Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapevine Material
2.2. Simple Sequence Repeat (SSR) Analysis
2.3. Ampelographic Characterization
2.4. Physicochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Simple Sequence Repeat (SSR) Analysis
3.2. Ampelographic Characterization
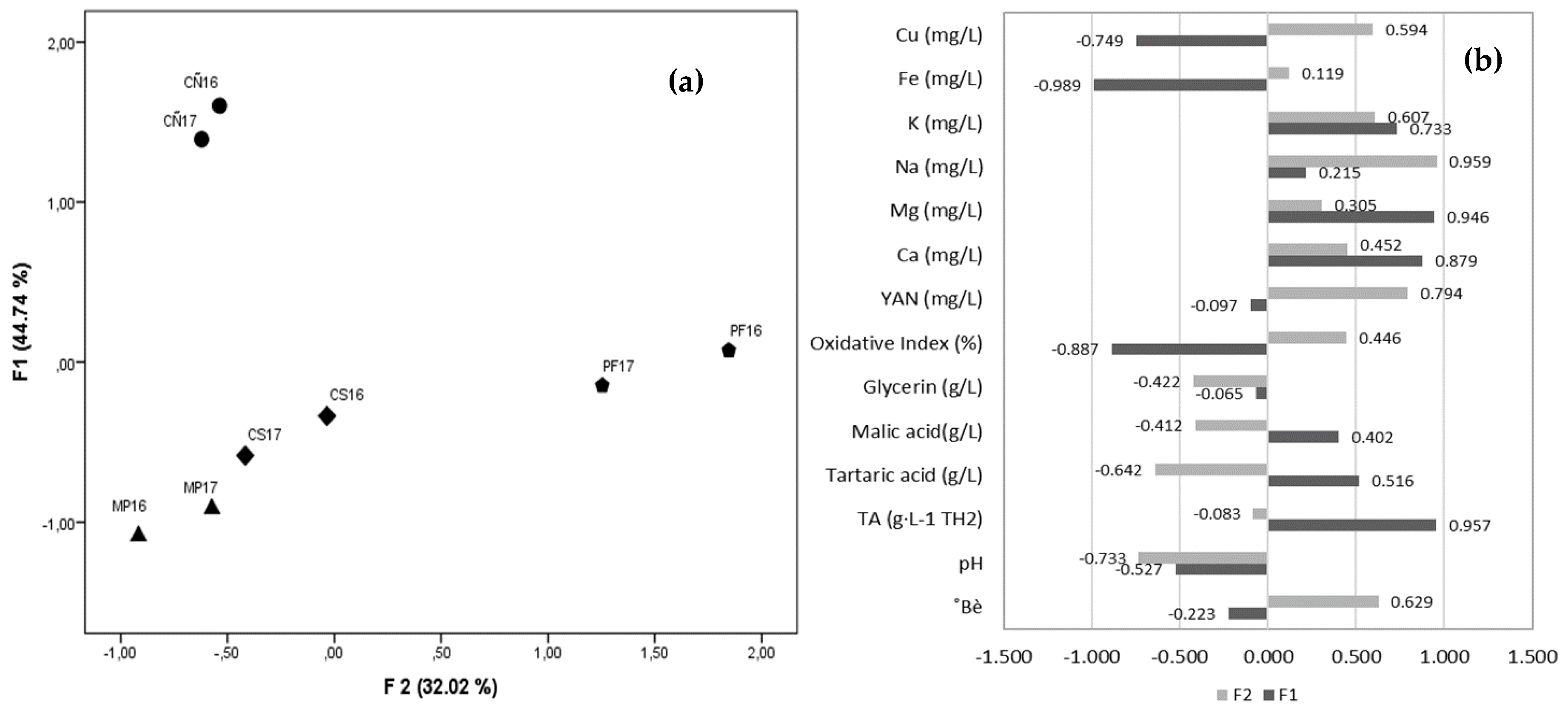
3.3. Grape Must Physicochemical Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estudillo, A.L. La vid y los viticultores de Jerez, la crisis comercial y el impacto de la filoxera: Un campo abierto a la investigación. Rev. Hist. Jerez 1992, 1, 43–71. [Google Scholar]
- Montañés, E. La industria vinícola del Jerez y la replantación del viñedo, 1894-1914: una aportación de historia empresarial. Hist. Agrar. Rev. Agric. Hist. Rural 2017, 71, 109–142. [Google Scholar]
- Roxas Clemente y Rubio, S. Ensayo Sobre las Variedades de vid que Vegetan en Andalucía, 1st ed.; Imprenta de Villapando: Madrid, Spain, 1807; pp. 111–113. [Google Scholar]
- De Bobadilla, G.F. Viníferas jerezanas y de Andalucía occidental; Instituto Nacional de Investigaciones Agronómicas: Madrid, Spain, 1956. [Google Scholar]
- Balda, P.; Ibáñez, J.; Sancha, C.; Martínez de Toda, F. Characterization and identification of minority red grape varieties recovered in Rioja, Spain. Am. J. Enol. Vitic. 2014, 65, 148–152. [Google Scholar] [CrossRef]
- García de Luján, A.; Jiménez-Cantizano, A. Consideraciones sobre la evolución de la viticultura del jerez en los últimos 80 años. In El Vino de Jerez en Los 80 años de la Denominación de Origen 1935–2015; Fundación Dialnet: Jerez, Spain, 2016; pp. 375–388. [Google Scholar]
- Jiménez-Cantizano, A.; García de Lujan, A.; Arroyo-García, R. Molecular characterization of table grape varieties preserved in the Rancho de la Merced Grapevine Germplasm Bank. Vitis 2018, 57, 93–101. [Google Scholar]
- González-Andrés, F.; Martín, J.P.; Yuste, J.; Rubio, J.A.; Arranz, C.; Ortiz, J.M. Identification and molecular biodiversity of autochthonous grapevine cultivars in the “Comarca del Bierzo”, León, Spain. Vitis 2007, 46, 71–76. [Google Scholar]
- Casanova, J.; Mozas, P.; Ortiz, J.M. Ampelography and microsatellite DNA analysis of autochthonous and endangered grapevine cultivars in the province of Huesca (Spain). Span. J. Agric. Res. 2011, 9, 790–800. [Google Scholar] [CrossRef]
- Lopes, M.S.; Rodrigues dos Santos, M.; Eiras Dias, J.E.; Mendonça, D.; da Câmara Machado, A. Discrimination of Portuguese graevines based on microsatellite markers. J. Biotechnol. 2006, 127, 34–44. [Google Scholar] [CrossRef]
- Gismondi, A.; Impei, S.; Di Marco, G.; Crespan, M.; Leonardi, D.; Canini, A. Detection of new genetic profiles and allelic variants improperly classified grapevine accessions. Genome 2014, 57, 111–118. [Google Scholar] [CrossRef]
- Costacurta, A.; Giannetto, S.; Meeghetti, S.; Crespan, M. Does it exist a Greek ampelographical heredity in South Italy? SSR profiles comparison of cultivars growing in both countries. In Proceedings of the Ampelos 2006, 2nd International Symposium on the Evaluation and Exploitation of Grapes of Corresponding Terroir through Winemaking and Commercialization of Wines, Santorini Island, Greece, 1–3 June 2006. [Google Scholar]
- Duchène, E.; Schneider, C. Grapevine and climatic changes: a glance at the situation in Alsace. Agron. Sustain. Dev. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Lopes, J.; Eiras-Dias, J.F.; Abreu, F.; Climaco, P.; Cunha, J.P.; Silvestre, J. Thermal requirements, duration and precocity of phenological stages of grapevine cultivars of the Portuguese collection. Ciéncia Téc. Vitiv. 2008, 1, 61–71. [Google Scholar]
- Fraga, H.; Santos, J.A.; Malheiro, A.C.; Oliveira, A.A.; Moutinho-Pereira, J.; Jones, G.V. Climatic suitability of Portuguese grapevine varieties and climate change adaptation. Int. J. Climatol. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Organisation Internationale de la Vigne et du Vin (OIV). OIV Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; OIV: Paris, France, 2009. [Google Scholar]
- Jiménez-Cantizano, A.; Amores-Arrocha, A.; Gutiérrez-Escobar, R.; Palacios, V. Identification and relationship of the autochthonous ’Romé’ and ’Rome Tinto’ grapevine cultivars. Span. J. Agric. Res. 2018, 16, e07SC02. [Google Scholar] [CrossRef]
- Marsal, G.; Méndez, J.J.; Mateo, J.M.; Ferrer, S.; Canals, J.M.; Zamora, F.; Fort, F. Molecular characterization of Vitis vinifera L. local cultivars from volcanic areas (Canary Islands and Madeira) using SSR markers. Oeno one 2019, 4, 667–680. [Google Scholar] [CrossRef]
- Jiménez-Cantizano, A.; Puertas, B.; Serrano, M.J. Adaptation and Selection of Cultivars of Grapevine Quality Wines in Warm Climate. In Proceedings of the IInd IS on Tropical Wines, Petrolina, Brasil, 25–28 May 2010; Pereira, G.E., Tonieto, J., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2011. [Google Scholar]
- Santesteban, L.G.; Miranda, C.; Royo, J.B. Vegetative Growth, Reproductive Development and Vineyard Balance. In Methodologies and Results in Grapevine Research, 1st ed.; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: New York, NY, USA, 2010; pp. 45–56. [Google Scholar]
- Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Genetical, Morphological and Physicochemical Characterization of the Autochthonous Cultivar ‘Uva Rey’ (Vitis vinifera L.). Agronomy 2019, 9, 563. [Google Scholar] [CrossRef]
- Park, S.D.E. Trypanotolerance in West African Cattle and the Population Genetic Effects of Selection. Ph.D. Thesis, University of Dublin, Dublin, Ireland, 2001. [Google Scholar]
- Lacombe, T.; Bourisquot, J.M.; Laucou, V.; Di Vecchi-Staraz, M.; Péros, J.P.; This, P. Large-Scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue. Available online: www.vivc.de (accessed on 21 September 2019).
- Jiménez-Cantizano, A. Caracterización Molecular del Banco de Germoplasma de vid del Rancho de la Merced. Ph.D. Thesis, Universidad de Cádiz, Cádiz, Spain, 2014. [Google Scholar]
- Ibáñez, J.; Vargas, M.A.; Palancar, M.; Borrego, J.; de Andrés, M.T. Genetic relationships among table-grape varieties. Am. J. Enol. Vitic. 2009, 60, 35–47. [Google Scholar]
- De Andrés, M.T.; Benito, A.; Pérez-Rivera, G.; Ocete, R.; López, M.A.; Gaforio, L.; Muñoz, G.; Cabello, F.; Martínez-Zapater, J.M.; Arroyo-García, R. Genetic diversity of wild grapevine populations in Spain and its genetic relationships with cultivated grapevines. Mol. Ecol. 2012, 21, 800–816. [Google Scholar] [CrossRef]
- Benito, A.; Muñoz-Organero, G.; de Andrés, M.T.; Ocete, R.; García-Muñoz, S.; López, M.A.; Arroyo-García, R.; Cabello, F. Ex situ ampelographical characterisation of wild Vitis vinifera from fifty-one Spanish populations. Aust. J. Grape Wine Res. 2017, 23, 143–152. [Google Scholar] [CrossRef]
- Recuéil des Méthodes Internationales D’analyse des vins et des Moûts; OIV Office International de la Vigne et du Vin: Paris, France, 2014.
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Use of Multiflora Bee Pollen as a Flor Velum Yeast Growth Activator in Biological Aging Wines. Molecules 2019, 24, 1763. [Google Scholar] [CrossRef]
- Aerny, J. Composés azotés des moûts et des vins. Rev. Suisse Vitic. Arboric. Hortic. 1997, 28, 161–168. [Google Scholar]
- AFNOR: Dosage des minéraux-méthodes par spectrométrie d’emission de flame, NF-T-90-019; Association Française de Normalisation: Paris, France, 1996.
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Constantini, K.; Crespan, M.; Dangl, G.S.; Eisenheid, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for indentification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, I.; Desalle, R. A Natural History of Wine, 2nd ed.; Yale University Press: New Haven, CT, USA, 2015; pp. 62–90. [Google Scholar]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, S.; Muñoz-Organero, G.; De Andrés, M.T.; Cabello, F. Ampelography: An old technique with future uses, the case of minor varieties of Vitis vinifera L. from the Balearic Islands. J. Int. Sci. Vigne Vin 2011, 45, 125–137. [Google Scholar] [CrossRef]
- García de Luján, A.; Puertas, B.; Lara, M. Variedades de vid en Andalucía, 1st ed.; Consejería de Agricultura y Pesca, Junta de Andalucía: Seville, Spain, 1990; pp. 69–76. [Google Scholar]
- Serrano, M.J.; Puertas, B.; Velasco, L.; Pérez, J.A.; Jimenez-Cantizano, A. Caracterización de la variedad de vid (Vitis vinifera L.) autóctona minoritaria andaluza C.V. Castellano. In Proceedings of the XVI Congreso Nacional de Enólogos, Jerez de la Frontera, Cádiz, 22–25 May 2014. [Google Scholar]
- Gago, P.; Conéjéro, G.; Martínez, M.C.; Boso, S.; This, P.; Verdeil, J.-L. Microanatomy of leaf trichomes: oportuities for improved ampelographic discrimination of grapevine (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 22, 494–503. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associate effects on grape and wine quality and production. Food Res. Intl. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Coombe, B. Influence of temperature on composition and quality of grapes. In Proceedings of the International Symposium on Grapevine Canopy and Vigor Management, Davis, CA, USA, 11 August 1986; International Society for Horticultural Science: Leuven, Belgium, 1987. [Google Scholar]
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture; University California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Lakso, A.N.; Kliewer, W.M. The influence of temperature on malic acid metabolism in grape berries. II. Temperature responses of net dark CO2 fixation and malic acids pools. Am. J. Enol. Vitic. 1977, 29, 145–148. [Google Scholar]
- Hidalgo-Togores, J. Vendimia. Recepción de Uva en la Bodega. Índices de maduración químicos. In Tratado de Enología, 5th ed.; Hernández-Úbeda, I., Ed.; Editorial Mundi-Prensa: Madrid, Spain, 2019; Volume I, pp. 238–240. [Google Scholar]
- Mullins, M.G.; Bouquet, A.; Williams, L.E. The Biology of the Grapevine, 1st ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 80–146. [Google Scholar]
- Geber, C. Researches sur la maduration des fruits charnus. Ann. Sci. Nat. Botan. 1897, 4, 1–6. [Google Scholar]
- Palacios, V.; Nebot, E.; Pérez-Rodríguez, L. Use of factor analysis for the characterization and modelling of Palomino fino grapes in the Jerez region. Am. J. Enol. Vitic. 1997, 48, 317–322. [Google Scholar]
- Puertas, B. Estudio sobre el potencial vitícola y enológico de quince variedades blancas de vid en la zona del Jerez. Ph.D. Thesis, Universidad de Cádiz, Cádiz, España, 1989. [Google Scholar]
- Belle, S.J. The Effect of Nitrogen Fertilisation on the Growth Yield and Juice Composition of Vitis vinifera cv. Cabernet Sauvignon Grapevines. Ph.D. Thesis, University of Western Australia, Perth, Australia, 1994. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donéche, B.; Lonvaud, A.; Glories, Y.; Maugean, A. Traité D’oenologie: Microbiologie du vin. Vinifications, 3rd ed.; Dunod Editions: Paris, France, 2017. [Google Scholar]
- Pinedo, J.M.; Maiquez, E.G.; Corral, L. The evolution of phenolic compounds during maturation in Palomino fino grape must. In Proceedings of the International Symposium on Viticulture and Enology, Córdoba, España, 1 April 1995; Pérez-Camacho, F., Medina, M., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2011. [Google Scholar]
- Cheynier, V.; Fulcrand, H.; Guyot, S.; Oszmianski, J.; Moutounet, M. Reactions of Enzymically Generated Wuinones in Relation to Browning in Grape Musts and Wines. In Enzymatic Browning and Its Prevention; Lee, C., Ed.; American Chemical Society: Washington, DC, USA, 1995; pp. 130–143. [Google Scholar]
- González-Mendoza, L.A.; Armas-Concepción, P.A.; González-Hernández, J.E.; García-Fernández, M.J.; Vidarte-Ramos, E.; Pomar-García, M. Estudio evolutivo de los cationes sodio, potasio, hierro y cobre, durante la maduración en cepas de las variedades listán negro, listán blanco y negramoll. D.O. Tacoronte-Acentejo. In Jornadas Técnicas Vitivinícolas Canarias; Servicio Técnico de Desarrollo Rural y Pesquero: Tenerife, España, 1997. [Google Scholar]
- Galani-Nikolakaki, S.; Kallithrakas-Kontos, N.; Katsanos, A.A. Trace element analysis of Cretan wines and wine products. Sci. Total Environ. 2002, 285, 155–163. [Google Scholar] [CrossRef]
| Autochthonous Variety | Reference Variety | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variety Code | CÑ | CS | MP | PF | CSV | CH | MPGB | PN | ||||||||
| Microsatellite Locus | ||||||||||||||||
| VVIB01 | 291 | 307 | 291 | 307 | 307 | 307 | 291 | 307 | 291 | 291 | 289 | 295 | 291 | 295 | 289 | 295 |
| VMC1b11 | 184 | 188 | 184 | 184 | 184 | 188 | 184 | 188 | 184 | 184 | 166 | 184 | 184 | 188 | 166 | 172 |
| VMC4F31 | 188 | 206 | 168 | 176 | 184 | 190 | 176 | 206 | 174 | 178 | 174 | 180 | 168 | 206 | 174 | 180 |
| VVMD5 | 232 | 234 | 220 | 224 | 224 | 232 | 226 | 238 | 228 | 236 | 232 | 236 | 226 | 234 | 226 | 236 |
| VVMD7 | 240 | 246 | 236 | 246 | 244 | 246 | 236 | 246 | 236 | 236 | 236 | 240 | 232 | 246 | 236 | 240 |
| VVMD21 | 249 | 255 | 243 | 265 | 243 | 249 | 243 | 249 | 249 | 257 | 249 | 249 | 249 | 265 | 249 | 249 |
| VVMD24 | 209 | 209 | 209 | 211 | 209 | 209 | 209 | 209 | 209 | 217 | 209 | 219 | 213 | 217 | 215 | 217 |
| VVMD25 | 240 | 252 | 252 | 252 | 238 | 252 | 240 | 240 | 238 | 246 | 238 | 252 | 240 | 246 | 238 | 246 |
| VVMD27 | 186 | 194 | 182 | 182 | 182 | 182 | 186 | 194 | 176 | 190 | 182 | 190 | 180 | 194 | 186 | 190 |
| VVMD28 | 236 | 250 | 246 | 246 | 246 | 248 | 238 | 250 | 236 | 238 | 220 | 230 | 248 | 270 | 220 | 238 |
| VVMD32 | 254 | 270 | 270 | 270 | 270 | 270 | 254 | 256 | 238 | 238 | 238 | 270 | 262 | 270 | 238 | 270 |
| VVIH54 | 166 | 166 | 166 | 168 | 166 | 168 | 166 | 166 | 166 | 182 | 164 | 168 | 166 | 166 | 164 | 168 |
| VVIN16 | 153 | 153 | 151 | 151 | 151 | 153 | 151 | 151 | 153 | 153 | 151 | 151 | 149 | 149 | 151 | 159 |
| VVIN73 | 264 | 264 | 264 | 264 | 264 | 264 | 264 | 264 | 264 | 268 | 264 | 266 | 264 | 264 | 264 | 266 |
| VVIP31 | 180 | 190 | 176 | 176 | 176 | 190 | 188 | 190 | 190 | 190 | 180 | 184 | 184 | 188 | 180 | 180 |
| VVIP60 | 318 | 326 | 322 | 322 | 318 | 326 | 318 | 322 | 306 | 314 | 318 | 322 | 318 | 318 | 318 | 320 |
| VVIQ52 | 85 | 89 | 85 | 89 | 85 | 89 | 85 | 85 | 83 | 89 | 83 | 89 | 83 | 83 | 89 | 89 |
| VVS2 | 142 | 144 | 142 | 142 | 131 | 142 | 131 | 144 | 137 | 151 | 135 | 142 | 131 | 131 | 135 | 151 |
| VVIV37 | 163 | 177 | 163 | 167 | 161 | 161 | 163 | 167 | 163 | 163 | 153 | 163 | 163 | 165 | 153 | 163 |
| VVIV67 | 358 | 372 | 366 | 375 | 372 | 375 | 364 | 366 | 364 | 372 | 364 | 372 | 364 | 375 | 364 | 372 |
| VrZAG62 | 187 | 203 | 187 | 193 | 187 | 193 | 187 | 193 | 187 | 193 | 187 | 195 | 185 | 195 | 187 | 193 |
| VrZAG79 | 236 | 246 | 236 | 258 | 242 | 248 | 250 | 260 | 246 | 246 | 242 | 244 | 250 | 254 | 238 | 244 |
| Code | Descriptor | CÑ | CS | MP | PF |
|---|---|---|---|---|---|
| OIV 001 | Young shoot: opening of the shoot tip; 1 closed, 3 half open, 5 fully open. | 5 | 5 | 5 | 5 |
| OIV 003 | Young shoot: intensity of anthocyanin coloration on prostrate hairs of the shoot tip; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 1 | 7 | 1 | 5 |
| OIV 004 | Young shoot: density of prostrate hairs on the shoot tip; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 5 | 5 | 7 | 5 |
| OIV 006 | Shoot: attitude (before tying); 1 erect, 3 semi-erect, 5 horizontal, 7 semi-drooping, 9 drooping. | 1 | 3 | 1 | 3 |
| OIV 007 | Shoot: color of the dorsal side of internodes; 1 green, 2 green and red, 3 red. | 1 | 2 | 1 | 2 |
| OIV 008 | Shoot: color of the ventral side of internodes; 1 green, 2 green and red, 3 red. | 1 | 2 | 1 | 2 |
| OIV 015-2 | Shoot: intensity of anthocyanin coloration on the bud scales; 1 none or very weak, 3 weak, 5 medium, 7 strong, 9 very strong. | 1 | 5 | 1 | 3 |
| OIV 016 | Shoot: number of consecutive tendrils; 1 two or less, 2 three or more. | 1 | 1 | 1 | 1 |
| OIV 051 | Young leaf: color of upper side of blade (4th leaf); 1 green, 2 yellow, 3 bronze, 4 copper-reddish. | 1 | 3 | 3 | 3 |
| OIV 053 | Young leaf: density of prostrate hairs between main veins on lower side of blade (4th leaf); 1 none or very low, 3 low, 5, medium, 7 high, 9 very high. | 7 | 7 | 9 | 5 |
| OIV 065 | Mature leaf: size of blade; 1 very small, 3, small, 5 medium, 7 large, 9 very large. | 5 | 5 | 5 | 7 |
| OIV 067 | Mature leaf: shape of blade; 1 cordate, 3 wedge-shaped, 3 pentagonal, 4 circular, 5 kidney-shaped. | 3 | 3 | 3 | 3 |
| OIV 068 | Mature leaf: number of lobes; 1 one, 2 three, 3 five, 4 seven, 5 more than seven. | 3 | 3 | 3 | 3 |
| OIV 070 | Mature leaf: area of anthocyanin coloration of main veins on upper side of blade; 1 absent, 2 only at the petiolar point, 3 up to the 1st bifurcation, 4 up to the 2nd bifurcation, 5 beyond the 2nd bifurcation. | 1 | 1 | 1 | 3 |
| OIV 074 | Mature leaf: profile of blade in cross section; 1 flat, 2 V-shaped, 3 involute, 4 revolute, 5 twisted. | 5 | 5 | 3 | 4 |
| OIV 075 | Mature leaf: blistering of upper side of blade; 1 absent or very weak, 2 weak, 3 medium, 4 strong, 9 very strong. | 5 | 3 | 5 | 3 |
| OIV 076 | Mature leaf: shape of teeth; 1 both sides concave, 2 both sides straight, 3 both sides convex, 4 one side concave on side convex, 5 mixture between both sides straight and both sides convex. | 3 | 3 | 2 | 3 |
| OIV 079 | Mature leaf: degree of opening/overlapping of petiole sinus; 1 very wide open, 3 open, 5 closed, 7 overlapped, 9 strongly overlapped. | 7 | 7 | 3 | 5 |
| OIV 080 | Mature leaf: shape of base petiole sinus; 1 U-shaped, 2 brace-shaped, 3 V-shaped. | 3 | 3 | 3 | 3 |
| OIV 081-1 | Mature leaf: teeth in the petiole sinus; 1 none, 9 present. | 1 | 2 | 1 | 1 |
| OIV 081-2 | Mature leaf: petiole sinus base limited by vein; 1 not limited, 3 on one side, 3 on both sides. | 1 | 1 | 1 | 1 |
| OIV 083-1 | Mature leaf: shape of the base of upper lateral sinuses; 1 U-shaped, 2 brace-shaped, 3 V-shaped. | 3 | 3 | 1 | 1 |
| OIV 083-2 | Mature leaf: teeth in the upper lateral sinuses; 1 none, 9 present. | 1 | 1 | 1 | 1 |
| OIV 084 | Mature leaf: density of prostrate hairs between main veins on lower side of blade; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 5 | 7 | 5 | 7 |
| OIV 085 | Mature leaf: density of erect hairs between main veins on lower side of blade; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 5 | 5 | 5 | 5 |
| OIV 086 | Mature leaf: density of prostrate hairs on main veins on lower side of blade; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 5 | 5 | 9 | 5 |
| OIV 087 | Mature leaf: density of erect hairs on main veins on lower side of blade; 1 none or very low, 3 low, 5 medium, 7 high, 9 very high. | 5 | 5 | 3 | 1 |
| OIV 151 | Flower: sexual organs; 1 fully developed stamens and no gynoecium, 2 fully developed stamens and reduced gynoecium, 3 fully developed stamens and fully developed gynoecium, 4 reflexed stamens and fully developed gynoecium. | 3 | 3 | 3 | 3 |
| OIV 202 | Bunch: length (peduncle excluded); 1 very short, 3 short, 5 medium, 7 long, 9 very long. | 7 | 7 | 7 | 7 |
| OIV 203 | Bunch: width; 1 very narrow, 3 narrow, 5 medium, 7 wide, 9 very wide. | 5 | 5 | 5 | 5 |
| OIV 204 | Bunch: density; 1 very loose, 3 loose, 5 medium, 7 dense, 9 very dense. | 3 | 5 | 5 | 5 |
| OIV 206 | Bunch: length of peduncle of primary bunch; 1 very short, 3 short, 5 medium, 7 long, 9 very long. | 1 | 1 | 1 | 1 |
| OIV 220 | Berry: length; 1 very short, 3 short, 5 medium, 7 long, 9 very long. | 5 | 5 | 5 | 3 |
| OIV 221 | Berry: width; 1 very narrow, 3 narrow, 5 medium, 7 wide, 9 very wide. | 5 | 5 | 5 | 3 |
| OIV 223 | Berry: shape; 1 obloid, 2 globose, 3 broad ellipsoid, 4 narrow ellipsoid, 5 cylindric, 6 obtuse ovoid, 7 ovoid, 8 obovoid, 9 horn shaped, 10 finger shaped. | 1 | 7 | 5 | 2 |
| OIV 225 | Berry: color of skin; 1 green yellow, 2 rose, 3 red, 4 grey, 5 dark red violet, 6 blue black. | 1 | 1 | 1 | 1 |
| Physicochemical Parameters | CÑ | CS | MP | PF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | ||||||||||||
| °Bé | 10.88 | ± | 0.02 a | 11.54 | ± | 0.01 b | 9.12 | ± | 0.02 c | 11.10 | ± | 0.01 d |
| pH | 3.72 | ± | 0.02 a | 3.94 | ± | 0.01 b | 3.93 | ± | 0.07 b | 3.77 | ± | 0.01 a |
| TA (g·L−1 TH2) | 3.79 | ± | 0.06 a | 3.47 | ± | 0.04 b | 3.51 | ± | 0.07 b | 3.24 | ± | 0.06 c |
| Ripening Index (RI) | 2.87 | ± | 0.02 a | 3.32 | ± | 0.06 b | 3.51 | ± | 0.01 c | 3.42 | ± | 0.04 b |
| Tartaric acid (g/L) | 2.80 | ± | 0.03 a | 2.96 | ± | 0.01 b | 2.88 | ± | 0.05 a | 2.37 | ± | 0.04 c |
| Malic acid(g/L) | 1.08 | ± | 0.08 a | 0.80 | ± | 0.03 b | 1.20 | ± | 0.10 c | 0.24 | ± | 0.00 d |
| Glycerin (g/L) | 0.26 | ± | 0.00 a | 0.35 | ± | 0.00 b | 0.33 | ± | 0.00 b | 0.19 | ± | 0.01 d |
| Oxidative index (%) | 10.45 | ± | 0.64 a | 29.97 | ± | 1.05 b | 21.18 | ± | 0.71 c | 56.32 | ± | 1.57 d |
| YAN (mg/L) | 184.73 | ± | 1,07 a | 188.50 | ± | 1.54 a,c | 143.57 | ± | 2.50 b | 192.57 | ± | 2.14 c |
| Calcium (mg/L) | 362.33 | ± | 1.00 a | 158.70 | ± | 1.00 b | 146.07 | ± | 1.00 c | 167.95 | ± | 1.00 b |
| Magnesium (mg/L) | 151.61 | ± | 0.30 a | 82.72 | ± | 0.20 b | 80.72 | ± | 0.40 b | 75.47 | ± | 0.20 c |
| Sodium (mg/L) | 13.16 | ± | 0.20 a | 9.24 | ± | 0.11 b | 6.06 | ± | 0.40 c | 13.82 | ± | 0.50 d |
| Potassium (mg/L) | 3228.55 | ± | 4.00 a | 2369.66 | ± | 9.01 b | 1749.59 | ± | 9.00 c | 2308.84 | ± | 12.00 b |
| Iron (mg/L) | 3.79 | ± | 0.01 a | 6.21 | ± | 0.04 b | 6.25 | ± | 0.10 c | 8.20 | ± | 0.20 d |
| Copper (mg/L) | 0.79 | ± | 0.01 a | 1.20 | ± | 0.02 b | 1.19 | ± | 0.04 b | 3.95 | ± | 0.03 c |
| 2017 | ||||||||||||
| °Bé | 9.05 | ± | 0.02 a | 9.40 | ± | 0.01 b | 8.65 | ± | 0.01 c | 10.65 | ± | 0.01 d |
| pH | 3.76 | ± | 0.01 a | 3.92 | ± | 0.01 b | 3.90 | ± | 0.01 b | 3.89 | ± | 0.01 b |
| TA (g·L−1 TH2) | 3.82 | ± | 0.02 a | 3.56 | ± | 0.01 b | 3.53 | ± | 0.01 b | 3.46 | ± | 0.01 b |
| Ripening Index (RI) | 2.37 | ± | 0.02 a | 2.64 | ± | 0.02 b | 2.45 | ± | 0.10 a | 3.07 | ± | 0.04 c |
| Tartaric acid (g/L) | 2.80 | ± | 0.01 a,c | 2.90 | ± | 0.01 b | 2.68 | ± | 0.01 a,b | 2.58 | ± | 0.01 c |
| Malic acid(g/L) | 0.37 | ± | 0.01 a | 0.34 | ± | 0.01 b | 0.46 | ± | 0.01 c | 0.31 | ± | 0.01 d |
| Glycerin (g/L) | 0.03 | ± | 0.01 a | 1.30 | ± | 0.01 b | 0.21 | ± | 0.01 c | 0.03 | ± | 0.01 a |
| Oxidative index (%) | 9.46 | ± | 0.10 a | 27.49 | ± | 0.97 b | 19.98 | ± | 0.89 c | 50.24 | ± | 1.42 d |
| YAN (mg/L) | 168.24 | ± | 0.98 a | 175.73 | ± | 1.24 b | 140.30 | ± | 2.80 c | 184.24 | ± | 1.70 d |
| Calcium (mg/L) | 371.22 | ± | 0.98 a | 159.22 | ± | 1.14 b | 154.28 | ± | 1.03 b | 179.91 | ± | 2.05 c |
| Magnesium (mg/L) | 148.72 | ± | 0.41 a | 78.74 | ± | 0.15 b | 79.70 | ± | 1.22 b | 71.52 | ± | 0.18 c |
| Sodium (mg/L) | 13.89 | ± | 0.18 a | 9.01 | ± | 0.09 b | 6.37 | ± | 0.34 c | 12.87 | ± | 0.38 d |
| Potassium (mg/L) | 3105.28 | ± | 7.06 a | 2472.02 | ± | 6.97 b | 1821.46 | ± | 11.06 c | 2340.51 | ± | 8.85 d |
| Iron (mg/L) | 4.02 | ± | 0.01 a | 6.23 | ± | 0.01 b | 6.21 | ± | 0.08 b | 7.98 | ± | 0.07 c |
| Copper (mg/L) | 0.82 | ± | 0.02 a | 0.99 | ± | 0.03 b | 1.27 | ± | 0.03 c | 4.11 | ± | 0.14 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho-Galán, P.; Amores-Arrocha, A.; Palacios, V.; Jiménez-Cantizano, A. Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain). Agronomy 2020, 10, 205. https://doi.org/10.3390/agronomy10020205
Sancho-Galán P, Amores-Arrocha A, Palacios V, Jiménez-Cantizano A. Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain). Agronomy. 2020; 10(2):205. https://doi.org/10.3390/agronomy10020205
Chicago/Turabian StyleSancho-Galán, Pau, Antonio Amores-Arrocha, Víctor Palacios, and Ana Jiménez-Cantizano. 2020. "Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain)" Agronomy 10, no. 2: 205. https://doi.org/10.3390/agronomy10020205
APA StyleSancho-Galán, P., Amores-Arrocha, A., Palacios, V., & Jiménez-Cantizano, A. (2020). Identification and Characterization of White Grape Varieties Autochthonous of a Warm Climate Region (Andalusia, Spain). Agronomy, 10(2), 205. https://doi.org/10.3390/agronomy10020205








