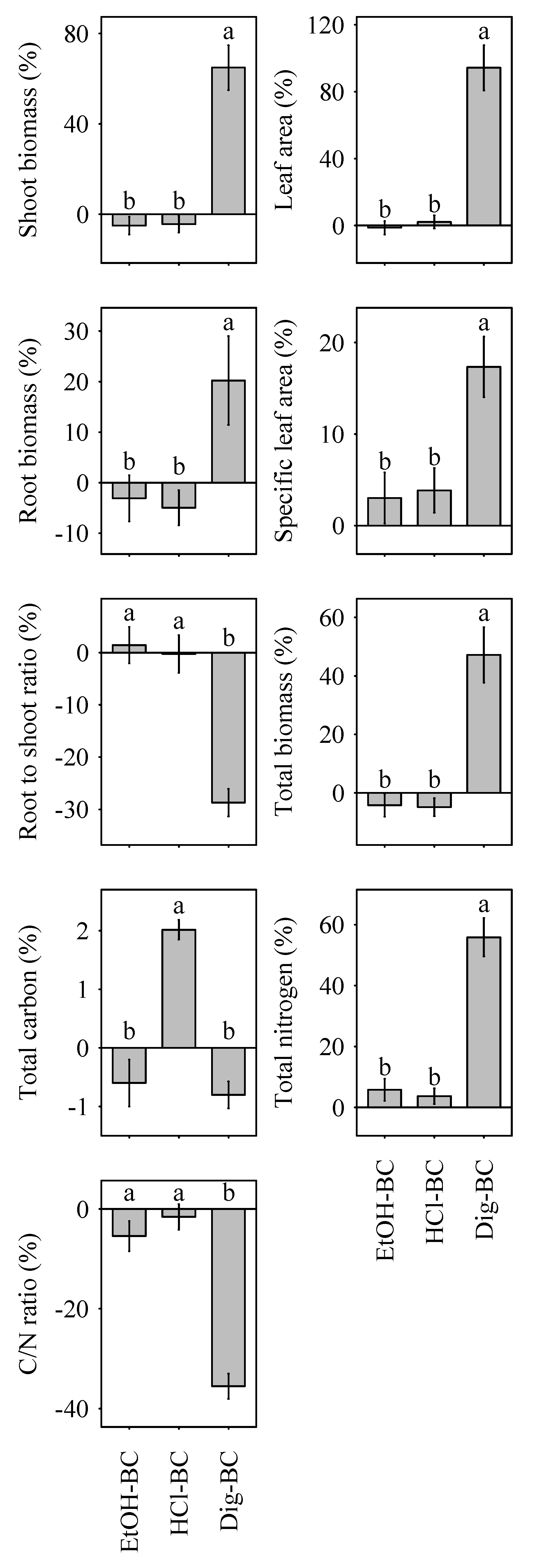
3.2. Plant Growth
3.3. Nutrient Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.; Bird, M.I.; Wurster, C.; Smernik, R. Rapid degradation of pyrogenic carbon. Glob. Chang. Biol. 2012, 18, 3306–3316. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8, 044049. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Biedermann, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Macdonald, L.M.; Farrell, M.; Van Zwieten, L.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source ofcontamination—Phytotoxic effects on cress seed (Lepidiumsativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar-Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Joseph, S.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Li, L.; Taylor, P.; et al. Shifting paradigms: Development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Novak, J.M.; Cantrell, K.B.; Watts, D.W.; Busscher, W.J.; Johnson, M.G. Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 2014, 14, 330–343. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2014, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Prost, K.; Borchard, N.; Siemens, J.; Kautz, T. Biochar Affected by Composting with Farmyard Manure. J. Environ. Qual. 2013, 42, 164–172. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, A.; Xu, R. Sorption of As(V) by Aluminum-Modified Crop Straw-Derived Biochars. Water Air Soil Pollut. 2013, 224, 1610. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Mohd, W.; Wan, A.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Patents Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Thring, R.W.; Arocena, J.M. Chemical Pretreatment of Rice Straw Biochar: Effect on Biochar Properties and Hexavalent Chromium Adsorption. Int. J. Environ. Res. 2019, 13, 91–105. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Kong, Z.; Li, C.Z.; Liu, D.; Yu, Y.; Gao, X. Removal and recycling of inherent inorganic nutrient species in mallee biomass and derived biochars by water leaching. Ind. Eng. Chem. Res. 2011, 50, 12143–12151. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Gonçalves, M.; Barbosa, R.; Mendes, B.; Pinto, F.; Gulyurtlu, I. Toxicity of char residues produced in the co-pyrolysis of different wastes. Waste Manag. 2010, 30, 628–635. [Google Scholar] [CrossRef]
- Lievens, C.; Mourant, D.; Hu, X.; Wang, Y.; Wu, L.; Rossiter, A.; Gunawan, R.; He, M.; Li, C.Z. A case study: What is leached from mallee biochars as a function of pH? Environ. Monit. Assess. 2018, 190, 294. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, L.; Xiao, D.; Lv, J.; Wen, B.; Ma, Y.; Zhang, S. Selected dark sides of biomass-derived biochars as environmental amendments. J. Environ. Sci. (China) 2017, 54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Taskin, M.B.; Kaya, E.C.; Atakol, O.; Emir, E.; Inal, A.; Gunes, A. Effect of acid modification of biochar on nutrient availability and maize growth in a calcareous soil. Soil Use Manag. 2017, 33, 447–456. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Nagel, K.A.; Temperton, V.M.; Dietrich, C.C.; Briese, C.; Jablonowski, N.D. Coming Late for Dinner: Localized Digestate Depot Fertilization for Extensive Cultivation of Marginal Soil With Sida hermaphrodita. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Temperton, V.M.; Jablonowski, N.D. Maize Silage Digestate Application Affecting Germination and Early Growth of Maize Modulated by Soil Type. Agronomy 2019, 9, 473. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Kabir, A.K.M.R.; Müller, J. Effect of self-purging pyrolysis on yield of biochar from maize cobs, husks and leaves. Bioresour. Technol. 2016, 218, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.; Levkovitch, I.; Graber, E.R. pH-Dependent Mineral Release and Surface Properties of Cornstraw Biochar: Agronomic Implications. Environ. Sci. Technol. 2010, 44, 9318–9323. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quat. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Van Der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; DuSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Gale, N.; Halim, A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A.; Johnson, M.G.; Colosky, E.C.; Ippolito, J.A.; Trigo, C. Physical Disintegration of Biochar: An Overlooked Process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Grassein, F.; Lemauviel-lavenant, S.; Lavorel, S.; Bahn, M.; Bardgett, R.D. Relationships between functional traits and inorganic nitrogen acquisition among eight contrasting European grass species Relationships between functional traits and inorganic nitrogen acquisition among eight contrasting European grass species. Ann. Bot. 2015, 115, 107–115. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
| NM-BC | EtOH-BC | HCl-BC | Dig-BC | UNI | |
|---|---|---|---|---|---|
| RE (%) | |||||
| pH | 22.6 ± 0.9 a | 16.8 ± 0.6 b | 3.3 ± 1.4 c | 19.4 ± 1.0 ab | Y |
| Csoil | 11,448.1 ± 1549.0 a | 10,273.8 ± 3265.6 a | 8659.7 ± 925.0 a | 8197.6 ± 1164.7 a | Y |
| Nsoil | 395.6 ± 77.3 a | 368.9 ± 49.9 a | 353.3 ± 40.1 a | 428.9 ± 48.2 a | Y |
| C/Nsoil | 2206.0 ± 278.1 a | 1867.0 ± 396.0 ab | 1745.8 ± 190.0 ab | 1399.5 ± 209.1 b | Y |
| Mgsoil | −22.3 ± 1.5 b | −18.9 ± 4.4 b | 2.6 ± 3.4 a | −12.8 ± 8.6 ab | N |
| Ksoil | 1374.7 ± 284.5 a | 895.3 ± 201.1 a | −34.9 ± 10.7 b | 1640.4 ± 413.4 a | N |
| DWshoot | 19.9 ± 5.0 b | 13.8 ± 5.0 b | 14.4 ± 4.6 b | 96.5 ± 11.9 a | Y |
| DWroot | −4.7 ± 4.5 b | −10.0 ± 3.2 b | −10.8 ± 2.9 b | 9.4 ± 5.9 a | N |
| DWSR | −21.0 ± 3.1 a | −20.4 ± 3.0 a | −22.2 ± 2.4 a | −44.2 ± 2.2 b | Y |
| DW | 8.8 ± 4.4 b | 3.4 ± 3.8 b | 3.1 ± 3.6 b | 57.2 ± 9.0 a | Y |
| LA | 7.4 ± 4.7 b | 6.6 ± 5.4 b | 9.8 ± 4.7 b | 113.8 ± 18.5 a | Y |
| SLA | −7.2 ± 2.7 b | −4.4 ± 3.1 b | −3.2 ±3.4 b | 8.8 ± 3.6 a | N |
| Cplant | −1.7 ± 0.4 b | −2.3 ± 0.5 b | 0.2 ± 0.4 a | −2.5 ± 0.4 b | N |
| Nplant | −12.3 ± 3.4 b | −7.4 ± 3.9 b | −9.6 ± 2.4 b | 35.9 ± 6.1 a | N |
| C/Nplant | 14.3 ± 5.1 b | 7.8 ± 4.8 b | 11.6 ± 3.3 b | −26.8 ± 2.9 a | N |
| Substrate | pH | N | C | K | Mg | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | |||||||||
| Biochar | 8.9 ± 0.1 | 9.8 | 786.0 | 21,206 | 543 | |||||
| Day 0 | Day 35 | Day 0 | Day 35 | Day 0 | Day 35 | Day 0 | Day 35 | Day 0 | Day 35 | |
| Sandy substrate | 7.1 ± 0.1 | 7.1 ± 0.2 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 4.2 ± 1.7 | 51.1 ± 24.0 | 2.3 ± 0.8 | 50.2 ± 4.2 |
| NM-BC | 8.6 ± 0.0 | 8.6 ± 0.1 | 0.7 ±0.0 | 0.6 ±0.0 | 31.5 ± 2.0 | 28.5 ± 5.0 | 31.4 ± 0.7 | 709.7 ± 113.1 | 1.8 ± 0.0 | 37.6 ± 1.0 |
| EtOH-BC | 8.1 ± 0.1 | 8.3 ±0.0 | 0.7 ±0.0 | 0.6 ±0.0 | 36.9 ± 2.6 | 20.1 ± 2.6 | 22.0 ± 0.2 | 465.3 ± 53.4 | 1.8 ± 0.0 | 35.0 ± 1.2 |
| HCl-BC | 7.5 ± 0.1 | 7.5 ± 0.1 | 0.7 ±0.0 | 0.6 ±0.0 | 41.2 ± 4.1 | 25.3 ± 1.0 | 6.8 ± 0.2 | 22.4 ± 1.2 | 2.2 ± 0.2 | 45.8 ± 0.6 |
| Dig-BC | 8.5 ±0.1 | 8.3 ± 0.1 | 0.8 ±0.1 | 0.7 ±0.0 | 31.4 ± 3.9 | 22.0 ± 4.5 | 35.4 ± 1.8 | 763.3 ± 91.3 | 1.8 ± 0.1 | 34.1 ± 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietrich, C.C.; Rahaman, M.A.; Robles-Aguilar, A.A.; Latif, S.; Intani, K.; Müller, J.; Jablonowski, N.D. Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.). Agronomy 2020, 10, 567. https://doi.org/10.3390/agronomy10040567
Dietrich CC, Rahaman MA, Robles-Aguilar AA, Latif S, Intani K, Müller J, Jablonowski ND. Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.). Agronomy. 2020; 10(4):567. https://doi.org/10.3390/agronomy10040567
Chicago/Turabian StyleDietrich, Charlotte C., Md Arifur Rahaman, Ana A. Robles-Aguilar, Sajid Latif, Kiatkamjon Intani, Joachim Müller, and Nicolai D. Jablonowski. 2020. "Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.)" Agronomy 10, no. 4: 567. https://doi.org/10.3390/agronomy10040567
APA StyleDietrich, C. C., Rahaman, M. A., Robles-Aguilar, A. A., Latif, S., Intani, K., Müller, J., & Jablonowski, N. D. (2020). Nutrient Loaded Biochar Doubled Biomass Production in Juvenile Maize Plants (Zea mays L.). Agronomy, 10(4), 567. https://doi.org/10.3390/agronomy10040567











