Ecosystem (Dis)benefits Arising from Formal and Informal Land-Use in Manchester (UK); a Case Study of Urban Soil Characteristics Associated with Local Green Space Management
Abstract
1. Introduction
2. Methods
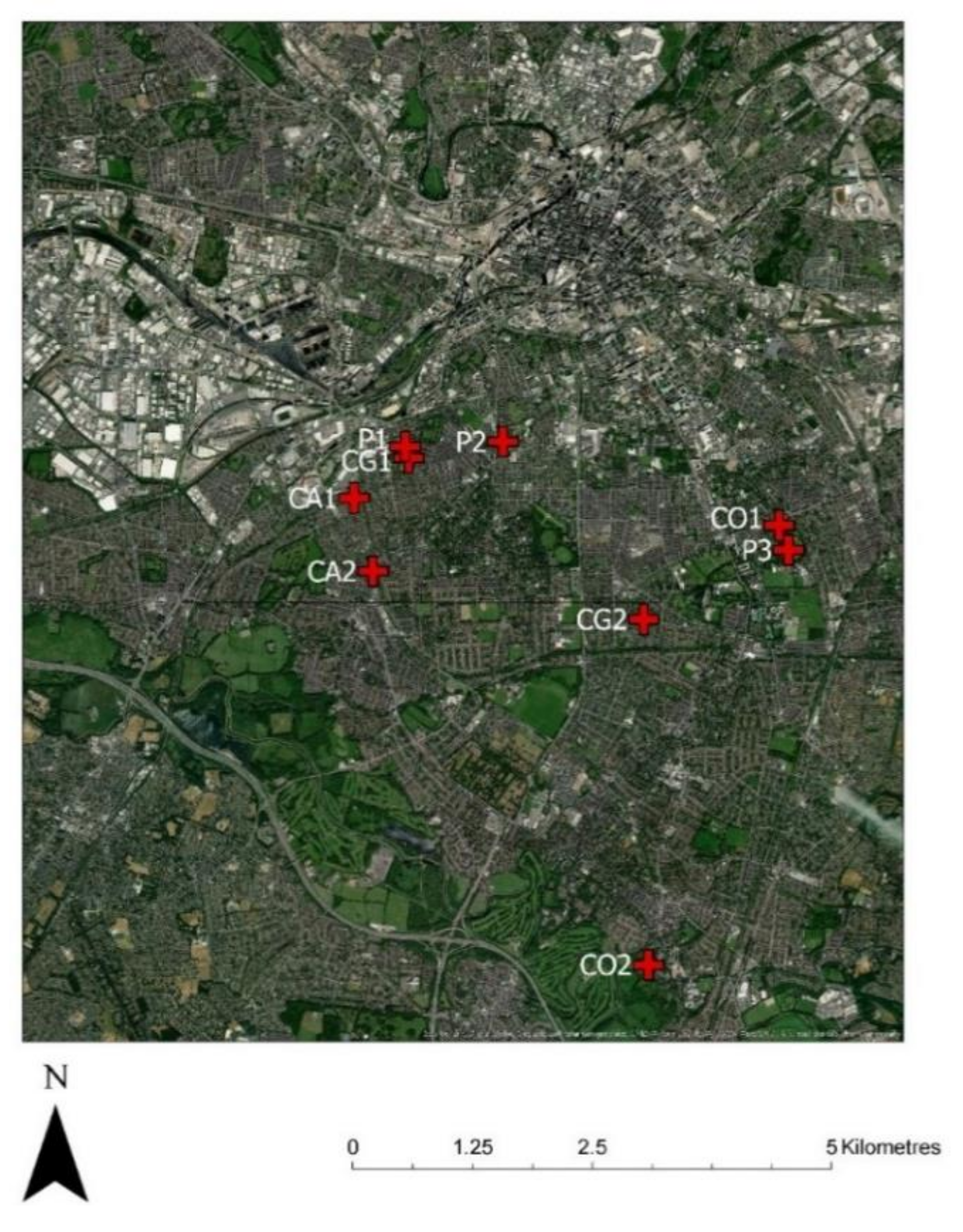
2.1. Site Selection
2.1.1. Community Gardens (CG)
2.1.2. Community Allotments (CA)
2.1.3. Community Orchards (CO)
2.1.4. Municipal Public Parks (P)
2.2. Soil Sampling
2.3. Soil Organic Matter and Carbon Determination
2.4. Determination of Soil pH, Total and Water-Soluble Lead and Zinc Concentrations
2.5. Measures of Soil CO2 Evolution
2.6. Statistical Analyses
3. Results
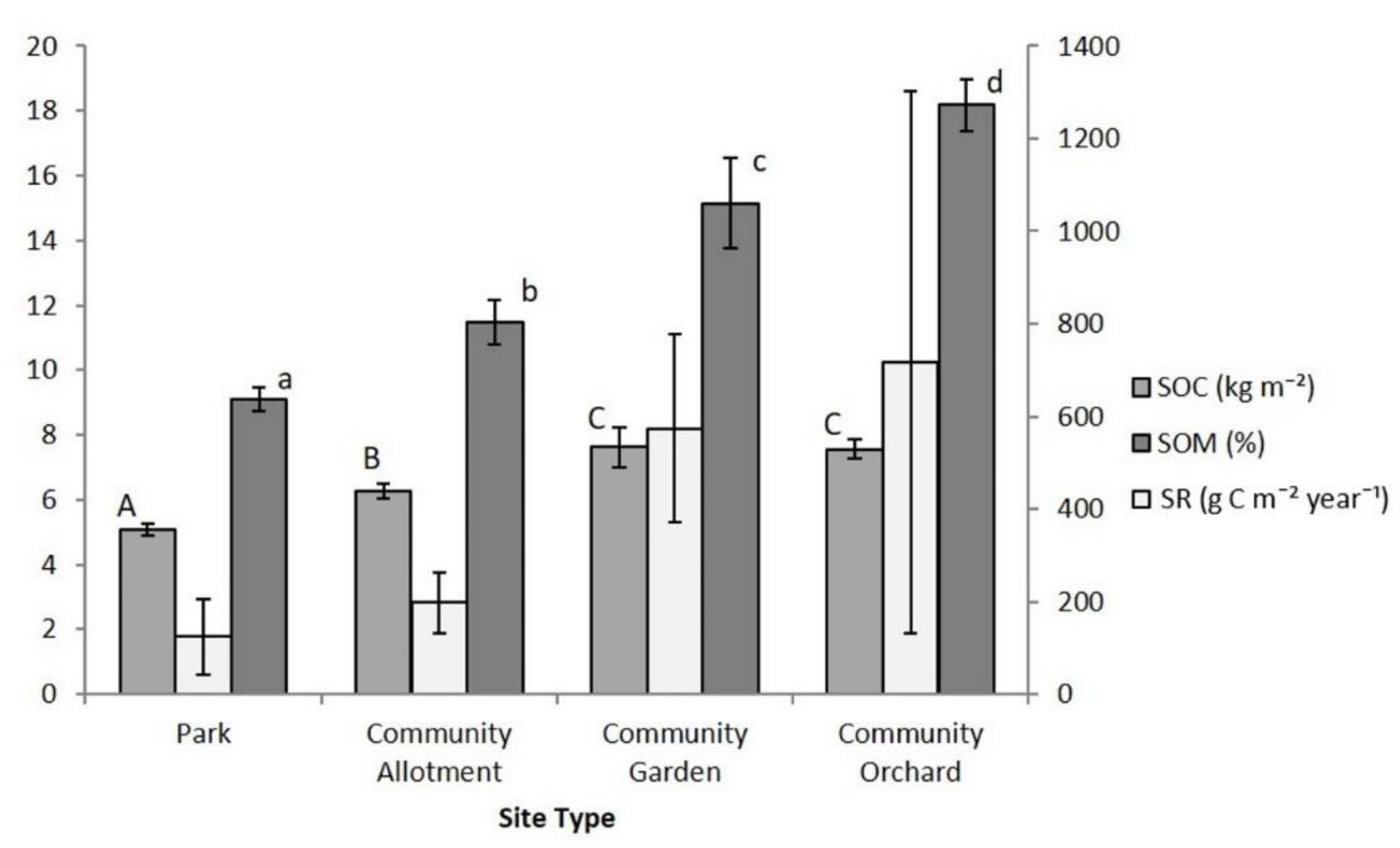
3.1. Soil Characteristics and Carbon Storage
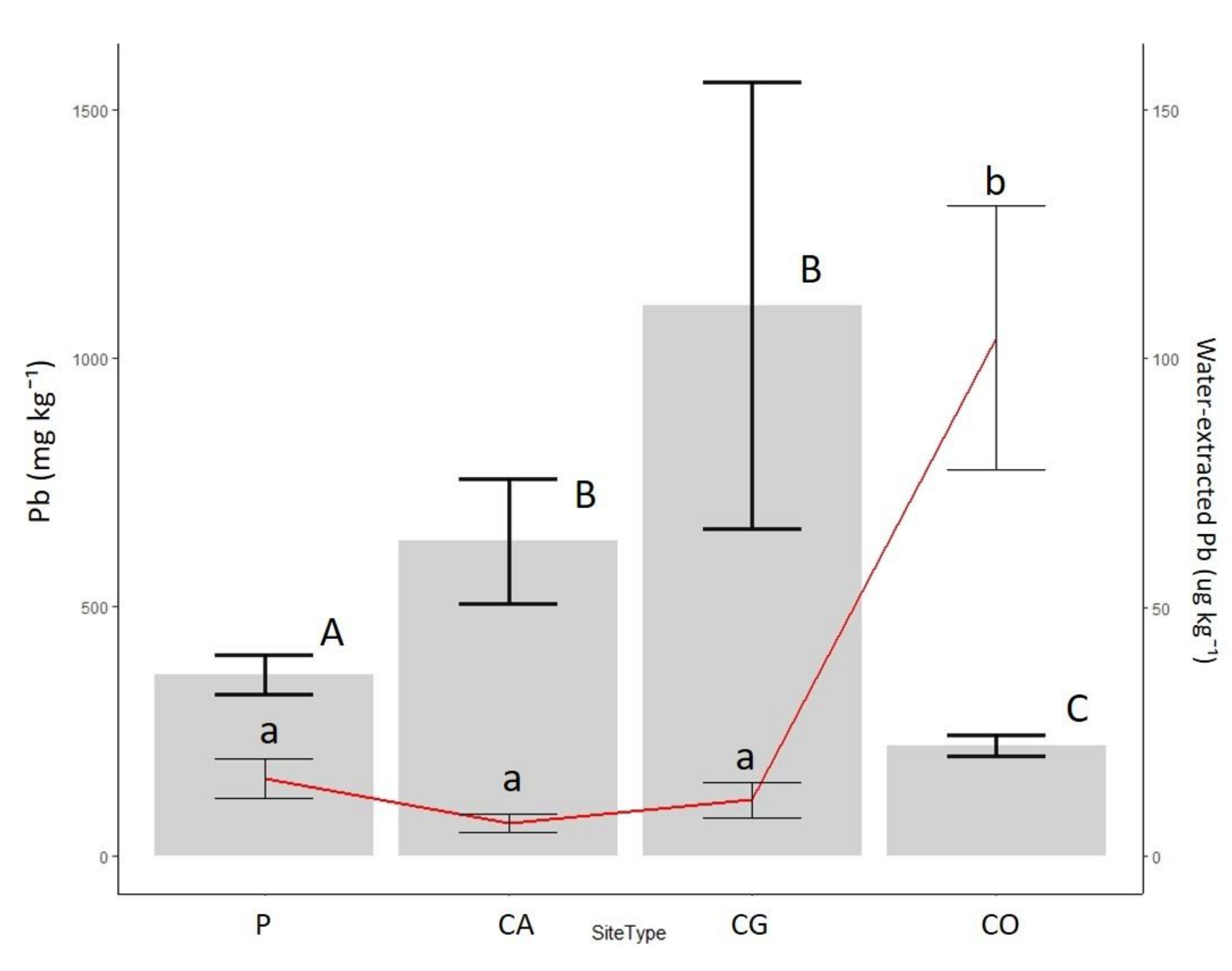
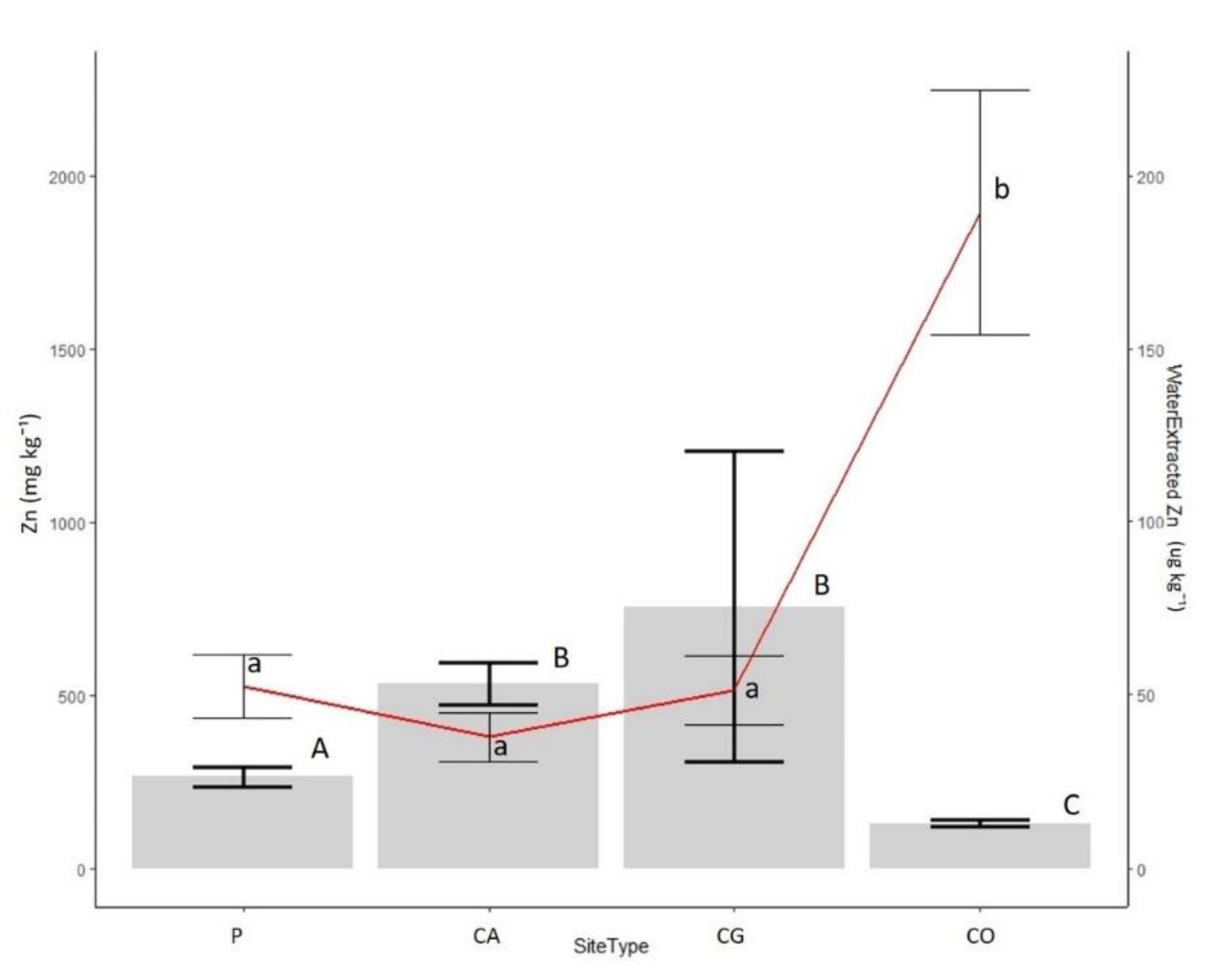
3.2. Lead and Zinc Concentrations
4. Discussion
4.1. Soil Carbon Storage and Respiration
4.2. Mobility of Lead and Zinc
4.3. Considerations for Urban Green Space Planning
5. Conclusions
Author Contributions
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs. World Urbanisation Prospects: 2007 Revision; United Nations: New York, NY, USA, 2007. [Google Scholar]
- Grimm, N.; Faeth, S.; Golubiewski, N.; Redman, C.; Wu, J.; Bai, X.; Briggs, J. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Krasny, M.; Tidball, K. Civic Ecology: Adaptation and Transformation from the Ground Up; MIT Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Folke, C.; Jansson, Å.; Larsson, J.; Costanza, R. Ecosystem appropriation by cities. Ambio 1997, 26, 167–172. [Google Scholar]
- Scott, A.J.; Carter, C.; Reed, M.R.; Larkham, P.; Adams, D.; Morton, N.; Waters, R.; Collier, D.; Crean, C.; Curzon, R.; et al. Disintegrated development at the rural–urban fringe: Re-connecting spatial planning theory and practice. Prog. Plan. 2013, 83, 1–52. [Google Scholar] [CrossRef]
- McDonnell, M.J.; Pickett, S.T. Ecosystem Structure and Function along Urban-Rural Gradients: An Unexploited Opportunity for Ecology. Ecology 1990, 71, 1232–1237. [Google Scholar] [CrossRef]
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Barton, D.N. Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 2013, 86, 235–245. [Google Scholar] [CrossRef]
- Chipungu, L.; Magidimisha, H.; Hardman, M.; Beesley, L. The importance of soil quality in the safe practice of urban agriculture in Zimbabwe, Kenya and South Africa. In Land-Use Change Impacts on Soil Processes in Tropical and Savannah Ecosystems; Brearley, F., Thomas, A., Eds.; Centre for Agriculture and Biosciences International: Wallingford, UK, 2015; pp. 72–84. [Google Scholar]
- Dennis, M.; Armitage, R.; James, P. Social-ecological innovation: Adaptive responses to urban environmental conditions. Urban Ecosyst. 2016, 19, 1063–1082. [Google Scholar] [CrossRef]
- Dennis, M.; Barlow, D.; Cavan, G.; Cook, P.; Gilchrist, A.; Handley, J.F.; James, P.; Thompson, J.; Tzoulas, K.; Wheater, C.P.; et al. Mapping Urban Green Infrastructure: A Novel Landscape-Based Approach to Incorporating Land Use and Land Cover in the Mapping of Human-Dominated Systems. Land 2018, 7, 17. [Google Scholar] [CrossRef]
- CIWEM (Chartered Institution of Water and Environmental Management). Multi-Functional Urban. Green Infrastructure. London: CIWEM. Available online: http://www.ciwem.org/FileGet.ashx?id=1875&library=Public%20 (accessed on 7 April 2020).
- Dennis, M.; James, P. User participation in urban green commons: Exploring the links between access, voluntarism, biodiversity and well being. Urban For. Urban Green. 2016, 15, 22–31. [Google Scholar] [CrossRef]
- MEA (Millenium Ecosystem Assessment). Ecosystems and Human Well-Being: Health Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- UK NEA (United Kingdom National Ecosystem Assessment). UK National Ecosystem Assessment: Understanding Nature’s Value to Society; UK NEA: London, UK, 2011. [Google Scholar]
- Nelson, E.; Mendoza, G.; Regetz, J.; Polasky, S.; Tallis, H.; Cameron, D.; Chan, K.M.A.; Daily, G.C.; Goldstein, J.; Kareiva, P.; et al. Modeling multiple ecosystem services, biodiversity conservation, commodity production, and tradeoffs at landscape scales. Front. Ecol. Environ. 2009, 7, 4–11. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Strohbach, M.; Kroll, F.; Schwarz, N.; Seppelt, R. Synergies, Trade-offs, and Losses of Ecosystem Services in Urban Regions: An Integrated Multiscale Framework Applied to the Leipzig-Halle Region, Germany. Ecol. Soc. 2012, 17, 22. [Google Scholar] [CrossRef]
- Howe, C.; Suich, H.; Vira, B.; Mace, G.M. Creating win-wins from trade-offs? Ecosystem services for human well-being: A meta-analysis of ecosystem service trade-offs and synergies in the real world. Glob. Environ. Chang. 2014, 28, 263–275. [Google Scholar] [CrossRef]
- Breuste, J.H.; Artmann, M. Allotment Gardens Contribute to Urban Ecosystem Service: Case Study Salzburg, Austria. J. Urban Plan. Dev. 2015, 141, A5014005. [Google Scholar] [CrossRef]
- Speak, A.; Mizgajski, A.; Borysiak, J. Allotment gardens and parks: Provision of ecosystem services with an emphasis on biodiversity. Urban For. Urban Green. 2015, 14, 772–781. [Google Scholar] [CrossRef]
- Cabral, I.; Keim, J.; Engelmann, R.; Kraemer, R.; Siebert, J.; Bonn, A. Ecosystem services of allotment and community gardens: A Leipzig, Germany case study. Urban For. Urban Green. 2017, 23, 44–53. [Google Scholar] [CrossRef]
- Dennis, M.; James, P. Site-specific factors in the production of local urban ecosystem services: A case study of community-managed green space. Ecosyst. Serv. 2016, 17, 208–216. [Google Scholar] [CrossRef]
- Charzynski, P.; Bednarek, R.; Hudańska, P.; Świtoniak, M. Issues related to classification of garden soils from the urban area of Toruń, Poland. Soil Sci. Plant Nutr. 2018, 64, 132–137. [Google Scholar] [CrossRef]
- Ernstson, H.; Sörlin, S.; Elmqvist, T. Social Movements and Ecosystem Services—The Role of Social Network Structure in Protecting and Managing Urban Green Areas in Stockholm. Ecol. Soc. 2008, 13, 39. [Google Scholar] [CrossRef]
- Barthel, S.; Folke, C.; Colding, J. Social–ecological memory in urban gardens—Retaining the capacity for management of ecosystem services. Glob. Environ. Chang. 2010, 20, 255–265. [Google Scholar] [CrossRef]
- Olsson, P.; Galaz, V. Social-Ecological Innovation and Transformation; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 223–247. [Google Scholar]
- Krasny, M.E.; Russ, A.; Tidball, K.; Elmqvist, T. Civic ecology practices: Participatory approaches to generating and measuring ecosystem services in cities. Ecosyst. Serv. 2014, 7, 177–186. [Google Scholar] [CrossRef]
- UK NEA (United Kingdom National Ecosystem Assessment). Follow-On UK National Ecosystem Assessment, Follow-On: Synthesis of the Key Findings; UK NEA: London, UK, 2014. [Google Scholar]
- Dennis, M.; James, P. Considerations in the valuation of urban green space: Accounting for user participation. Ecosyst. Serv. 2016, 21, 120–129. [Google Scholar] [CrossRef]
- Klimas, C.; Williams, A.; Hoff, M.; Lawrence, B.; Thompson, J.; Montgomery, J. Valuing Ecosystem Services and Disservices across Heterogeneous Green Spaces. Sustainabiluty 2016, 8, 853. [Google Scholar] [CrossRef]
- Greinert, A. The heterogeneity of urban soils in the light of their properties. J. Soils Sediments 2015, 15, 1725–1737. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Jenn, P.; Lepp, N. Carbon and Metal(loid)s in Parkland and Road Verge Surface Soils in the City of Liverpool, UK. Agronomy 2020, 10, 335. [Google Scholar] [CrossRef]
- Jo, H.-K.; McPherson, G.E. Carbon Storage and Flux in Urban Residential Greenspace. J. Environ. Manag. 1995, 45, 109–133. [Google Scholar] [CrossRef]
- Pouyat, R.; Groffman, P.M.; Yesilonis, I.; Hernandez, L. Soil carbon pools and fluxes in urban ecosystems. Environ. Pollut. 2002, 116, S107–S118. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Nowak, D.J. Carbon Storage by Urban Soils in the United States. J. Environ. Qual. 2006, 35, 1566–1575. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Golubiewski, N.E. A comparison of soil organic carbon stocks between residential turf grass and native soil. Urban Ecosyst. 2008, 12, 45–62. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Szlavecz, K.; Yesilonis, I.D.; Groffman, P.M.; Schwarz, K. Urban Ecosystem Ecology; Aitkenhead-Peterson, J., Volder, J., Eds.; American Society of Agronomy: Madison, WI, USA, 2015; pp. 119–152. [Google Scholar]
- Kaye, J.P.; McCulley, R.L.; Burke, I.C. Carbon fluxes, nitrogen cycling, and soil microbial communities in adjacent urban, native and agricultural ecosystems. Glob. Chang. Biol. 2005, 11, 575–587. [Google Scholar] [CrossRef]
- Golubiewski, N.E. Urbanization Increases Grassland Carbon Pools: Effects of Landscaping in Colorado’s Front Range. Ecol. Appl. 2006, 16, 555–571. [Google Scholar] [CrossRef]
- Parry, G.; Johnson, M.; Bell, R. Trace metal surveys of soil as a component of strategic and local planning policy development. Environ. Pollut. Ser. B Chem. Phys. 1981, 2, 97–107. [Google Scholar] [CrossRef]
- Beesley, L.; Dickinson, N. Carbon and trace element fluxes in the pore water of an urban soil following greenwaste compost, woody and biochar amendments, inoculated with the earthworm Lumbricus terrestris. Soil Biol. Biochem. 2011, 43, 188–196. [Google Scholar] [CrossRef]
- Stringer, L.C.; Dougill, A.J.; Fraser, E.D.; Hubacek, K.; Prell, C.; Reed, M.S. Unpacking “Participation” in the Adaptive Management of Social–ecological Systems: A Critical Review. Ecol. Soc. 2006, 11, 39. [Google Scholar] [CrossRef]
- Kabisch, N.; Frantzeskaki, N.; Pauleit, S.; Naumann, S.; Davis, M.; Artmann, M.; Haase, D.; Knapp, S.; Korn, H.; Stadler, J.; et al. Nature-based solutions to climate change mitigation and adaptation in urban areas: Perspectives on indicators, knowledge gaps, barriers, and opportunities for action. Ecol. Soc. 2016, 21, 15. [Google Scholar] [CrossRef]
- European Commission. Hard Surfaces. In Hidden Costs-Searching for Alternatives to Land Take and Soil Sealing; European Union: Luxembourg, 2013. [Google Scholar] [CrossRef]
- Ritvo, H. The Dawn of Green: Manchester, Thirlmere, and Modern Environmentalism; University of Chicago Press: Chicago, IL, USA, 2009. [Google Scholar]
- Cranfield Soil and Agrifood Institute. Soilscapes. Available online: http://www.landis.org.uk/soilscapes/ (accessed on 7 April 2020).
- Rossiter, D.G. Classification of Urban and Industrial Soils in the World Reference Base for Soil Resources. J. Soils Sediments 2007, 7, 96–100. [Google Scholar] [CrossRef]
- UK Soils Portal Map Viewer. Available online: http://mapapps2.bgs.ac.uk/ukso/home.html (accessed on 7 April 2020).
- Holland, L. Diversity and connections in community gardens: A contribution to local sustainability. Local Environ. 2004, 9, 285–305. [Google Scholar] [CrossRef]
- Cox, R.J.; Peterson, H.L.; Young, J.; Cusik, C.; Espinoza, E. The forensic analysis of soil organic by FTIR. Forensic Sci. Int. 2000, 108, 107–116. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Jeroen, J.G.; Tittonell, P.A. Estimating soil organic carbon through loss on ignition: Effects of ignition conditions and structural water loss. Eur. J. Soil Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis: Part 3, Chemical Methods; Sparks, D., Page, A., Helmke, P., Loeppert, T., Eds.; Soil Science Society of America; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Clark, S.; Menrath, W.; Chen, M.; Roda, S.; Succop, P. Use of a field portable X-ray fluorescence analyzer to determine the concentration of lead and other metals in soil samples. Ann. Agric. Environ. Med. 1999, 6, 27–32. [Google Scholar]
- Markey, A.M.; Clark, C.S.; Succop, P.A.; Roda, S. Determination of the feasibility of using a portable X-ray fluorescence (XRF) analyzer in the field for measurement of lead content of sieved soil. J. Environ. Health 2008, 70, 24–29. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 10 April 2020).
- Defra and Environment Agency. Soil Guideline Values for Lead Contamination; R&D Publication SGV London; Environment Agency: London, UK, 2002. [Google Scholar]
- Beesley, L. Carbon storage and fluxes in existing and newly created urban soils. J. Environ. Manag. 2012, 104, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Lal, R.; Follett, R.F.; Stewart, B.A.; Kimble, J.M. Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci. 2007, 172, 943–956. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier: Burlington, MA, USA, 2006. [Google Scholar]
- Blagodatskaya, E.; Yuyukina, T.; Blagodatsky, S.; Kuzyakov, Y. Three-source-partitioning of microbial biomass and of CO2 efflux from soil to evaluate mechanisms of priming effects. Soil Biol. Biochem. 2011, 43, 778–786. [Google Scholar] [CrossRef]
- Beesley, L. Respiration (CO2 flux) from urban and peri-urban soils amended with green waste compost. Geoderma 2014, 223, 68–72. [Google Scholar] [CrossRef]
- Davies, D.; Alloway, B.J. (Eds.) Heavy Metals in Soils; Blackie: London, UK, 1990; pp. 177–196. [Google Scholar]
- Madrid, F.; Reinoso, R.; Florido, M.; Barrientos, E.D.; Ajmone-Marsan, F.; Davidson, C.; Madrid, L. Estimating the extractability of potentially toxic metals in urban soils: A comparison of several extracting solutions. Environ. Pollut. 2007, 147, 713–722. [Google Scholar] [CrossRef]
- Ruiz-Cortés, E.; Reinoso, R.; Díaz-Barrientos, E.; Madrid, L. Concentrations of Potentially Toxic Metals in Urban Soils of Seville: Relationship with Different Land Uses. Environ. Geochem. Health 2005, 27, 465–474. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Beesley, L.; Lepp, N.W.; Dickinson, N.; Hartley, W.; Clemente, R. Field sampling of soil pore water to evaluate trace element mobility and associated environmental risk. Environ. Pollut. 2011, 159, 3078–3085. [Google Scholar] [CrossRef]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Roig, A.; Bernal, M.P. Heavy metal bioavailability in a soil affected by mineral sulphides contamination following the mine spillage at Aznalcóllar (Spain). Biodegradation 2003, 14, 199–205. [Google Scholar] [CrossRef]
- Ross, S. Sources and Forms of Potentially Toxic Metals in Soil-Plant Systems. In Toxic Metals in Soil-Plant Systems; Ross, S., Ed.; John Wiley and Sons Ltd: Chichester, UK, 1994; pp. 3–25. [Google Scholar]
- Kabata-Pendias, A. Soil–plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Walker, D.J.; Clemente, R.; Bernal, M.P. Contrasting effects of manure and compost on soil pH, heavy metal availability and growth of Chenopodium album L. in a soil contaminated by pyritic mine waste. Chemosphere 2004, 57, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Mauro, S.E.-D. An exploratory study of potential As and Pb contamination by atmospheric deposition in two urban vegetable gardens in Rome, Italy. J. Soils Sediments 2016, 18, 426–430. [Google Scholar] [CrossRef]
- Spliethoff, H.; Mitchell, R.G.; Shayler, H.; Marquez-Bravo, L.G.; Russell-Anelli, J.; Ferenz, G.; McBride, M. Estimated lead (Pb) exposures for a population of urban community gardeners. Environ. Geochem. Health 2016, 38, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Vasenev, V.; Kuzyakov, Y. Urban soils as hot spots of anthropogenic carbon accumulation: Review of stocks, mechanisms and driving factors. Land Degrad. Dev. 2018, 29, 1607–1622. [Google Scholar] [CrossRef]
- Lepp, N.W.; Hartley, J.; Toti, M.; Dickinson, N.M. Patterns of soil copper contamination in the vicinity of a copper rod rolling factory. Environ. Pollut. 1997, 95, 363–369. [Google Scholar] [CrossRef]
- Pulford, I.; Watson, C. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]

| Site | N | Area (m2) | Area Cultivated (m2) | Percent Cultivated | Bulk Density (g cm−3) | SOM (%) | pH | Total Lead (mg kg−1) | Total Zinc (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 5 | 55,000 | 0 | 0 | 0.92 (0.42) | 8.55 (0.72) | 5.84 (0.43) | 390.68 (37.71) | 336.60 (48.33) |
| P2 | 5 | 29,000 | 0 | 0 | 0.99 (0.02) | 9.28 (0.29) | 5.50 (0.27) | 387.05 (86.39) | 267.67 (42.50) |
| P3 | 5 | 130,000 | 0 | 0 | 1.00 (0.06) | 9.41 (0.61) | 5.63 (0.26) | 313.65 (85.69) | 188.98 (38.40) |
| CG1 | 5 | 936 | 36 | 4 | 0.87 (0.06) | 13.54 (1.16) | 6.36 (0.21) | 1394.87 (836.01) | 721.79 (371.48) |
| CG2 | 5 | 1530 | 80 | 5 | 0.88 (0.03) | 16.75 (2.49) | 6.63 (0.16) | 817.95 (406.24) | 788.21 (306.02) |
| CA1 | 5 | 950 | 403 | 42 | 0.96 (0.04) | 11.57 (1.29) | 6.37 (0.26) | 668.21 (261.24) | 465.11 (104.12) |
| CA2 | 5 | 630 | 195 | 31 | 0.94 (0.04) | 11.38 (0.70) | 6.55 (0.13) | 596.72 (46.29) | 601.51 (58.10) |
| CO1 | 5 | 1734 | 552 | 32 | 0.73 (0.03) | 19.45 (1.28) | 5.00 (0.34) | 213.15 (39.69) | 104.82 (11.36) |
| CO2 | 5 | 1044 | 260 | 25 | 0.71 (0.03) | 16.91 (0.63) | 4.27 (0.14) | 228.37 (22.40) | 153.90 (6.94) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, M.; Beesley, L.; Hardman, M.; James, P. Ecosystem (Dis)benefits Arising from Formal and Informal Land-Use in Manchester (UK); a Case Study of Urban Soil Characteristics Associated with Local Green Space Management. Agronomy 2020, 10, 552. https://doi.org/10.3390/agronomy10040552
Dennis M, Beesley L, Hardman M, James P. Ecosystem (Dis)benefits Arising from Formal and Informal Land-Use in Manchester (UK); a Case Study of Urban Soil Characteristics Associated with Local Green Space Management. Agronomy. 2020; 10(4):552. https://doi.org/10.3390/agronomy10040552
Chicago/Turabian StyleDennis, Matthew, Luke Beesley, Michael Hardman, and Philip James. 2020. "Ecosystem (Dis)benefits Arising from Formal and Informal Land-Use in Manchester (UK); a Case Study of Urban Soil Characteristics Associated with Local Green Space Management" Agronomy 10, no. 4: 552. https://doi.org/10.3390/agronomy10040552
APA StyleDennis, M., Beesley, L., Hardman, M., & James, P. (2020). Ecosystem (Dis)benefits Arising from Formal and Informal Land-Use in Manchester (UK); a Case Study of Urban Soil Characteristics Associated with Local Green Space Management. Agronomy, 10(4), 552. https://doi.org/10.3390/agronomy10040552






