Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass
Abstract
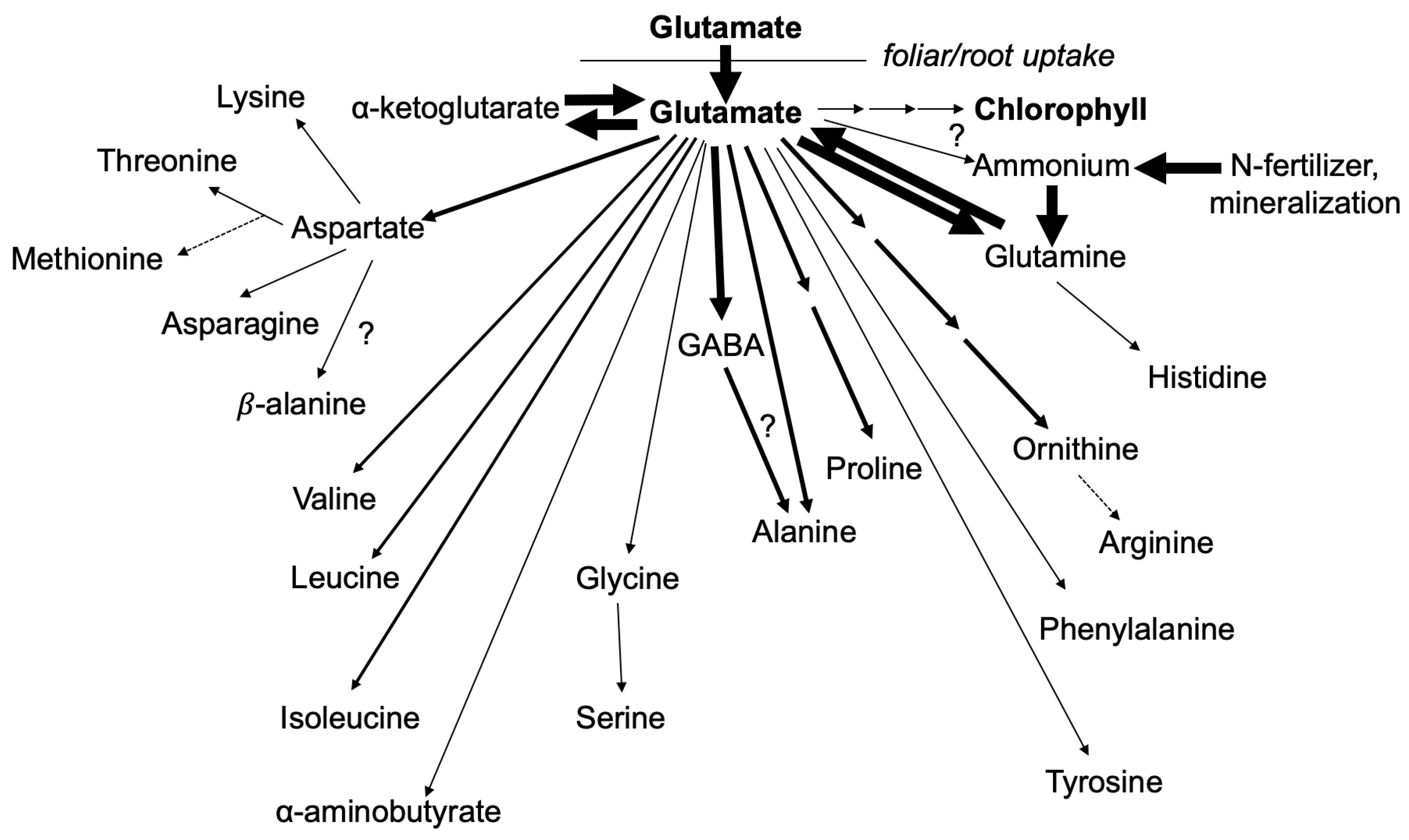
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions, General Experimental Procedures, and Reagents
2.2. Stable Isotope Labeling of Turfgrass
2.3. Extraction and Quantification of Amino Acids
3. Results and Discussion
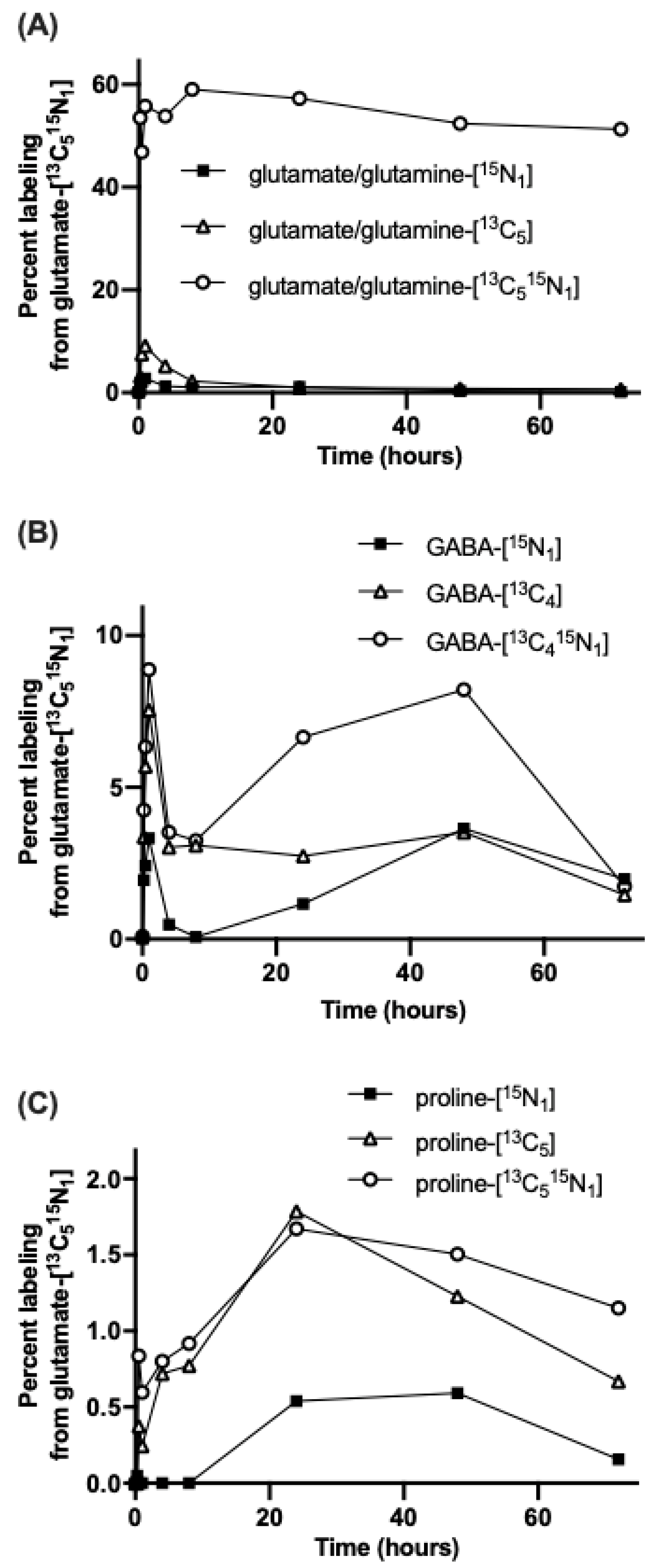
3.1. Nitrogen from Foliar Applied Glutamate is Incorporated into Proline and γ-aminobutyric acid (GABA)
3.2. The Carbon Skeleton from Foliar Applied Glutamate is also Incorporated into Proline and GABA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Markets & Markets Biostimulants Market by Active Ingredient (Humic Substances, Amino Acids, Seaweed Extracts, Microbial Amendments), Crop Type (Fruits & Vegetables, Cereals, Turf & Ornamentals), Application Method, Form, and Region – Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/biostimulant-market-1081.html?gclid=EAIaIQobChMI08H2466A6AIVUdHeCh0yBg7xEAAYASAAEgKOuPD_BwE (accessed on 4 March 2020).
- Zhang, X.; Schmidt, R.E. Hormone-containing products’ impact on antioxidant status of tall fescue and creeping bentgrass subjected to drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Stiegler, J.C.; Richardson, M.D.; Karcher, D.E.; Roberts, T.L.; Norman, R.J. Foliar Absorption of Various Inorganic and Organic Nitrogen Sources by Creeping Bentgrass. Crop Sci. 2013, 53, 1148. [Google Scholar] [CrossRef]
- Rhodes, D.; Hogan, A.L.; Deal, L.; Jamieson, G.C.; Haworth, P. Amino acid metabolism of Lemna minor L.: II. responses to chlorsulfuron. Plant Physiol. 1987, 84, 775–780. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.M.; Utturkar, S.M.; Crook, J.W.; Thimmapuram, J.; Widhalm, J.R. The origin and biosynthesis of the naphthalenoid moiety of juglone in black walnut. Hortic. Res. 2018, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Shelp, B.J. Plant GABA: Not Just a Metabolite. Trends Plant Sci. 2016, 21, 811–813. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.C.; Chung, T.Y.; Wu, H.Y.; Juo, Y.A.; Hsieh, M.H. Exogenous glutamate rapidly induces the expression of genes involved in metabolism and defense responses in rice roots. BMC Genomics 2017, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Szepesi, Á.; Szollosi, R. Mechanism of Proline Biosynthesis and Role of Proline Metabolism Enzymes Under Environmental Stress in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 337–353. [Google Scholar]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCoy, R.M.; Meyer, G.W.; Rhodes, D.; Murray, G.C.; Sors, T.G.; Widhalm, J.R. Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass. Agronomy 2020, 10, 358. https://doi.org/10.3390/agronomy10030358
McCoy RM, Meyer GW, Rhodes D, Murray GC, Sors TG, Widhalm JR. Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass. Agronomy. 2020; 10(3):358. https://doi.org/10.3390/agronomy10030358
Chicago/Turabian StyleMcCoy, Rachel M., George W. Meyer, David Rhodes, George C. Murray, Thomas G. Sors, and Joshua R. Widhalm. 2020. "Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass" Agronomy 10, no. 3: 358. https://doi.org/10.3390/agronomy10030358
APA StyleMcCoy, R. M., Meyer, G. W., Rhodes, D., Murray, G. C., Sors, T. G., & Widhalm, J. R. (2020). Exploratory Study on the Foliar Incorporation and Stability of Isotopically Labeled Amino Acids Applied to Turfgrass. Agronomy, 10(3), 358. https://doi.org/10.3390/agronomy10030358




