Efficient Physiological and Nutrient Use Efficiency Responses of Maize Leaves to Drought Stress under Different Field Nitrogen Conditions
Abstract
1. Introduction
2. Materials and Methods
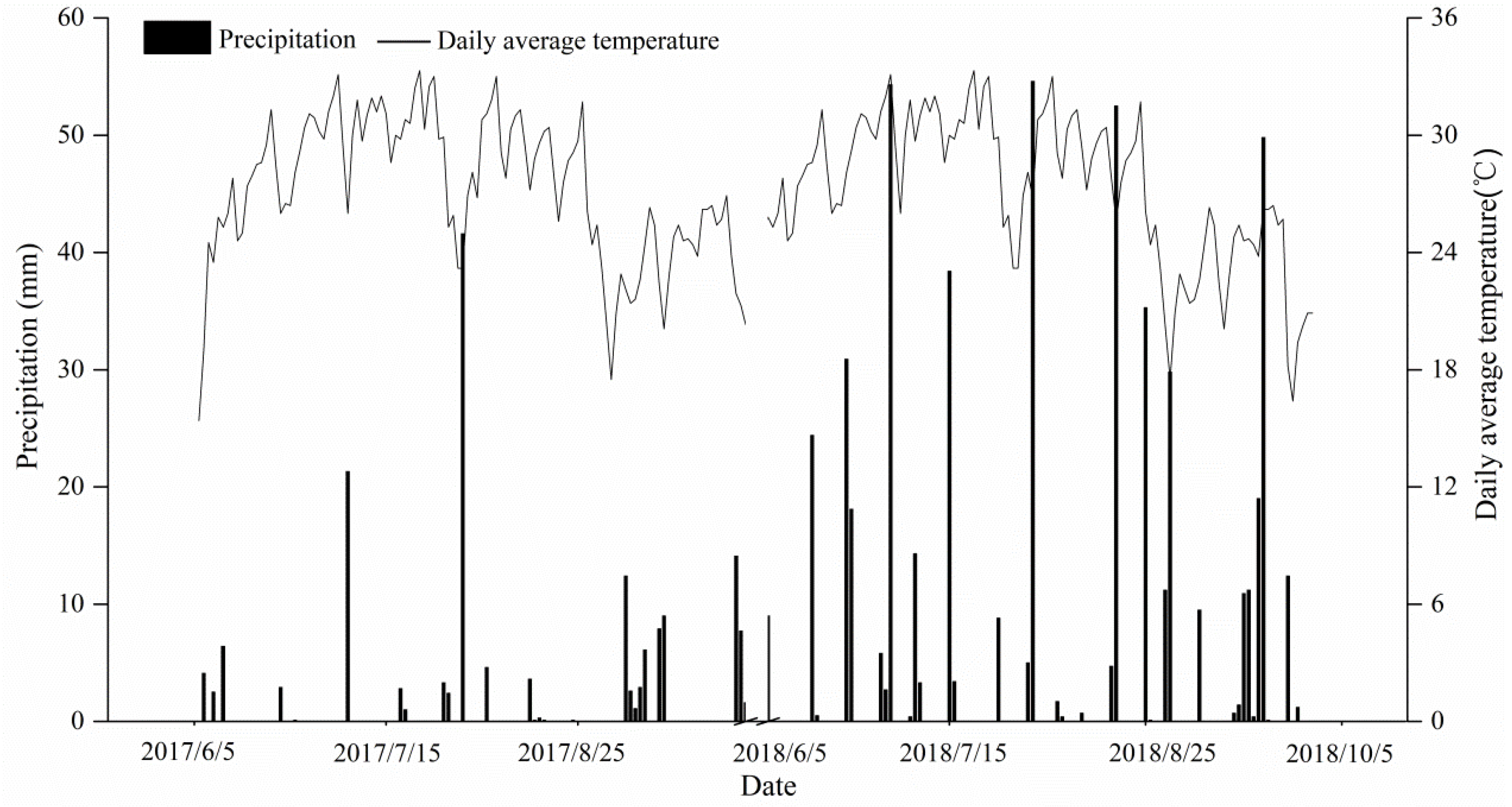
2.1. Site Description
2.2. Experimental Design and Management
2.3. Remote Sensing Image
2.4. Leaf Curling, Thickness and Relative Water Content (RWC) Measurements
2.5. Field Soil Water Consumption Measurements
2.6. Determination of the Net Photosynthetic Rate and the Transpiration Rate
2.7. Plant N Measurements
2.8. Data Analysis
3. Results
3.1. Leaf Morphology
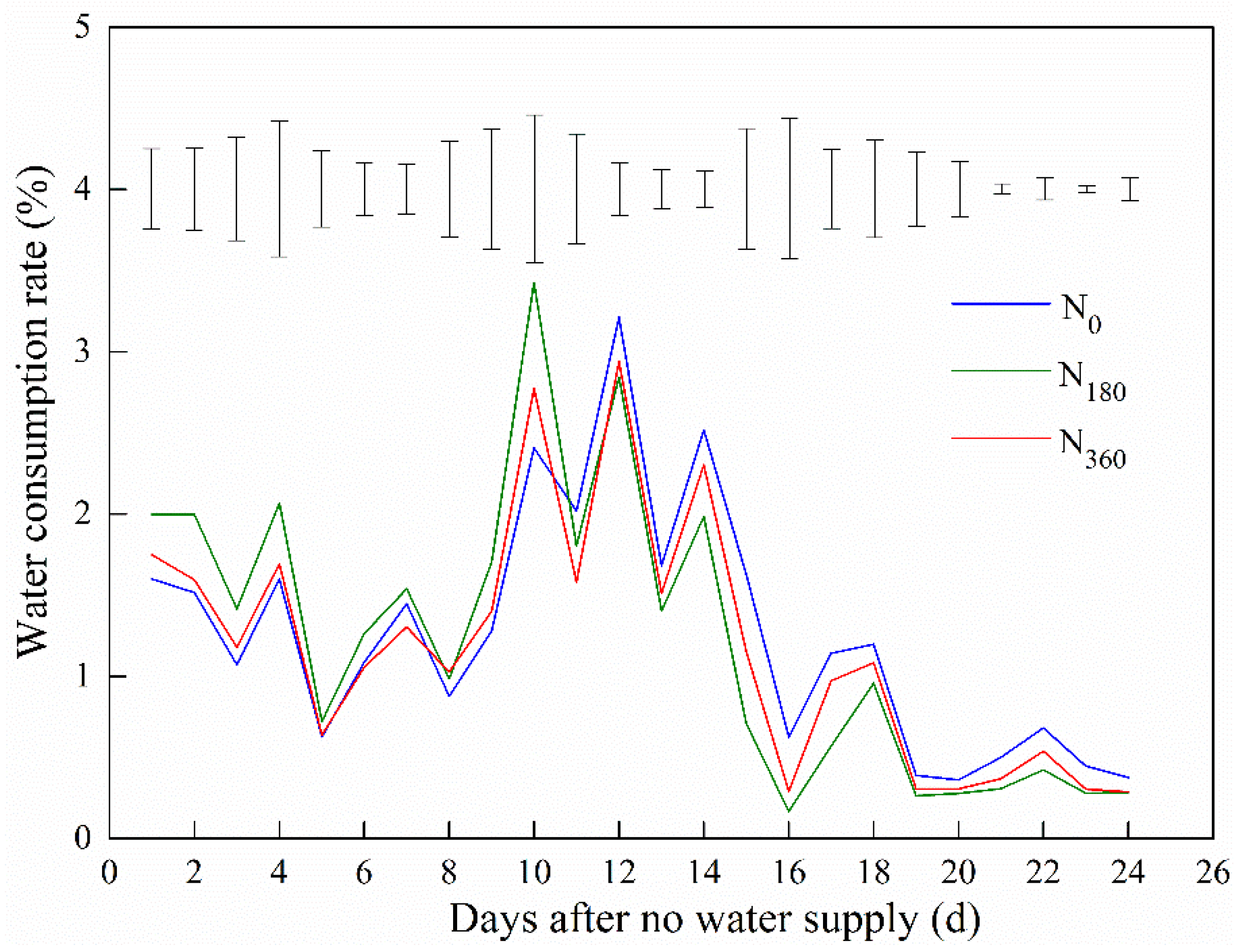
3.2. Soil Water Consumption Rate
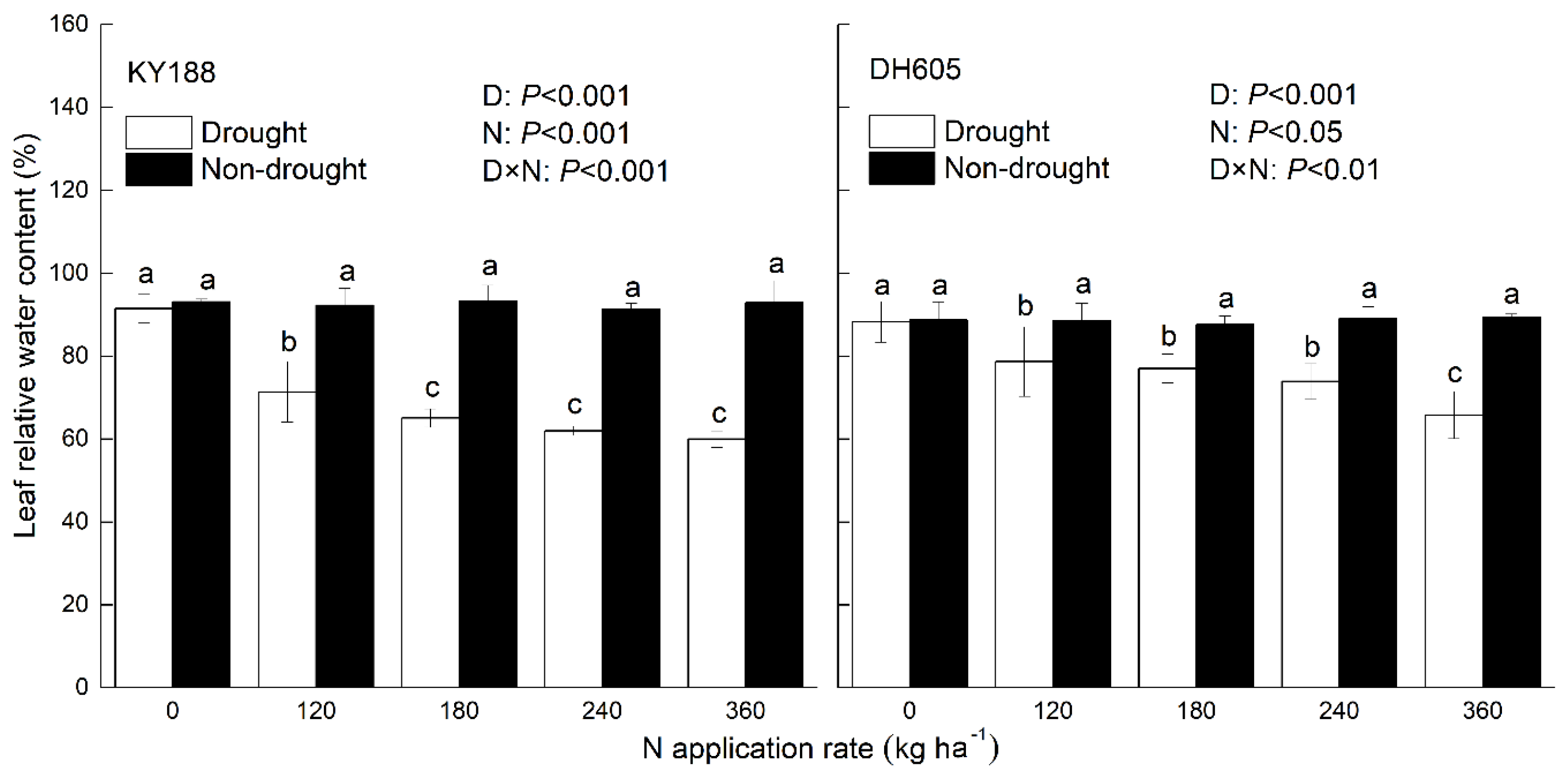
3.3. RWC of Leaves
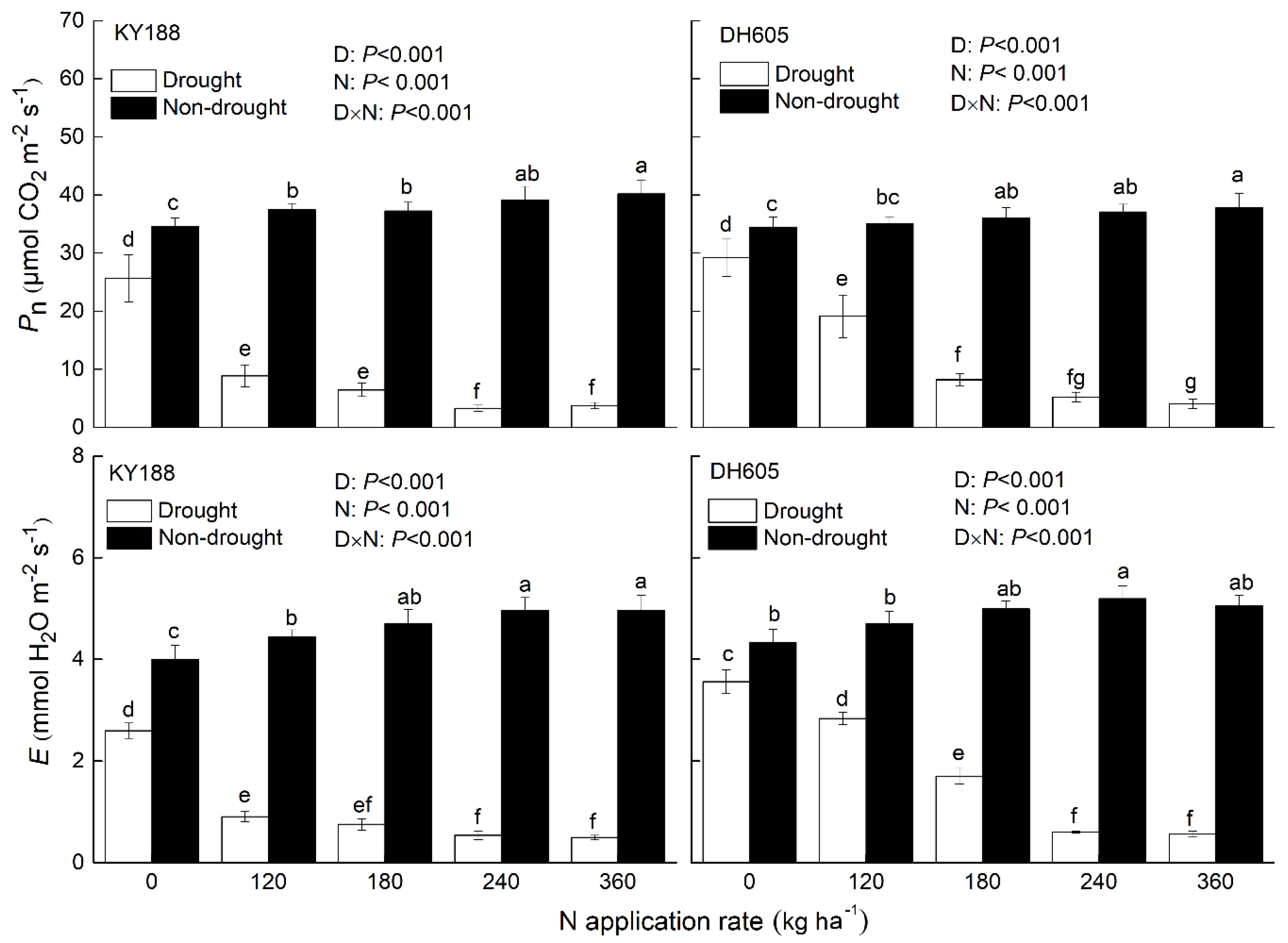
3.4. Gas Exchange Parameters
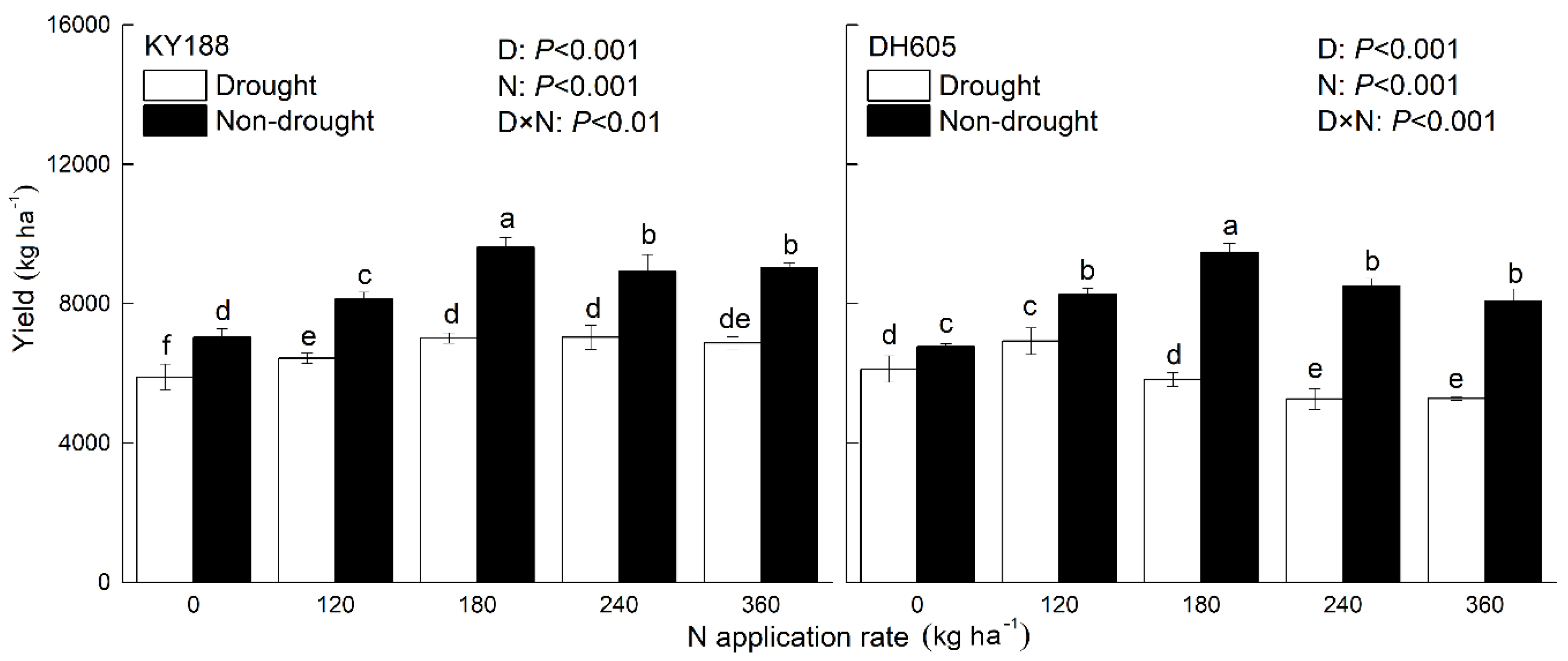
3.5. Corn Grain Yield
3.6. N Use Efficiency
4. Discussion
4.1. Physiological Performance Response of Maize Leaves to Drought Stress Under Different N Conditions
4.2. Yield and N Use Efficiency of Maize Subjected to Drought Under Different N Treatement Conditions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campos, H.; Cooper, A.; Habben, J.E.; Edmeades, G.O.; Schussler, J.R. Improving drought tolerance in maize: A view from industry. Field Crop. Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Min, H.W.; Chen, C.X.; Wei, S.W.; Shang, X.L.; Sun, M.Y.; Xia, R.; Liu, X.G.; Hao, D.Y.; Chen, H.B.; Xie, Q. Identification of drought tolerant mechanisms in maize seedlings based on transcriptome analysis of recombination inbred lines. Front. Plant Sci. 2016, 7, 1080. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. CIMMYT 1999–2000 Facts and Trends. Meeting World Maize Needs: Technological Opportunities and Priorities for the Public Sector; CIMMYT: Mexico City, Mexico, 2001. [Google Scholar]
- Ertiro, B.T.; Beyene, Y.; Das, B.; Mugo, S.; Olsen, M.; Oiken, S.; Juma, C.; Labuschagne, M.; Prasanna, B.M. Combining ability and testcross performance of drought-tolerant maize inbred lines under stress and non-stress environments in Kenya. Plant Breed. 2017, 136, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.S.; Min, J.Z.; Ding, Y.H. Relationship between reduction of summer precipitation in North China and atmospheric circulation anomalies. J. Water Resour. Prot. 2010, 2, 569–576. [Google Scholar] [CrossRef][Green Version]
- Shen, H.; Leblanc, M.; Tweed, S.; Liu, W.Z. Groundwater depletion in the Hai River Basin, China, from in situ and GRACE observations. Hydrolog. Sci. J. 2015, 60, 671–687. [Google Scholar] [CrossRef]
- Curá, J.A.; Franz, D.R.; Filosofía, J.E. Inoculation with Azospirillum sp. and Herbaspirillum sp. Bacteria increases the tolerance of maize to drought stress. Microorganisms 2017, 5, 41. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.X.; Pierre-André, J. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Dai, A.G. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Checovich, M.L.; Galatro, A.; Moriconi, J.I.; Simontacchi, M.; Dubcovsky, J.; Santa-María, G.E. The stay-green phenotype of TaNAM-RNAi wheat plants is associated with maintenance of chloroplast structure and high enzymatic antioxidant activity. Plant Physiol. Biochem. 2016, 104, 257–265. [Google Scholar] [CrossRef]
- Albacete, A.A.; Martínez-Andújar, C.; Pérez-Alfocea, F. Hormonal and metabolic regulation of source-sink relations under salinity and drought: From plant survival to crop yield stability. Biotechnol. Adv. 2014, 32, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, L.; Wang, J.; Wang, J.; Xie, H. The genotoxic and cytotoxic effects of 1-butyl-3-methylimidazolium chloride in soil on Vicia faba seedlings. J. Hazard. Mater. 2015, 285, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B.; Bains, N.; Farooq, M. Nitrogen nutrition, its assimilation and remobilization in diverse wheat genotypes. Int. J. Agric. Biol. 2015, 17, 531–538. [Google Scholar] [CrossRef]
- Wang, X.B.; Wang, L.F.; Shangguan, Z.P. Leaf gas exchange and fluorescence of two winter wheat varieties in response to drought stress and nitrogen supply. PLoS ONE 2016, 11, e0165733. [Google Scholar] [CrossRef]
- Gou, W.; Zheng, P.F.; Tian, L.; Gao, W.; Zhang, L.X.; Akram, N.A.; Ashraf, M. Exogenous application of urea and a urease inhibitor improves drought stress tolerance in maize (Zea mays L.). J. Plant Res. 2017, 130, 599–609. [Google Scholar] [CrossRef]
- Jin, L.; Cui, H.; Li, B.; Zhang, J.W.; Dong, S.T.; Liu, P. Effects of integrated agronomic management practices on yield and nitrogen efficiency of summer maize in North China. Field Crop. Res. 2012, 134, 30–35. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ying, Y.Q.; Zhang, Y.B.; Bi, Y.F.; Wang, A.K.; Du, X.H. Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci. Rep. 2018, 8, 228. [Google Scholar] [CrossRef]
- Li, Y.J.; Wang, M.; Zhang, F.X.; Xu, Y.D.; Chen, X.H.; Qin, X.L.; Wen, X.X. Effect of post-silking drought on nitrogen partitioning and gene expression patterns of glutamine synthetase and asparagine synthetase in two maize (Zea mays L.) varieties. Plant Physiol. Biochem. 2016, 102, 62–69. [Google Scholar] [CrossRef]
- Entringer, G.C.; Guedes, F.L.; Oliveira, A.A.; Nascimento, J.P.; Souza, J.C. Genetic control of leaf curl in maize. Genet. Mol. Res. 2014, 13, 1672–1678. [Google Scholar] [CrossRef]
- Guo, J.Y.; Yang, Y.; Wang, G.X.; Yang, L.D.; Sun, X.Y. Ecophysiological responses of Abies fabri seedlings to drought stress and nitrogen supply. Physiol. Plant. 2010, 139, 335–347. [Google Scholar]
- Kübert, A.; Götz, M.; Kuester, E.; Piayda, A.; Werner, C.; Rothfuss, Y.; Dubbert, M. Nitrogen loading enhances stress impact of drought on a semi-natural temperate grassland. Front. Plant Sci. 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Copper, P.J.M.; Gregory, P.J.; Tully, D.; Harris, H.C. Improving water use efficiency of annual crops in the rainfed farming systems of West Asia and North Africa. Exp. Agric. 1987, 23, 113–158. [Google Scholar] [CrossRef]
- Pilbeam, C.J.; Simmonds, L.P.; Kavilu, A.W. Transpiration efficiencies of maize and beans in semi-arid Kenya. Field Crop. Res. 1995, 41, 179–188. [Google Scholar] [CrossRef]
- Ferreira, T.C.; Carr, M.K.V. Responses of potatoes (Solanum tuberosum L.) to irrigation and nitrogen in a hot, dry climate: I. Water use. Field Crop. Res. 2002, 78, 51–64. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Saifullah; Ahmad, A. Water stress and nitrogen management effects on gas exchange, water relations, and water use efficiency in wheat. J. Plant Nutr. 2011, 34, 1867–1882. [Google Scholar] [CrossRef]
- Peña-Rojas, K.; Aranda, X.; Joffre, R.; Fleck, J. Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Funct. Plant Biol. 2005, 32, 117–130. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Bulliform cells. In Anatomical Adaptations of Halophytes; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Heckathorn, S.A.; DeLucia, E.H. Effect of leaf rolling on gas exchange and leaf temperature of andropogon gerardii and spartina pectinata. Bot. Gaz. 1991, 152, 263–268. [Google Scholar] [CrossRef]
- Bahrun, A.; Jensen, C.R.; Asch, F.; Mogensen, V.O. Drought-induced changes in xylem pH, ionic composition, and ABA concentration act as early signals in field-grown maize (Zea mays L.). J. Exp. Bot. 2002, 53, 251–263. [Google Scholar] [CrossRef]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D.; Zhou, X. Arbuscular mycorrhizae improve photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012, 58, 186–191. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Javawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of cytokinin and nitrogen on drought tolerance of Creeping Bentgrass. PLoS ONE 2016, 11, e0154005. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Wang, Y.; Pan, Y.H.; Pang, J.Y.; Zhang, X.; Fan, J.F.; Zhang, Y. The influence of nitrogen availability on anatomical and physiological responses of Populus alba × P. glandulosa to drought stress. BMC Plant Biol. 2019, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Lada, R.R.; Caldwell, C.D.; Falk, K.C. Water-stress and N-nutrition effects on photosynthesis and growth of Brassica carinata. Photosynthetica 2011, 49, 309–315. [Google Scholar] [CrossRef]
- Yang, M.; Geng, M.Y.; Shen, P.F.; Chen, X.H.; Li, Y.J.; Wen, X.X. Effect of post-silking drought stress on the expression profiles of genes involved in carbon and nitrogen metabolism during leaf senescence in maize (Zea mays L.). Plant Physiol. Biochem. 2019, 135, 304–309. [Google Scholar] [CrossRef]
- Shi, H.L.; Ma, W.J.; Song, J.Y.; Lu, M.; Rahman, S.U.; Bui, T.T.X.; Vu, D.D.; Zheng, H.F.; Wang, J.H.; Zhang, Y. Physiological and transcriptional responses of Catalpa bungei to drought stress under sufficient- and deficient-nitrogen conditions. Tree Physiol. 2017, 37, 1457–1468. [Google Scholar] [CrossRef]
- Dziedek, C.; Oheimb, G.; Calvo, L.; Fichtner, A.; Kriebitzsch, W.U.; Marcos, E.; Pitz, W.T.; Hardtle, W. Does excess nitrogen supply increase the drought sensitivity of European beech (Fagus sylvatica L.) seedlings? Plant Ecol. 2016, 217, 393–405. [Google Scholar] [CrossRef]

| Cultivar | N Rate (kg ha−1) | Leaf Margin (mm) | Leaf Center (mm) | Margin/Center Ratio |
|---|---|---|---|---|
| KY188 | ||||
| Drought | 0 | 2.16 ± 0.12 ab | 1.68 ± 0.08 b | 1.29 ± 0.06 ab |
| 120 | 1.69 ± 0.04 d | 1.29 ± 0.03 c | 1.31 ± 0.06 a | |
| 180 | 1.55 ± 0.07 de | 1.22 ± 0.03 c | 1.27 ± 0.06 ab | |
| 240 | 1.44 ± 0.09 e | 1.15 ± 0.09 c | 1.26 ± 0.05 ab | |
| 360 | 1.38 ± 0.11 e | 1.13 ± 0.08 c | 1.22 ± 0.03 b | |
| Nondrought | 0 | 2.23 ± 0.04 a | 2.35 ± 0.03 a | 0.95 ± 0.02 c |
| 120 | 2.07 ± 0.04 abc | 2.35 ± 0.15 a | 0.88 ± 0.04 cd | |
| 180 | 1.99 ± 0.16 bc | 2.23 ± 0.09 a | 0.89 ± 0.04 cd | |
| 240 | 1.98 ± 0.14 c | 2.41 ± 0.23 a | 0.82 ± 0.04 de | |
| 360 | 1.96 ± 0.10 c | 2.46 ± 0.23 a | 0.80 ± 0.03 e | |
| Mean | 1.85 ± 0.02 B | 1.83 ± 0.06 B | 1.07 ± 0.02 A | |
| Drought (D) | ** | ** | ** | |
| Nitrogen (N) | ** | ** | ** | |
| D × N | ** | ** | ns | |
| DH605 | ||||
| Drought | 0 | 2.03 ± 0.11 a | 1.29 ± 0.08 d | 1.57 ± 0.04 a |
| 120 | 1.85 ± 0.07 b | 1.31 ± 0.07 d | 1.42 ± 0.09 b | |
| 180 | 1.78 ± 0.08 b | 1.30 ± 0.06 d | 1.37 ± 0.12 b | |
| 240 | 1.75 ± 0.03 b | 1.29 ± 0.04 d | 1.36 ± 0.02 b | |
| 360 | 1.48 ± 0.10 c | 1.24 ± 0.05 d | 1.20 ± 0.09 c | |
| Nondrought | 0 | 2.10 ± 0.05 a | 2.60 ± 0.02 c | 0.81 ± 0.03 d |
| 120 | 2.07 ± 0.11 a | 2.73 ± 0.16 abc | 0.76 ± 0.02 d | |
| 180 | 2.05 ± 0.13 a | 2.65 ± 0.13 bc | 0.77 ± 0.07 d | |
| 240 | 2.14 ± 0.05 a | 2.78 ± 0.14 ab | 0.77 ± 0.05 d | |
| 360 | 2.17 ± 0.04 a | 2.86 ± 0.10 a | 0.76 ± 0.01 d | |
| Mean | 1.94 ± 0.03 A | 2.00 ± 0.01 A | 1.08 ± 0.01 A | |
| Drought (D) | ** | ** | ** | |
| Nitrogen (N) | ** | ns | ** | |
| D × N | ** | ns | ** | |
| Cultivar | N rate (kg ha−1) | NAE (%) | NPFP (kg kg−1) | NHI (%) |
|---|---|---|---|---|
| KY188 | ||||
| Drought | 0 | - | - | 64.3 ± 2.4 a |
| 120 | 34.9 ± 3.2 c | 53.6 ± 1.3 b | 61.8 ± 1.4 ab | |
| 180 | 32.4 ± 3.9 cd | 39.0 ± 0.8 c | 60.0 ± 1.3 b | |
| 240 | 29.7 ± 4.2 cd | 29.3 ± 1.5 d | 60.7 ± 1.4 b | |
| 360 | 27.6 ± 2.4 de | 19.1 ± 0.5 f | 60.2 ± 1.1 b | |
| Nondrought | 0 | - | - | 64.3 ± 2.6 a |
| 120 | 49.5 ± 2.1 a | 67.8 ± 1.7 a | 62.5 ± 1.8 ab | |
| 180 | 45.9 ± 3.5 ab | 53.5 ± 1.5 b | 53.6 ± 2.4 c | |
| 240 | 40.8 ± 4.1 b | 37.2 ± 2.0 c | 54.4 ± 2.7 c | |
| 360 | 23.7 ± 2.5 e | 25.1 ± 0.4 e | 54.3 ± 0.6 c | |
| Mean | 35.6 ± 1.2 A | 40.6 ± 0.4 A | 59.6 ± 0.5 B | |
| Drought (D) | ** | ** | ** | |
| Nitrogen (N) | ** | ** | ** | |
| D × N | ** | ** | ** | |
| DH605 | ||||
| Drought | 0 | - | - | 70.0 ± 1.8 a |
| 120 | 57.1 ± 4.5 a | 51.0 ± 3.2 b | 66.0 ± 1.2 b | |
| 180 | 40.0 ± 3.7 c | 38.5 ± 2.1 c | 58.8 ± 0.5 d | |
| 240 | 16.3 ± 4.4 e | 24.3 ± 0.8 e | 57.8 ± 2.9 d | |
| 360 | 10.6 ± 4.5 e | 14.6 ± 0.8 f | 60.3 ± 1.8 cd | |
| Nondrought | 0 | - | - | 68.2 ± 2.6 ab |
| 120 | 56.7 ± 2.9 a | 69.0 ± 1.4 a | 69.8 ± 0.7 a | |
| 180 | 49.1 ± 3.8 b | 52.6 ± 1.5 b | 62.8 ± 1.8 c | |
| 240 | 47.0 ± 4.8 b | 35.4 ± 0.9 d | 61.0 ± 1.5 cd | |
| 360 | 30.0 ± 3.2 d | 22.4 ± 1.0 e | 60.5 ± 2.8 cd | |
| Mean | 38.3 ± 3.2 A | 38.5 ± 0.9 B | 63.5 ± 0.6 A | |
| Drought (D) | ** | ** | * | |
| Nitrogen (N) | ** | ** | ** | |
| D × N | ** | ** | * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, Y.; Fu, W.; Guo, W.; Ren, N.; Zhao, Y.; Ye, Y. Efficient Physiological and Nutrient Use Efficiency Responses of Maize Leaves to Drought Stress under Different Field Nitrogen Conditions. Agronomy 2020, 10, 523. https://doi.org/10.3390/agronomy10040523
Wang Y, Huang Y, Fu W, Guo W, Ren N, Zhao Y, Ye Y. Efficient Physiological and Nutrient Use Efficiency Responses of Maize Leaves to Drought Stress under Different Field Nitrogen Conditions. Agronomy. 2020; 10(4):523. https://doi.org/10.3390/agronomy10040523
Chicago/Turabian StyleWang, Yang, Yufang Huang, Wen Fu, Wenqing Guo, Ning Ren, Yanan Zhao, and Youliang Ye. 2020. "Efficient Physiological and Nutrient Use Efficiency Responses of Maize Leaves to Drought Stress under Different Field Nitrogen Conditions" Agronomy 10, no. 4: 523. https://doi.org/10.3390/agronomy10040523
APA StyleWang, Y., Huang, Y., Fu, W., Guo, W., Ren, N., Zhao, Y., & Ye, Y. (2020). Efficient Physiological and Nutrient Use Efficiency Responses of Maize Leaves to Drought Stress under Different Field Nitrogen Conditions. Agronomy, 10(4), 523. https://doi.org/10.3390/agronomy10040523




