Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characteristics of the Experimental Location
2.2. Soil Sampling
2.3. Pot Experiment
2.4. Sample Preparation and Analysis Methods
2.5. Preparation of the Amendment Materials
2.6. Analysis of Total Elements in Plant and Soil Samples
2.7. Analysis of Extratcable Elements in Soil Samples
2.8. Soil pH and Eh
2.9. Statistical Analysis
3. Results
3.1. Principal Component Analysis and Interpretation
3.2. The Content of Toxic Elements in Plants and Soil Affected by Modified Hairs-Fe2O3 (X2)
3.3. The Content of Toxic Elements in Plants and Soil Affected by Zeolite-KMnO4 (X2)
3.4. The Content of Toxic Elements in Plants and Soil Affected by Modified Hairs-Fe2O3 (X1)
3.5. The Content of Toxic Elements in Plants and Soil Affected by Zeolite-KMnO4 (X1)
3.6. The Content of Toxic Elements in Plants and Soil Affected by MnSO4 (X2)
3.7. The Content of Toxic Elements in Plants and Soil Affected by KMnO4 (X2)
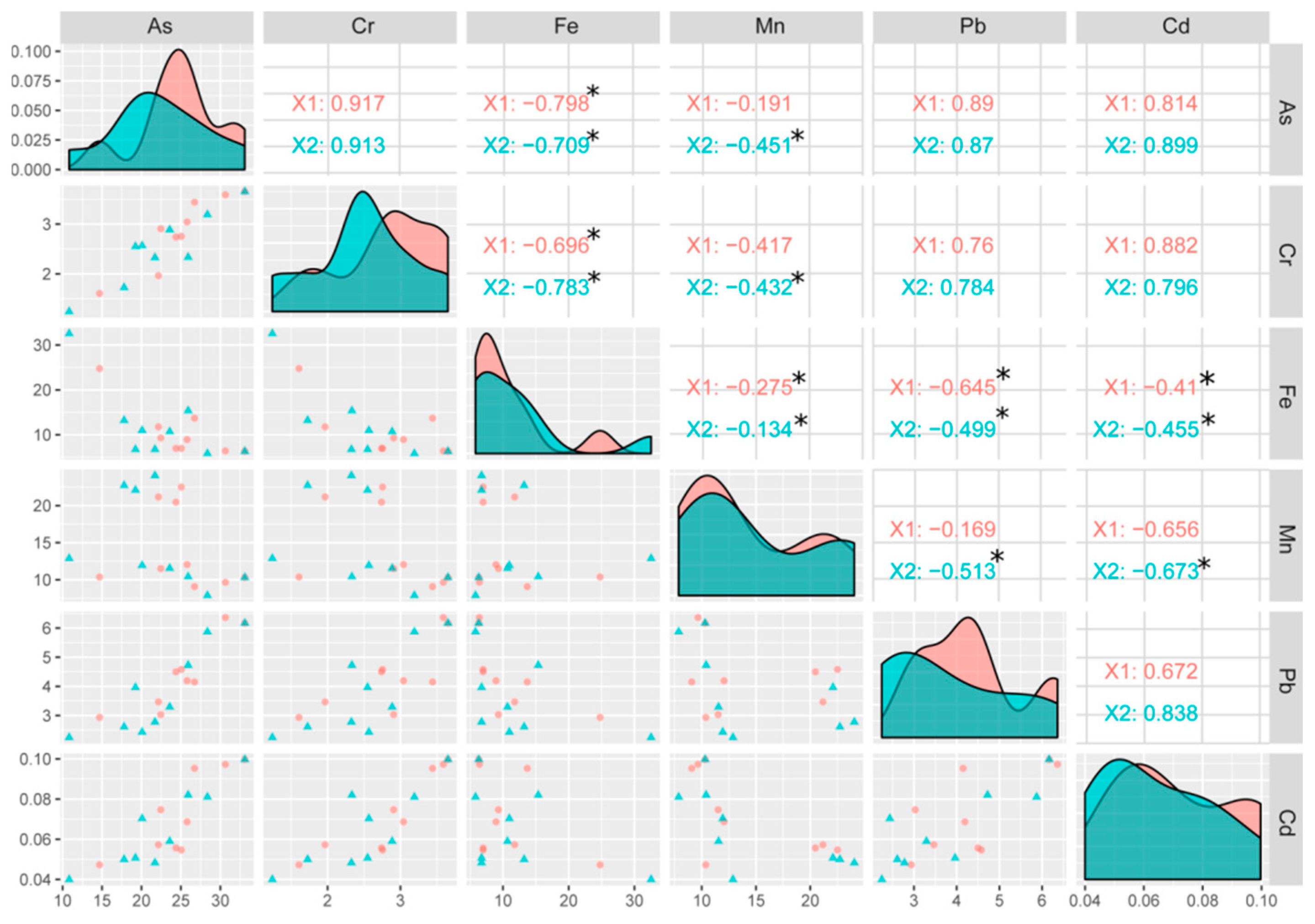
3.8. Correlation between Different Elements in the Rice Plant
4. Discussion
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vandenbossche, M.; Jimenez, M.; Casetta, M.; Traisnel, M. Remediation of heavy metals by biomolecules: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1644–1704. [Google Scholar] [CrossRef]
- Sun, L.; Wu, Q.; Liao, K.; Yu, P.; Cui, Q.; Rui, Q.; Wang, D. Contribution of heavy metals to toxicity of coal combustion related fine particulate matter (PM2.5) in Caenorhabditis elegans with wild-type or susceptible genetic background. Chemosphere 2016, 144, 2392–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Cotter-Howells, J.; Caporn, S. Remediation of contaminated land by formation of heavy metal phosphates. Appl. Geochem. 1996, 11, 335–342. [Google Scholar] [CrossRef]
- Javadian, H.; Taghavi, M. Application of novel Polypyrrole/thiol-functionalized zeolite Beta/MCM-41 type mesoporous silica nanocomposite for adsorption of Hg2+ from aqueous solution and industrial wastewater: Kinetic, isotherm and thermodynamic studies. Appl. Surf. Sci. 2014, 289, 487–494. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A. Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 2005, 277, 127–138. [Google Scholar] [CrossRef]
- Zhang, H.; Carrillo, F.; López-Mesas, M.; Palet, C. Valorization of keratin biofibers for removing heavy metals from aqueous solutions. Text. Res. J. 2019, 89, 1153–1165. [Google Scholar] [CrossRef]
- Armentrout, P.B.; Chen, Y.; Rodgers, M.T. Metal cation dependence of interactions with amino acids: Bond energies of Cs + to Gly, Pro, Ser, Thr, and Cys. J. Phys. Chem. A 2012, 116, 3989–3999. [Google Scholar] [CrossRef]
- Armentrout, P.B.; Armentrout, E.I.; Clark, A.A.; Cooper, T.E.; Stennett, E.M.S.; Carl, D.R. An experimental and theoretical study of alkali metal cation interactions with cysteine. J. Phys. Chem. B 2010, 114, 3927–3937. [Google Scholar] [CrossRef]
- Ashmawy, A.M.; Ibrahim, H.S.; Moniem, S.M.A.; Saleh, T.S. Immobilization of some metals in contaminated sludge by zeolite prepared from local materials. Toxicol. Environ. Chem. 2012, 94, 1657–1669. [Google Scholar] [CrossRef]
- Egashira, R.; Tanabe, S.; Habaki, H. Adsorption of heavy metals in mine wastewater by Mongolian natural zeolite. Procedia Eng. 2012, 42, 49–57. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.Y.; Shao, H.B.; Shao, M.A. The remediation of the lead-polluted garden soil by natural zeolite. J. Hazard. Mater. 2009, 169, 1106–1111. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Hajabbasi, M.A.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Aydinalp, C.; Marinova, S. Distribution and forms of heavy metals in some agricultural soils. Pol. J. Environ. Stud. 2003, 12, 629–633. [Google Scholar]
- Krishnamurti, G.S.R.; Huang, P.M.; Kozak, L.M. Sorption and desorption kinetics of cadmium from soils: Influence of phosphate. Soil Sci. 1999, 164, 888–898. [Google Scholar] [CrossRef]
- Antoniadis, V.; Robinson, J.S.; Alloway, B.J. Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 2008, 71, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Khan, K.; Lu, Y.; Khan, H.; Ishtiaq, M.; Khan, S.; Waqas, M.; Wei, L.; Wang, T. Heavy metals in agricultural soils and crops and their health risks in Swat District, northern Pakistan. Food Chem. Toxicol. 2013, 58, 449–458. [Google Scholar] [CrossRef]
- Kubo, K.; Iinuma, M.; Chen, H. Mastication as a Stress-Coping Behavior. Biomed Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef]
- Wang, G.; Fowler, B.A. Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicol. Appl. Pharmacol. 2008, 233, 92–99. [Google Scholar] [CrossRef]
- Guo, T.R.; Zhang, G.P.; Zhang, Y.H. Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf. B Biointerfaces 2007, 57, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy metal contamination in rice-producing soils of Hunan province, China and potential health risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhou, Q.; Liu, J.; Liu, W.; Wang, T. High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere 2008, 71, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Watanabe, T.; Shimbo, S.; Higashikawa, K.; Ikeda, M. Lead and cadmium contents in cereals and pulses in north-eastern China. Sci. Total Environ. 1998, 220, 137–145. [Google Scholar] [CrossRef]
- Zhang, M.; Wilson, M.J.; He, Z. Mineralogy of Red Soils in Southern China in Relation to Their Development and Charge Characteristics. In The Red Soils of China; Springer: Dordrecht, The Netherlands, 2004; pp. 35–61. [Google Scholar]
- Takahashi, G. Damage and Heavy Metal Pollution in China’s Far mLand: Reality and Solutions. J. Contemp. East Asia Stud. 2016, 5, 11–25. [Google Scholar] [CrossRef]
- Ge, S.; Xu, H.; Ji, M.; Jiang, Y. Characteristics of Soil Organic Carbon, Total Nitrogen, and C/N Ratio in Chinese Apple Orchards. Open J. Soil Sci. 2013, 03, 213–217. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3-Chemical Methods, SSSA Book Series, Madison, 961–1010; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Cucarella, V.; Zaleski, T.; Mazurek, R.; Renman, G. Fertilizer potential of calcium-rich substrates used for phosphorus removal from wastewater. Pol. J. Environ. Stud. 2007, 16, 817–822. [Google Scholar]
- Cordell, D.; White, S. Sustainable Phosphorus Measures: Strategies and Technologies for Achieving Phosphorus Security. Agronomy 2013, 3, 86–116. [Google Scholar] [CrossRef]
- Walker, J.M.; Barber, S.A. Absorption of potassium and rubidium from the soil by corn roots. Plant Soil 1962, 17, 243–259. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef]
- Burridge, J.C.; Hewitt, I.J. A comparison of two soil-extraction procedures for the determination of edta-extractable copper and manganese. Commun. Soil Sci. Plant Anal. 1987, 18, 301–310. [Google Scholar] [CrossRef]
- Palaniswamy, K.M.; Gomez, K.A. Length-Width Method for Estimating Leaf Area of Rice1. Agron. J. 1974, 66, 430. [Google Scholar] [CrossRef]
- Kataby, G.; Prozorov, T.; Koltypin, Y.; Cohen, H.; Sukenik, C.N.; Ulman, A.; Gedanken, A. Self-Assembled Monolayer Coatings on Amorphous Iron and Iron Oxide Nanoparticles: Thermal Stability and Chemical Reactivity Studies. Langmuir 1997, 13, 6151–6158. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Q.C.; Zhang, S.Q.; Sun, X.J.; Zhang, Z.S. Stabilization/solidification (S/S) of mercury-contaminated hazardous wastes using thiol-functionalized zeolite and Portland cement. J. Hazard. Mater. 2009, 168, 1575–1580. [Google Scholar] [CrossRef]
- Mohsenibandpei, A.; Ghaderpoori, M.; Hassani, G.; Bahrami, H.; Bahmani, Z.; Alinejad, A.A. Water solution polishing of nitrate using potassium permanganate modified zeolite: Parametric experiments, kinetics and equilibrium analysis. Glob. Nest J. 2016, 18, 546–558. [Google Scholar]
- Smith, C.J.; Hopmans, P.; Cook, F.J. Accumulation of Cr, Pb, Cu, Ni, Zn and Cd in soil following irrigation with treated urban effluent in Australia. Environ. Pollut. 1996, 94, 317–323. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. ; J. Environ. Qual. 2002, 31, 581–589. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Wang, X.; Hussain, B.; Yaseen, M.; Aziz, M.Z.; Yang, X. Immobilization of cadmium and lead in contaminated paddy field using inorganic and organic additives. Sci. Rep. 2018, 8, 17839. [Google Scholar] [CrossRef]
- Lee, M.; Paik, I.S.; Kim, I.; Kang, H.; Lee, S. Remediation of heavy metal contaminated groundwater originated from abandoned mine using lime and calcium carbonate. J. Hazard. Mater. 2007, 144, 208–214. [Google Scholar] [CrossRef]
- Krebs, R.; Gupta, S.K.; Furrer, G.; Schulin, R. Solubility and plant uptake of metals with and without liming of sludge- amended soils. J. Environ. Qual. 1998, 27, 18–23. [Google Scholar] [CrossRef]
- Xie, L.; Hao, P.; Cheng, Y.; Ahmed, I.M.; Cao, F. Effect of combined application of lead, cadmium, chromium and copper on grain, leaf and stem heavy metal contents at different growth stages in rice. Ecotoxicol. Environ. Saf. 2018, 162, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Chen, X.; Yang, Y.; Wang, S.; Li, Y.; Hu, H.; Ge, Y.; Cheng, W. Effects of pH, Fe, and Cd on the uptake of Fe2+ and Cd2+ by rice. Environ. Sci. Pollut. Res. 2013, 20, 8947–8954. [Google Scholar] [CrossRef] [PubMed]
- Ascar, L.; Ahumada, I.; Richter, P. Influence of redox potential (Eh) on the availability of arsenic species in soils and soils amended with biosolid. Chemosphere 2008, 72, 1548–1552. [Google Scholar] [CrossRef]
- Zou, Q.; An, W.; Wu, C.; Li, W.; Fu, A.; Xiao, R.; Chen, H.; Xue, S. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition. Environ. Chem. Lett. 2018, 16, 615–622. [Google Scholar] [CrossRef]
- Bishop, M.E.; Glasser, P.; Dong, H.; Arey, B.; Kovarik, L. Reduction and immobilization of hexavalent chromium by microbially reduced Fe-bearing clay minerals. Geochim. Cosmochim. Acta 2014, 133, 186–203. [Google Scholar] [CrossRef]
- Popenda, A. Desalination and Water Treatment Effect of redox potential on heavy metals and As behavior in dredged sediments. Desalin. Water Treat. 2014, 52, 3918–3927. [Google Scholar] [CrossRef]
- Gambrell, R.P.; Wiesepape, J.B.; Patrick, W.H.; Duff, M.C. The effects of pH, redox, and salinity on metal release from a contaminated sediment. Water. Air. Soil Pollut. 1991, 57–58, 359–367. [Google Scholar] [CrossRef]
- Reddy, C.N.; Patrick, W.H. Effect of Redox Potential and pH on the Uptake of Cadmium and Lead by Rice Plants1. J. Environ. Qual. 1977, 6, 259. [Google Scholar] [CrossRef]
- Leila Mahdavian Effects of magnetic field, pH and retention time on the lead (Pb2+) adsorption by modified human hair, goat hair and sheep wool. Afr. J. Microbiol. Res. 2012, 6, 183–189.
- Salem Attia, T.M.; Hu, X.L.; Yin, D.Q. Synthesised magnetic nanoparticles coated zeolite (MNCZ) for the removal of arsenic (As) from aqueous solution. J. Exp. Nanosci. 2014, 9, 551–560. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Li, J.; Wei, D.; Chen, S.; Guo, Q.; Ma, Y. Effects of rape straw and red mud on extractability and bioavailability of cadmium in a calcareous soil. Front. Environ. Sci. Eng. 2014, 9, 419–428. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibia, S.; Farida, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [PubMed]
- Shao, G.; Chen, M.; Wang, W.; Mou, R.; Zhang, G. Iron nutrition affects cadmium accumulation and toxicity in rice plants. Plant Growth Regul. 2007, 53, 33–42. [Google Scholar] [CrossRef]
- Tamez, C.; Hernandez, R.; Parsons, J.G. Removal of Cu (II) and Pb (II) from aqueous solution using engineered iron oxide nanoparticles. Microchem. J. 2016, 125, 97–104. [Google Scholar] [CrossRef]
- Ghosh, A.; Collie, S.R. Keratinous materials as novel absorbent systems for toxic pollutants. Def. Sci. J. 2014, 64, 209–221. [Google Scholar] [CrossRef]
- Patil, K.; Smith, S.V.; Rajkhowa, R.; Tsuzuki, T.; Wang, X.; Lin, T. Milled cashmere guard hair powders: Absorption properties to heavy metal ions. Powder Technol. 2012, 218, 162–168. [Google Scholar] [CrossRef]
- Mondal, N.K.; Basu, S. Potentiality of waste human hair towards removal of chromium(VI) from solution: Kinetic and equilibrium studies. Appl. Water Sci. 2019, 9, 49. [Google Scholar] [CrossRef]
- Enkhzaya, S.; Shiomori, K.; Oyuntsetseg, B. Removal of heavy metals from aqueous solution by adsorption using livestock biomass of Mongolia. J. Environ. Sci. Technol. 2017, 10, 107–119. [Google Scholar]
- Tan, T.C.; Chia, C.K.; Teo, C.K. Uptake of metal ions by chemically treated human hair. Water Res. 1985, 19, 157–162. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal complexes of amino acids and amino acid side chain groups. Structures and properties. Dalt. Trans. 2009, 7854–7869. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, O.; Odani, A.; Takani, M. Metal-amino acid chemistry. Weak interactions and related functions of side chain groups. J. Chem. Soc. Dalt. Trans. 2002, 3411–3421. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Wang, J.; Zheng, G.; Wu, J.; Dai, H. Flower-, wire-, and sheet-like MnO2-deposited diatomites: Highly efficient absorbents for the removal of Cr(VI). J. Environ. Sci. 2015, 29, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zheng, G.; Wang, J.; Wang, L.; Wu, J.; Dai, H. MnO2 nanowires in situ grown on diatomite: Highly efficient absorbents for the removal of Cr(VI) and As(V). Microporous Mesoporous Mater. 2014, 200, 27–34. [Google Scholar] [CrossRef]
- Chang, F.; Qu, J.; Liu, H.; Liu, R.; Zhao, X. Fe-Mn binary oxide incorporated into diatomite as an adsorbent for arsenite removal: Preparation and evaluation. J. Colloid Interface Sci. 2009, 338, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Al-Degs, Y.S.; Tutunju, M.F.; Shawabkeh, R.A. The feasibility of using diatomite and Mn-diatomite for remediation of Pb2+, Cu2+, and Cd2+ from water. Sep. Sci. Technol. 2000, 35, 2299–2310. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Kragović, M.; Pašalić, S.; Marković, M.; Petrović, M.; Nedeljković, B.; Momčilović, M.; Stojmenović, M. Natural and modified zeolite—Alginate composites. Application for removal of heavy metal cations from contaminated water solutions. Minerals 2018, 8, 11. [Google Scholar] [CrossRef]
- Panayotova, M. Use of zeolite for cadmium removal from wastewater. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2000, 35, 1591–1601. [Google Scholar] [CrossRef]
- Remenárová, L.; Pipíška, M.; Florková, E.; Horník, M.; Rozložník, M.; Augustín, J. Zeolites from coal fly ash as efficient sorbents for cadmium ions. Clean Technol. Environ. Policy 2014, 16, 1551–1564. [Google Scholar] [CrossRef]
- Yu, X.; Muhammad, F.; Yan, Y.; Yu, L.; Li, H.; Huang, X.; Jiao, B.; Lu, N.; Li, D. Effect of chemical additives on electrokinetic remediation of Cr-contaminated soil coupled with a permeable reactive barrier. R. Soc. Open Sci. 2019, 6, 182138. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M. Solid-liquid-solid extraction of heavy metals (Cr, Cu, Cd, Ni and Pb) in aqueous systems of zeolite-sewage sludge. J. Hazard. Mater. 2009, 161, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Allen, H.E.; Cowan, C.E. Adsorption of cadmium and copper by manganese oxide. Soil Sci. 1991, 152, 72–81. [Google Scholar] [CrossRef]
- Ye, X.; Li, H.; Ma, Y.; Wu, L.; Sun, B. The bioaccumulation of Cd in rice grains in paddy soils as affected and predicted by soil properties. J. Soils Sediments 2014, 14, 1407–1416. [Google Scholar] [CrossRef]
- Burke, D.J.; Pietrasiak, N.; Situ, S.F.; Abenojar, E.C.; Porche, M.; Kraj, P.; Lakliang, Y.; Samia, A.C.S. Iron oxide and titanium dioxide nanoparticle effects on plant performance and root associated microbes. Int. J. Mol. Sci. 2015, 16, 23630–23650. [Google Scholar] [CrossRef]
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T.; et al. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Silva, J.L.; Patel, M.; Stojanovic, J.; Lu, Y.; Kim, T.; Horgan, T. Human Hair as a Nutrient Source for Horticultural Crops. Horttechnology 2008, 18, 592–596. [Google Scholar] [CrossRef]
- Zheljazkov, V.D. Assessment of wool waste and hair waste as soil amendment and nutrient source. J. Environ. Qual. 2005, 34, 2310–2317. [Google Scholar] [CrossRef]
- Wu, Q.; Xia, G.; Chen, T.; Zheng, J.; Bu, F.; Chi, D. Effects of Nitrogen and Zeolite on Rice Grain Yield, Water and Nitrogen Use, and Soil Total Nitrogen in Coastal Region of Northeast China. Commun. Soil Sci. Plant Anal. 2016, 47, 2103–2114. [Google Scholar] [CrossRef]
- Sepaskhah, A.R.; Yousefi, F. Effects of zeolite application on nitrate and ammonium retention of a loamy soil under saturated conditions. Aust. J. Soil Res. 2007, 45, 368–373. [Google Scholar] [CrossRef]
- Ghanbari, M.; Ariafar, S. The study of different levels of zeolite application on quantitative and qualitative parameters in basil (Ocimum basilicum L) under drought conditions. Int. J. Agric. Res. Rev. 2013, 3, 844–853. [Google Scholar]
- Yang, J.X.; Wang, L.Q.; Wei, D.P.; Chen, S.B.; Ma, Y.B. Foliar Spraying and Seed Soaking of Zinc Fertilizers Decreased Cadmium Accumulation in Cucumbers Grown in Cd-Contaminated Soils. Soil Sediment Contam. 2011, 20, 400–410. [Google Scholar] [CrossRef]
- Akkajit, P.; Tongcumpou, C. Fractionation of metals in cadmium contaminated soil: Relation and effect on bioavailable cadmium. Geoderma 2010, 156, 126–132. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Yuan, Y.; Wang, X.; Li, X. Heavy metal accumulation and its spatial distribution in agricultural soils: Evidence from Hunan province, China. RSC Adv. 2018, 8, 10665–10672. [Google Scholar] [CrossRef]
- Shuman, L.M. Effect of organic waste amendments on zinc adsorption by two soils. Soil Sci. 1999, 164, 197–205. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Wang, L.; Liang, X.; Lin, D. Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 2013, 193–194, 149–155. [Google Scholar] [CrossRef]
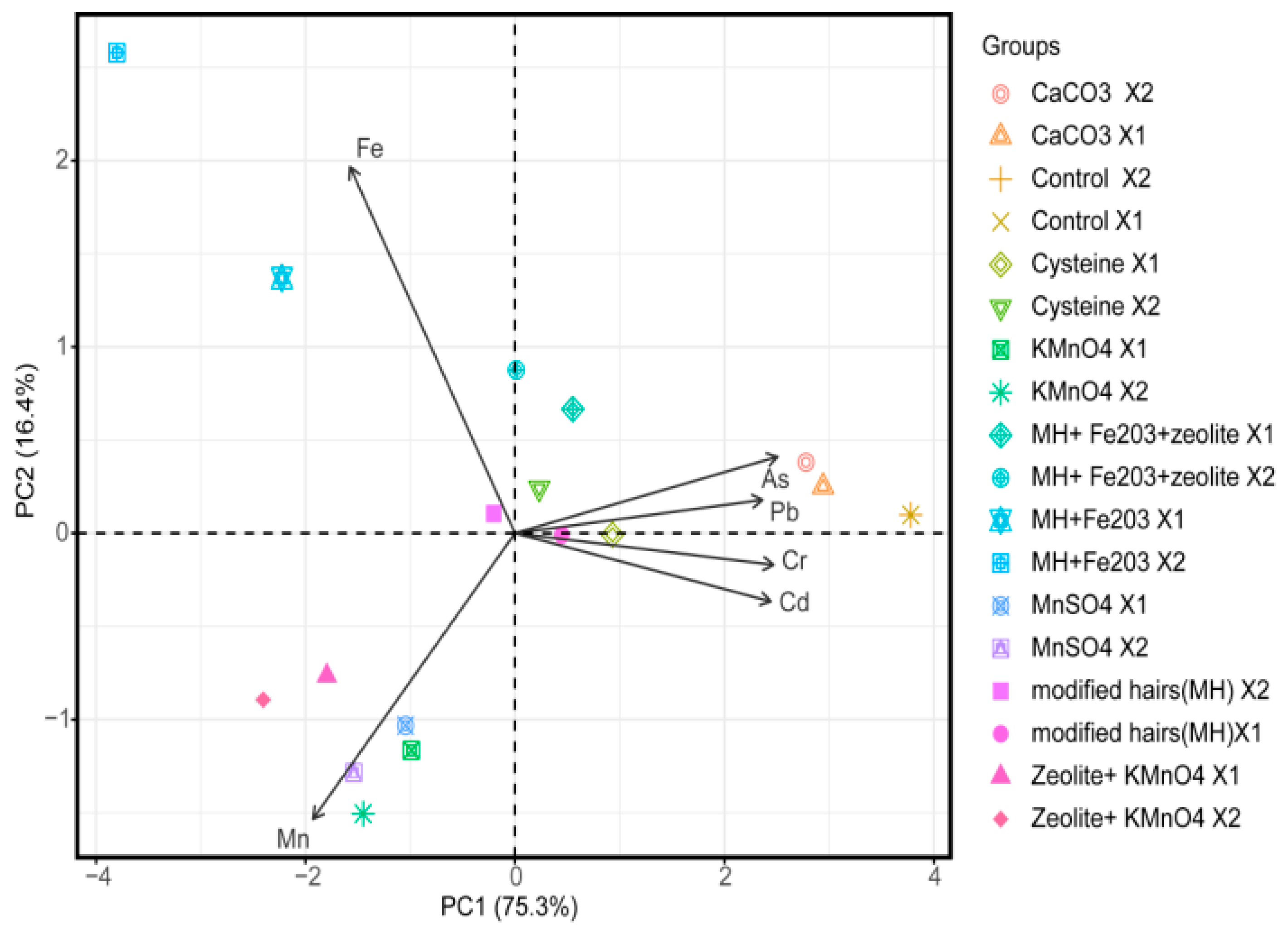
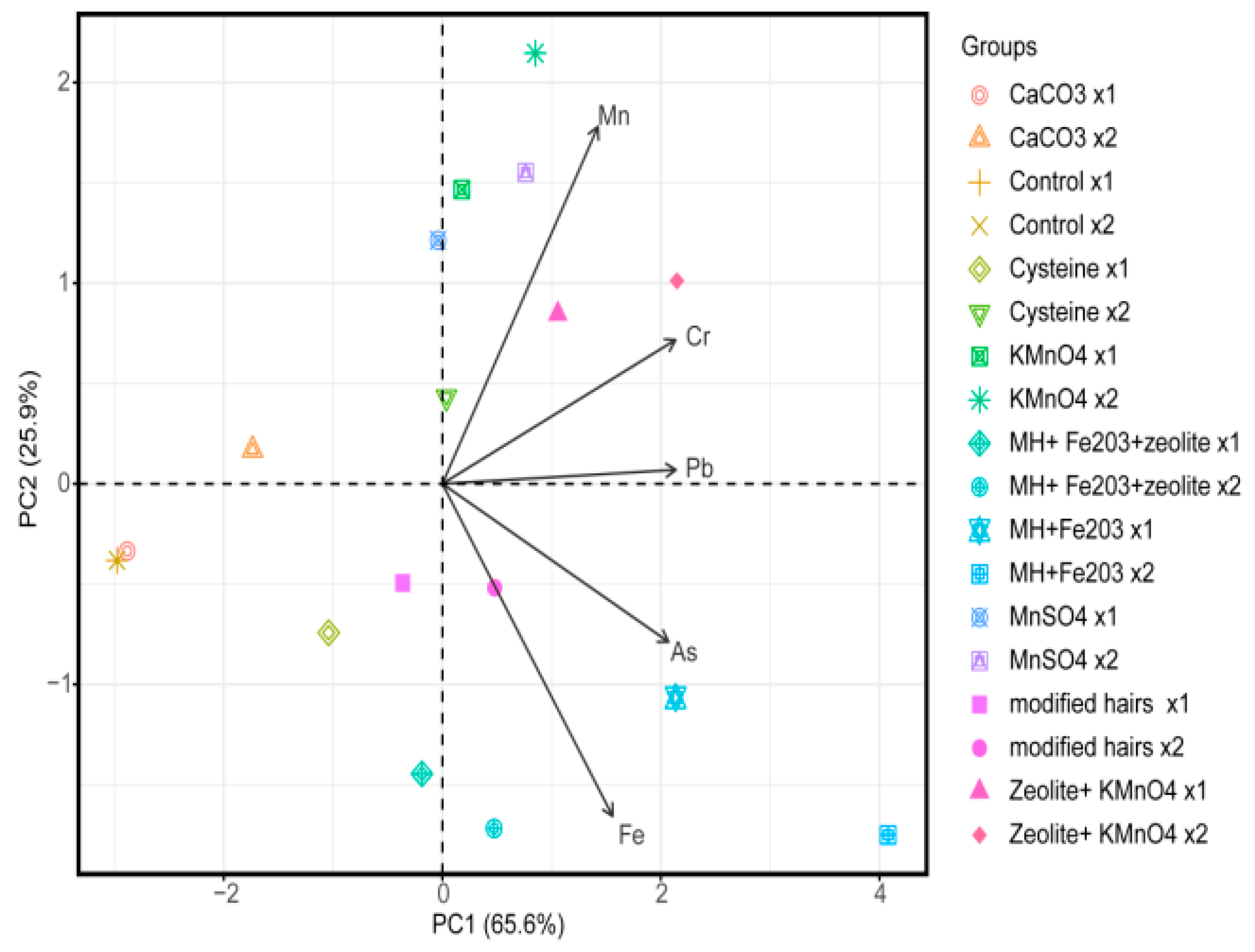
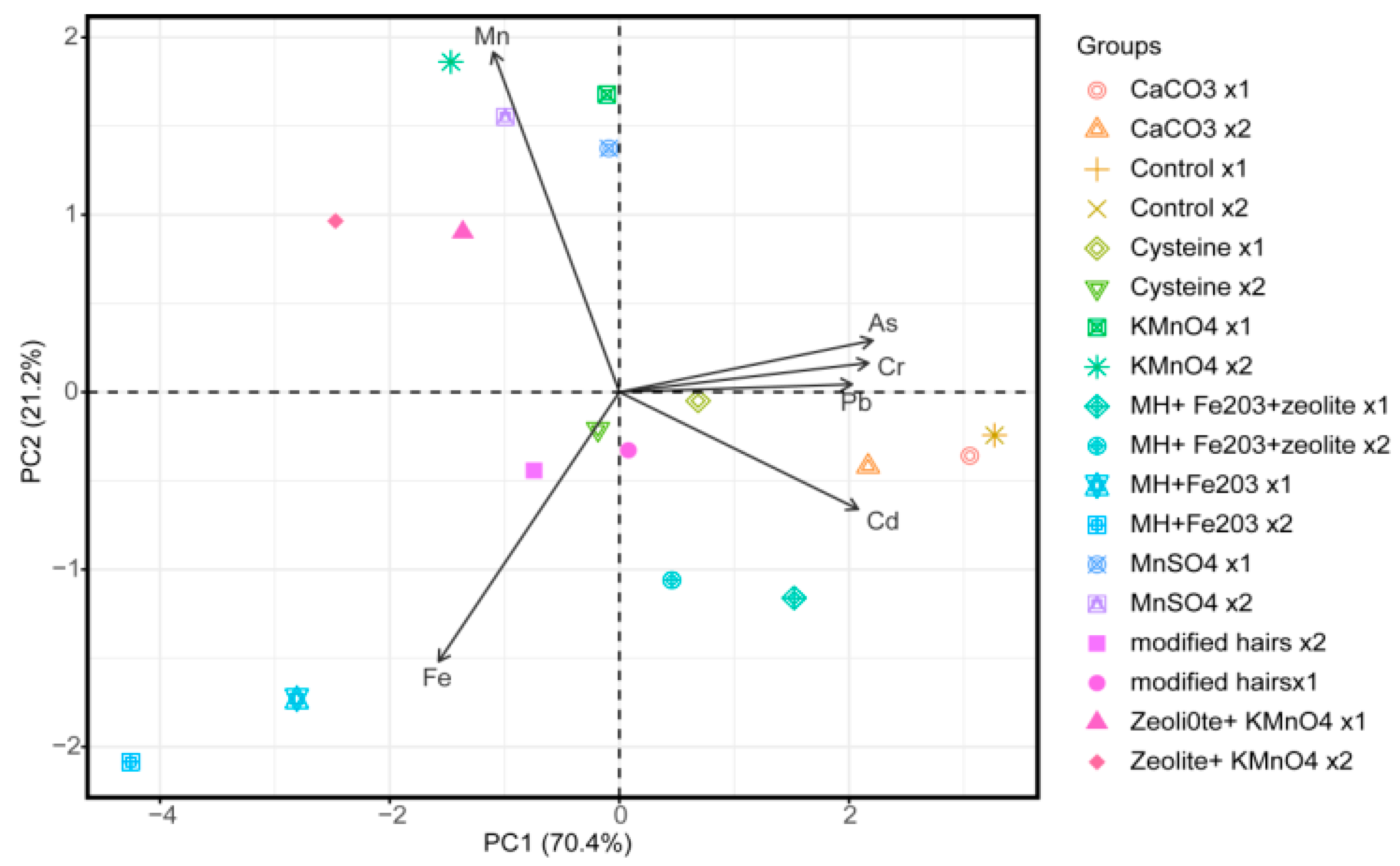
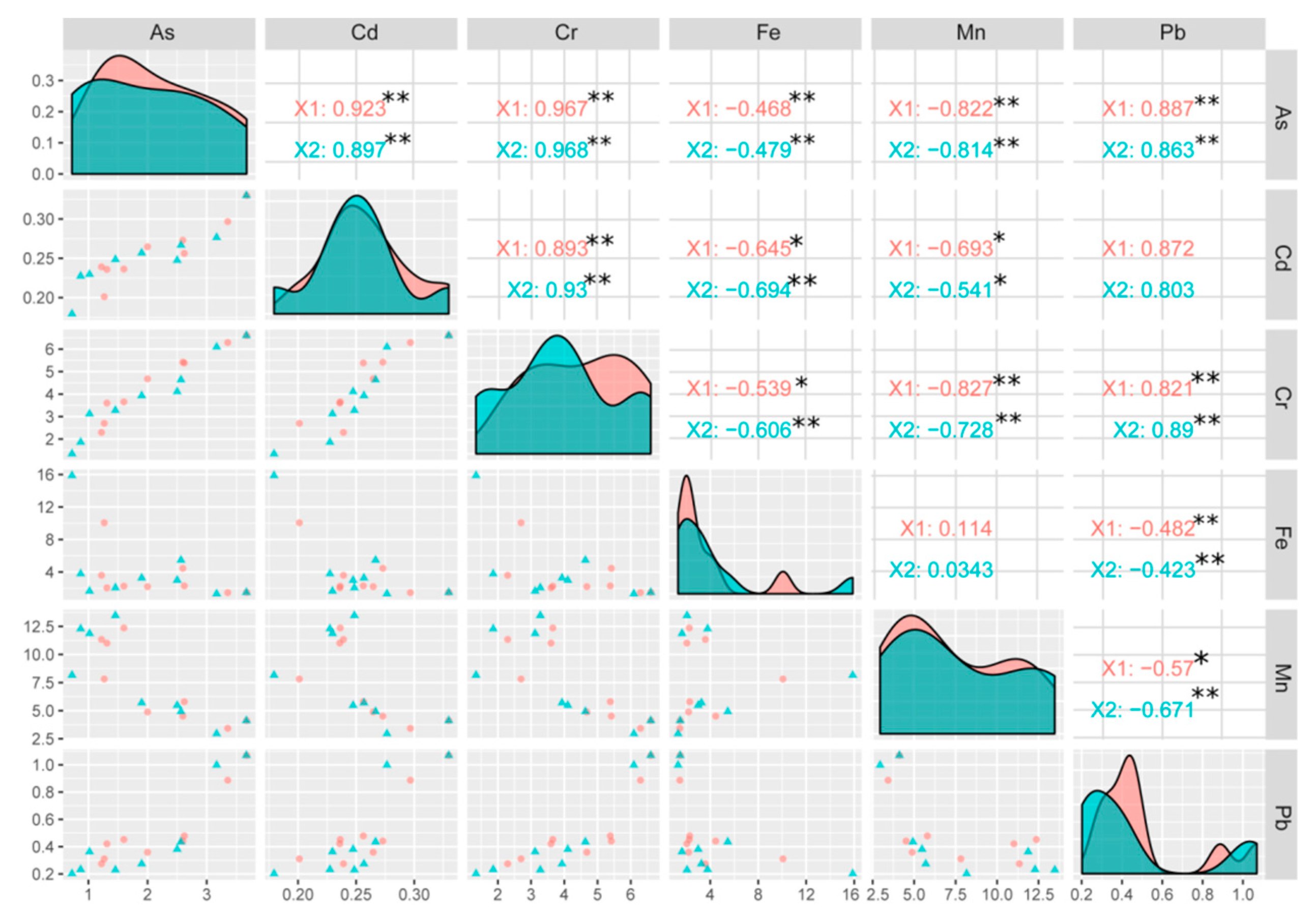

| Treatments | Rate for Level 1 (X1) (g/pot) | Rate for Level 2 (X2) (g/pot) |
|---|---|---|
| T1 | 0 | 0 |
| T2 | 5 g CaCO3 | 10 g CaCO3 |
| T3 | 0.25 g modified hairs + 5 g CaCO3 | 0.5 g modified hairs + 10 g CaCO3 |
| T4 | 0.25 g cysteine + 5 g CaCO3 | 0.5 g cysteine + 10 g CaCO3 |
| T5 | 0.5 g MnSO4 + 5 g CaCO3 | 1 g MnSO4+ 10 g CaCO3 |
| T6 | 0.5 g KMnO4 + 5 g CaCO3 | 1 g KMnO4 + 10 g CaCO3 |
| T7 | 0.5 g modified hairs-Fe2O3 + 5 g CaCO3 | 1 g modified hairs-Fe2O3 + 10 g CaCO3 |
| T8 | 0.5 g zeolite-modified hairs-Fe2O3 + 5 g CaCO3 | 1 g zeolite-modified hairs-Fe2O3 + 10 g CaCO3 |
| T9 | 0.5 g zeolite-KMnO4 + 5 g CaCO3 | 1 g zeolite-KMnO4 + 10 g CaCO3 |
| X1 | As (mg kg−1) | Cr (mg kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Pb (mg kg−1) | Cd (mg kg−1) |
|---|---|---|---|---|---|---|
| T1 | 43 ± 2 | 34 ± 3 | 15 ± 1 | 210 ± 17 | 308 ± 10 | 0.51 ± 0.02 |
| T2 | 44 ± 1 | 34 ± 3 | 16 ± 1 | 218 ± 18 | 309 ± 15 | 0.53 ± 0.02 |
| T3 | 54 ± 3 | 37 ± 3 | 23 ± 2 | 231 ± 16 | 341 ± 16 | 0.56 ± 0.01 |
| T4 | 52 ± 2 | 36 ± 2 | 29 ± 2 | 233 ± 18 | 326 ± 18 | 0.59 ± 0.01 |
| T5 | 51 ± 2 | 39 ± 2 | 14 ± 1 | 276 ± 17 | 235 ± 10 | 0.59 ± 0.01 |
| T6 | 49 ± 1 | 39 ± 2 | 15 ± 1 | 286 ± 18 | 354 ± 10 | 0.59 ± 0.01 |
| T7 | 61 ± 3 | 41 ± 3 | 48 ± 3 | 251 ± 10 | 362 ± 14 | 0.69 ± 0.01 |
| T8 | 53 ± 2 | 36 ± 3 | 39 ± 3 | 221 ± 11 | 352 ± 11 | 0.59 ± 0.01 |
| T9 | 55 ± 2 | 39 ± 3 | 23 ± 2 | 282 ± 10 | 361 ± 16 | 0.68 ± 0.01 |
| X2 | ||||||
| T1 | 43 ± 1 | 34 ± 2 | 15 ± 1 | 210 ± 11 | 308 ± 10 | 0.51 ± 0.02 |
| T2 | 51 ± 1 | 35 ± 2 | 17 ± 1 | 225 ± 14 | 310 ± 11 | 0.56 ± 0.01 |
| T3 | 58 ± 2 | 39 ± 3 | 25 ± 2 | 234 ± 14 | 350 ± 9 | 0.55 ± 0.01 |
| T4 | 53 ± 3 | 37 ± 3 | 25 ± 1 | 273 ± 13 | 340 ± 7 | 0.59 ± 0.01 |
| T5 | 55 ± 2 | 42 ± 4 | 16 ± 1 | 294 ± 15 | 329 ± 10 | 0.60 ± 0.01 |
| T6 | 47 ± 2 | 40 ± 3 | 16 ± 1 | 307 ± 14 | 366 ± 11 | 0.61 ± 0.01 |
| T7 | 67 ± 2 | 42 ± 3 | 71 ± 1 | 267 ± 13 | 378 ± 7 | 0.71 ± 0.01 |
| T8 | 57 ± 3 | 37 ± 2 | 45 ± 2 | 223 ± 13 | 351 ± 7 | 0.59 ± 0.01 |
| T9 | 58 ± 2 | 41 ± 3.12 | 26 ± 1.38 | 294 ± 12 | 375 ± 13 | 0.70 ± 0.01 |
| X1 | As (mg kg−1) | Cr (mg kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Pb (mg kg−1) | Cd (mg kg−1) |
|---|---|---|---|---|---|---|
| T1 | 3.7 ± 0.2 | 6.6 ± 0.3 | 1.5 ± 0.3 | 4.1 ± 0.3 | 1.1 ± 0.2 | 0.33 ± 0.02 |
| T2 | 3.4 ± 0.3 | 6.3 ± 0.2 | 1.4 ± 0.2 | 3.4 ± 0.3 | 0.8 ± 0.2 | 0.30 ± 0.01 |
| T3 | 2 ± 0.3 | 4.6 ± 0.1 | 2.2 ± 0.2 | 4.8 ± 0.3 | 0.3 ± 0.1 | 0.26 ± 0.01 |
| T4 | 2.6 ± 0.3 | 5.3 ± 0.2 | 2.2 ± 0.1 | 5.8 ± 0.3 | 0.4 ± 0.2 | 0.25 ± 0.02 |
| T5 | 1.3 ± 0.2 | 3.6 ± 0.4 | 2 ± 0.3 | 11 ± 0.3 | 0.4 ± 0.1 | 0.23 ± 0.01 |
| T6 | 1.6 ± 0.2 | 3.6 ± 0.4 | 2.2 ± 0.3 | 12.3 ± 0.3 | 0.4 ± 0.1 | 0.23 ± 0.01 |
| T7 | 1.3 ± 0.3 | 2.6 ± 0.4 | 10 ± 0.6 | 7.8 ± 0.3 | 0.3 ± 0.10 | 0.201 ± 0.02 |
| T8 | 2.6 ± 0.3 | 3.7 ± 0.5 | 4.4 ± 0.1 | 4.5 ± 0.2 | 0.4 ± 0.05 | 0.273 ± 0.02 |
| T9 | 1.2 ± 0.4 | 2.3 ± 0.4 | 3.5 ± 0.1 | 11.3 ± 0.3 | 0.2 ± 0.07 | 0.239 ± 0.01 |
| X2 | ||||||
| T1 | 3.6 ± 0.2 | 6.6 ± 0.3 | 1.5 ± 0.3 | 4.1 ± 0.3 | 1.0 ± 0.09 | 0.329 ± 0.02 |
| T2 | 3.1 ± 0.3 | 6.1 ± 0.3 | 1.3 ± 0.2 | 2.9 ± 0.3 | 0.9 ± 0.08 | 0.276 ± 0.01 |
| T3 | 1.9 ± 0.4 | 3.9 ± 0.2 | 3.2 ± 0.2 | 5.7 ± 0.1 | 0.2 ± 0.08 | 0.256 ± 0.01 |
| T4 | 2.5 ± 0.3 | 4.1 ± 0.3 | 2.9 ± 0.2 | 5.4 ± 0.3 | 0.3 ± 0.08 | 0.247 ± 0.01 |
| T5 | 1.0 ± 0.4 | 3.1 ± 0.4 | 1.6 ± 0.3 | 11.8 ± 0.2 | 0.3 ± 0.08 | 0.229 ± 0.01 |
| T6 | 1.4 ± 0.3 | 3.2 ± 0.3 | 2.1 ± 0.3 | 13.4 ± 0.3 | 0.2 ± 0.07 | 0.248 ± 0.01 |
| T7 | 0.7 ± 0.1 | 1.3 ± 0.1 | 15.8 ± 0.3 | 8.1 ± 0.3 | 0.2 ± 0.06 | 0.179 ± 0.01 |
| T8 | 2.5 ± 0.2 | 2.6 ± 0.3 | 5.4 ± 0.4 | 4.9 ± 0.3 | 0.4 ± 0.09 | 0.266 ± 0.01 |
| T9 | 0.8 ± 0.2 | 1.8 ± 0.3 | 3.7 ± 0.3 | 12.2 ± 0.3 | 0.2 ± 0.10 | 0.227 ± 0.01 |
| X1 | As (mg kg−1) | Cr (mg kg−1) | Fe (mg kg−1) | Mn (mg kg−1) | Pb (mg kg−1) | Cd (mg kg−1) |
|---|---|---|---|---|---|---|
| T1 | 33 ± 0.5 | 3.6 ± 0.4 | 6 ± 0.39 | 10 ± 0.5 | 6.2 ± 0.3 | 0.10 ± 0.01 |
| T2 | 31 ± 0.5 | 3.6 ± 0.6 | 6 ± 0.46 | 9 ± 0.3 | 6.3 ± 0.2 | 0.09 ± 0.01 |
| T3 | 22 ± 0.8 | 2.9 ± 0.5 | 9 ± 0.49 | 11 ± 0.2 | 3.0 ± 0.2 | 0.07 ± 0.01 |
| T4 | 26 ± 0.6 | 3.0 ± 0.5 | 9 ± 0.56 | 12 ± 0.3 | 4.2 ± 0.3 | 0.07 ± 0.01 |
| T5 | 24 ± 3.3 | 2.7 ± 0.5 | 7 ± 0.42 | 20 ± 0.4 | 4.5 ± 0.1 | 0.05 ± 0.01 |
| T6 | 25 ± 0.6 | 2.7 ± 0.7 | 7 ± 0.42 | 22 ± 0.3 | 4.6 ± 0.1 | 0.05 ± 0.010 |
| T6 | 15 ± 1.2 | 1.6 ± 0.2 | 24 ± 1.25 | 10 ± 0.2 | 2.9 ± 0.4 | 0.04 ± 0.010 |
| T8 | 27 ± 1.0 | 3.4 ± 0.5 | 13 ± 0.94 | 9 ± 0.3 | 4.1 ± 0.3 | 0.09 ± 0.010 |
| T9 | 22 ± 2.3 | 1.9 ± 0.3 | 11 ± 0.50 | 21 ± 0.3 | 3.4 ± 0.4 | 0.05 ± 0.010 |
| X2 | ||||||
| T1 | 33 ± 0.5 | 3.6 ± 0.3 | 6 ± 0.3 | 10 ± 0.5 | 6.2 ± 0.1 | 0.10 ± 0.01 |
| T2 | 28 ± 0.6 | 3.2 ± 0.3 | 5 ± 0.3 | 7 ± 0.2 | 5.8 ± 0.4 | 0.08 ± 0.01 |
| T3 | 20 ± 0.6 | 2.5 ± 0.2 | 11 ± 0.3 | 12 ± 0.4 | 2.4 ± 0.2 | 0.07 ± 0.01 |
| T4 | 23 ± 0.7 | 2.9 ± 0.2 | 10 ± 0.3 | 11 ± 0.1 | 3.3 ± 0.1 | 0.06 ± 0.01 |
| T5 | 19 ± 1.1 | 2.5 ± 0.2 | 7 ± 0.3 | 22 ± 0.3 | 3.9 ± 0.2 | 0.05 ± 0.01 |
| T6 | 21 ± 1.4 | 2.3 ± 0.2 | 7 ± 0.4 | 24 ± 0.3 | 2.7 ± 0.2 | 0.05 ± 0.01 |
| T7 | 10 ± 1.1 | 1.2 ± 0.1 | 32 ± 0.8 | 13 ± 0.2 | 2.2 ± 0.2 | 0.04 ± 0.01 |
| T8 | 26 ± 1.2 | 2.3 ± 0.5 | 15 ± 0.4 | 10 ± 0.2 | 4.7 ± 0.4 | 0.08 ± 0.01 |
| T9 | 18 ± 1.4 | 1.7 ± 0.1 | 13 ± 0.6 | 22 ± 0.2 | 2.6 ± 0.3 | 0.05 ± 0.02 |
| X1 | Leaf Area (cm2) | Dry Weight (g) | Wet Weight (g) | pH | Eh (mV) |
|---|---|---|---|---|---|
| T1 | 1.74 | 1.31 | 8.64 | 6.6 | −20 |
| T2 | 1.83 | 1.82 | 11.70 | 7.9 | 37 |
| T3 | 2.12 | 4.91 | 18.01 | 7.8 | 160 |
| T4 | 2.11 | 4.14 | 16.94 | 8.0 | 150 |
| T5 | 2.39 | 4.01 | 14.03 | 7.9 | 208 |
| T6 | 2.38 | 3.92 | 17.91 | 8.0 | 207 |
| T7 | 2.84 | 6.14 | 26.54 | 7.8 | 267 |
| T8 | 2.53 | 2.93 | 15.83 | 7.9 | 250 |
| T9 | 2.58 | 4.71 | 23.31 | 7.8 | 272 |
| X2 | |||||
| T1 | 1.74 | 1.31 | 8.64 | 6.6 | −20 |
| T2 | 1.98 | 3.64 | 17.83 | 8.3 | 35 |
| T3 | 2.70 | 5.71 | 22.54 | 8.1 | 165 |
| T4 | 2.40 | 4.43 | 22.81 | 8.1 | 152 |
| T5 | 2.63 | 5.22 | 21.12 | 7.5 | 283 |
| T6 | 2.76 | 6.24 | 25.10 | 7.7 | 248 |
| T7 | 3.20 | 8.44 | 36.71 | 7.6 | 297 |
| T8 | 2.86 | 4.41 | 22.91 | 8.0 | 265 |
| T9 | 3.00 | 7.53 | 33.12 | 8.0 | 270 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Ma, Y.; Li, J.; Tahir, N.; Hussain, B. Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety. Agronomy 2020, 10, 359. https://doi.org/10.3390/agronomy10030359
Ullah A, Ma Y, Li J, Tahir N, Hussain B. Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety. Agronomy. 2020; 10(3):359. https://doi.org/10.3390/agronomy10030359
Chicago/Turabian StyleUllah, Aman, Yibing Ma, Jumei Li, Nazia Tahir, and Babar Hussain. 2020. "Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety" Agronomy 10, no. 3: 359. https://doi.org/10.3390/agronomy10030359
APA StyleUllah, A., Ma, Y., Li, J., Tahir, N., & Hussain, B. (2020). Effective Amendments on Cadmium, Arsenic, Chromium and Lead Contaminated Paddy Soil for Rice Safety. Agronomy, 10(3), 359. https://doi.org/10.3390/agronomy10030359




