Abstract
The boron (B) concentration surpasses the plant need in arid and semi-arid regions of the world, resulting in phyto-toxicity. Salicylic acid (SA) is an endogenous signaling molecule responsible for stress tolerance in plants and is a potential candidate for ameliorating B toxicity. In this study, the effects of seed priming with SA (0, 50, 100 and 150 µM for 12 h) on the growth, pigmentation and mineral concentrations of maize (Zea mays L.) grown under B toxicity were investigated. One-week old seedlings were subjected to soil spiked with B (0, 15 and 30 mg kg−1 soil) as boric acid. Elevating concentrations of B reduced the root and shoot length, but these losses were significantly restored in plants raised from seeds primed with 100 µM of SA. The B application decreased the root and shoot fresh/dry biomasses significantly at 30 mg kg−1 soil. The chlorophyll and carotenoid contents decreased with increasing levels of B, while the contents of anthocyanin, H2O2, ascorbic acid (ASA) and glycinebetaine (GB) were enhanced. The root K and Ca contents were significantly increased, while a reduction in the shoot K contents was recorded. The nitrate concentration was significantly higher in the shoot as compared to the root under applied B toxic regimes. However, all of these B toxicity effects were diminished with 100 µM SA applications. The current study outcomes suggested that the exogenously applied SA modulates the response of plants grown under B toxic conditions, and hence could be used as a plant growth regulator to stimulate plant growth and enhance mineral nutrient uptake under B-stressed conditions.
1. Introduction
Abrupt changes in climate along with the potential abiotic and biotic stresses are serious challenges for plant growth and production worldwide [1]. Environmental stresses negatively influence the germination, growth and yield of the crop plants. The continuous yield losses caused by abiotic stresses are one of the important reasons for socioeconomic imbalance [2]. Drought reduces the yield of staple food crops throughout the world up to 70% [3], and the effects of drought and salt stress on plant growth mechanisms and patterns have been discussed [3,4]. In the last few decades, soil and water resources are being contaminated with toxic elements due to industrial revolution and urbanization together with the use of artificial fertilizers [5,6]. Increasing levels of these toxic elements are imposing harmful effects on plants, plant-dependent animals and ultimately human health [7].
Boron is an important micronutrient in many plants for their normal functioning [8]. It is also considered to be an essential element for vascular plants according to the defined criteria for essentiality. The indirect association of B with photosynthesis has been reported in crop plants—e.g., soybean [9]. However, the rate of emergence and productivity is also decreased in many plants, including tomato, maize, wheat, alfalfa and carrot under B toxicity [10]. The B toxicity significantly reduces the yield of crop plants in relatively dry areas of the world [11]. Some of the factors contributing to the elevating levels of B are the use of fertilizers, mining and irrigation [12,13]. The B-induced toxicity occurs more commonly in saline soil in semi-arid geographical zones [14]. The interplay between salt stress and B nutrition in plants has been described, with contrasting results showing antagonistic and synergistic relations even within the same plant species [15]. It has been observed that salinity increases B toxicity [16], but the interaction of salinity and B is not fully understood [17], making it an important area of research in plant physiology and ecotoxicology.
Oxidative stress may result from a deficiency or excess of B, which triggers the over-production of reactive oxygen species (ROS). The ROS and their derivatives are highly toxic agents and damage cellular membranes due to lipid peroxidation, causing protein denaturation and mutations in DNA [18]. Different nutrients such as silicon (Si) [19], zinc (Zn) [20,21], potassium (K) [22] and calcium (Ca) [23] can ameliorate B toxicity in different crop plants. The SA signal molecule [24] plays an important role in reducing the hazardous effects posed by biotic and abiotic stresses. Thus, SA has been used by many researchers to reduce the hazardous effects of different stresses such as osmotic stress [25], heat, saline and B toxicity in wheat [26].
Among the most important staple foods, maize holds an important position after wheat and rice [27]. Maize is well known for its high potential of extracting heavy metals from soil [28]. Despite this phytoextraction ability, maize is affected by various environmental stresses along with the high metal concentrations. The abiotic stress effects on maize growth and yield have been studied [29,30]. In the current study, the main objective was to assess the effects of high B toxicity under the remodeling effects of SA in terms of physio-biochemical improvements in the maize cultivar Gohar-19.
2. Results
For assessing the effects of SA on mitigating the effects of B toxicity, plants were supplied with 0, 50, 100 and 150 µM of SA. The B toxicity levels were 0, 15 and 30 mg kg−1 soil. Roots transport B via passive diffusion or facilitate transport [30] in the plant body through transpiration streams and it is accumulated in older shoots without being translocated [31], therefore the study parameters include both the root and shoot data of maize cv. Gohar-19.
2.1. Root and Shoot Length
The B toxicity significantly reduced the root and shoot length of maize seedlings. High B concentrations in soil inhibit the root and shoot growth due to the decreased photosynthetic activity and net plant productivity. Elevating the B concentration in soil decreased the root and shoot length up to 21.77% and 25.25%, respectively, which are significant reductions (Table 1, Figure 1). The priming of seeds with SA reduced the B toxic effects and retained the root and shoot lengths. Plant seeds that were primed with various concentrations (0, 50, 100 and 150 µM) of SA improved the root and shoot lengths. Significant increases in the root and shoot lengths were observed at 100 µM SA (Figure 2, Table 1). A 23.8% increase in root length was observed with the application of 100 µM of SA in 30 mg kg−1 of B-treated plants, while a 26.7% decrease was observed in the shoot length of 30 mg kg−1 B-treated plants as compared with the control. The SA application at 100 µM was found to be the best treatment and caused increases in the shoot length in 30 mg kg−1 B-treated plants up to 31.8%.

Table 1.
Effects of SA (0, 50, 100 and 150 µM) on the plant root and shoot length of maize cultivar Gohar-19 under different B toxicity levels (0, 15 and 30 mg kg−1).
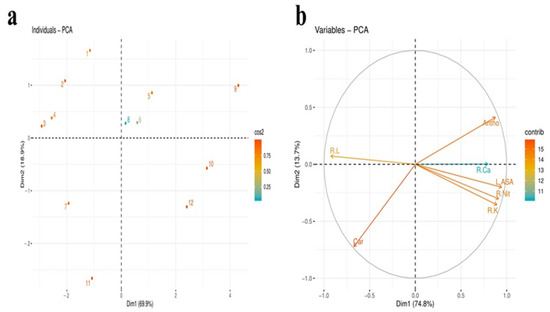
Figure 1.
Score (a) and loading plot (b) of principal component analysis (PCA) on different attributes of maize cultivar Gohar-19 plants supplemented with and without SA while grown under B stress. Score plot represents the separation of treatments as T1: 0 mg B without SA; T2: 0 mg B with 50 µM SA; T3: 0 mg B with 100 µM SA; T4: 0 mg B with 150 µM SA; T5: 15 mg/kg B without SA; T6: 15 mg/kg B with 50 µM SA; T7: 15 mg/kg B with 100 µM SA; T8: 15 mg/kg B with 150 µM SA; T9: 30 mg/kg B without SA; T10: 30 mg/kg B with 50 µM SA; T11: 30 mg/kg B with 100 µM SA; T12: 30 mg/kg B with 150 µM SA. Attributes evaluated include R L = root length; Car = carotenoids; R Nit = root nitrate; R K = root potassium; R Ca = root calcium, Antho = anthocyanin; L ASA = leaf ascorbic acid.
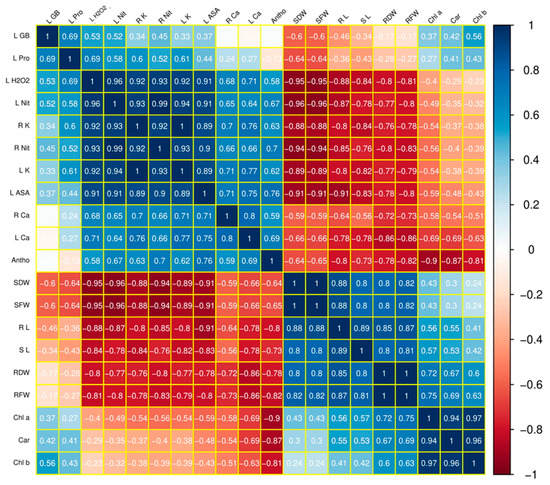
Figure 2.
Correlations (r values) among the different studied parameters of maize cultivar Gohar-19 grown under different B stress levels and fertigated with and without SA. R L = root length; S L = shoot length; RFW=root fresh weight; RDW = root dry weight; SFW = shoot fresh weight; SDW = shoot dry weight; Chl a = chlorophyll a; Chl b = chlorophyll b; Car = carotenoids; Antho = anthocyanin; L ASA = leaf ascorbic acid; L H2O2 = leaf hydrogen peroxide; L Pro = leaf proline; L GB = leaf glycine betaine; R K = root potassium; L K = leaf potassium; R Ca = root calcium; L Ca = leaf calcium; R Nit = root nitrate; L Nit = leaf nitrate.
2.2. Plant Biomass
Plant fresh and dry biomass were also reduced in response to increasing the B treatment levels. The results obtained exhibited a positive correlation with the root and shoot lengths. Reducing and increasing patterns in plant fresh and dry biomass were observed in the root and shoot lengths. We observed 30% and 32.89% decreases in the root and shoot fresh biomass, respectively. The maximum increase in plant biomass was noted in plants raised from seeds primed with 100 µM of SA, as shown in Table 2. This gain in the plant growth biomarkers was due to the enhanced photosynthetic activity and improved antioxidant status of the plant body (Figure 2).

Table 2.
Effect of SA (0, 50, 100 and 150 µM) on the plant fresh and dry weight of maize cultivar Gohar-19 under varying B toxicity levels (0, 15 and 30 mg kg−1).
2.3. Photosynthetic Pigments
Elevated B levels significantly reduced the photosynthetic pigment contents of maize seedlings. It was observed that the chl a contents were reduced with increasing B treatment levels. The 30 mg kg−1 B imposed deteriorative effects and reduced the chl a contents effectively (Figure 3a). An improvement in the chl a concentration was recorded through priming seeds with SA. Seeds primed with 100 µM of SA expressed the maximum chl a content, which suggests reduced toxicity effects.
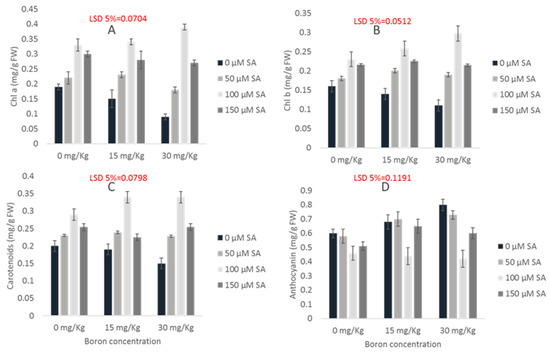
Figure 3.
Effect of SA on chlorophyll a (A), chlorophyll b (B), carotenoids (C) and anthocyanin (D) contents of maize cultivar Gohar-19 under varying B toxicity levels.
The chl b contents were also reduced under B toxicity as compared to the control. An increase in the chl b contents was observed with respect to the control in the plants emerging from primed seeds. An increase of 30.4% in the chl b contents was observed at 100 µM SA treatment, while a non-significant increase was observed at 150 µM SA treatment as compared to the control (Figure 3b). The carotenoid contents were reduced effectively under 30 mg kg−1 B. The B application at 30 mg Kg−1 caused reductions of 52.6%, 31.3% and 45% in the chla, chlb and carotenoids, respectively. The SA priming improved the carotenoid contents by reducing the drastic effects of B toxicity. A non-significant change in the carotenoid contents was observed in 50 and 150 µM SA primed seeds, while 100 µM SA significantly enhanced the carotenoid contents as compared to the control (Figure 2 and Figure 3c).
2.4. Anthocyanin
The anthocyanin contents increased with increasing the levels of B toxicity. Significant increases in the anthocyanin contents were observed in plants treated with 15 and 30 mg kg−1 B. There was a 33.33% increase in anthocyanin contents when 30 mg kg−1 soil B was applied, as compared to the control. The SA priming reduced the anthocyanin contents overall, but only 100 µM of SA caused a 47.5% reduction in the anthocyanin contents (Figure 2 andFigure 3c).
2.5. Ascorbic Acid
The toxic effects of B increased the ASA contents of maize seedlings. The B treatment of 30 mg kg−1 significantly increased the ASA content up to 44% as compared with the control (Figure 4). Priming with SA reduced the B toxic effects. Only 100 and 150 µM of SA effectively mitigated the toxic effects on plants grown in pots containing 15 mg kg−1 B. However, under a high boron toxicity, only 100 µM of SA significantly reduced the ASA content up to 36% as compared to the control, as shown in Table 3, Figure 2.
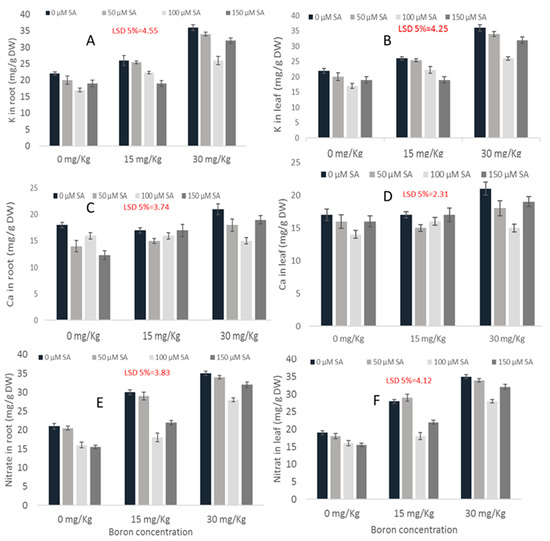
Figure 4.
Effect of SA on the K (A,B), Ca (C,D) and nitrate (E,F) contents in the roots and leaves of maize cultivar Gohar-19 under varying B toxicity levels.

Table 3.
Effect of SA (0, 50, 100 and 150 µM) on the leaf ascorbic acid, H2O2, proline and glycine betaine contents of maize cultivar Gohar-19 under varying B toxicity levels (0, 15 and 30 mg kg−1).
2.6. H2O2 Concentration
2.7. Proline Content
The toxic effects of B significantly increased the proline contents. The B treatment of 30 mg kg−1 increased the proline contents up to 43.75% as compared to the control. Priming with SA remained productive in reducing the B toxic effects. Only 100 and 150 µM SA effectively mitigated the toxic effects in plants grown in pots containing 15 mg kg−1 B. However, under a high boron toxicity, only 150 µM SA significantly reduced the toxic effects by increasing the proline content in comparison with the control, as shown in Table 3, Figure 2.
2.8. Glycine Betaine
An outstanding improvement was noted in the leaf GB contents of plants grown under B stress. Exogenous applications of SA further increased the GB content in the leaves of plants experiencing B toxicity stress. All the treatments of SA affected the GB level, however 100 µM SA increased the GB contents up to 100% under 30 mg kg−1 of B treatment as compared to the control, as indicated in Table 3, Figure 2.
2.9. Potassium Content
An increase in the K contents was observed in response to the B toxicity. Applications of SA reduced the K contents and a maximum reduction of up to 27.8% was noted in the plants primed with 100 µM of SA. The K uptake and accumulation exhibited quite similar patterns in the plant root and shoot (Figure 2 and Figure 4a,b).
2.10. Calcium Content
Boron toxicity significantly influenced the Ca accumulation in the root of the maize cultivar Gohar-19. The Ca content was reduced at a lower SA treatment level as compared to the control. With increasing the SA concentration up to 100 and 150 µM, higher increments in the Ca contents relative to the control were recorded. A total of 100 µM SA treatment was found to be effective in reducing toxic effects by lowering the Ca content up to 28.7% in the 30 mg kg−1 B group. The Ca accumulation in the plant leaves was reduced due to the B toxicity even at high concentrations of SA applications. No significant effects of SA on Ca contents were observed (Figure 2 and Figure 4a,b).
2.11. Nitrate Concentration
An increase in the nitrate contents was observed with an increasing B level. Various SA concentrations (0, 50, 100 and 150 µM) reduced the nitrate content. The application of 100 µM of SA reduced the nitrate content up to 20% in the most destructive B treatment of 30 mg kg−1 (Figure 2 and Figure 4a,b).
The relationship between B toxicity and the morpho-physiological attributes of maize under SA application is illustrated in Figure 2.
A Pearson correlation analysis was conducted to quantify the interactive effects of B toxicity and SA application on plant growth and biomass, chlorophyll contents, lipid peroxidation and the antioxidant and nutrient uptake of maize (Figure 2). B toxicity was negatively correlated with plant growth and biomass, photosynthetic pigments, oxidative stress and antioxidative response. Chlorophyll contents were positively correlated with plant biomass accumulation. Positive correlations were also identified among growth attributes and K, Ca and nitrate contents.
2.12. Principal Component Analysis
The combinatorial effect of B toxicity and SA application was evaluated on important attributes of maize plants by the synthesis of the score and loading plots of PCA, as presented in Figure 4. All the three applied B treatments with and without SA were successfully dispersed by the first two principal components (Figure 1a). The maximum variance among all the components was based on extracted components—i.e., PC1 (Dim1) and PC2 (Dim2), where component Dim1 contributed 69.9% while the contribution of Dim2 was 18.9% (Figure 1b).
3. Discussion
The only non-metallic element of group 13 of the periodic Table is B, which exhibits a trivalent oxidation state. Naturally, B is present in the form of borate, boric acid and borosilicate mineral. In the Earth crust, the B level varies between 1–500 mg kg−1 and 2–100 mg kg-1 as per the geographical region and soil composition status [32].
B has a considerable importance due to its supportive role in plant development and growth. It helps in the processes of cell division, the formation of cell wall and the elongation of cells [33]. However, B causes toxic effects at very high or very low levels. B toxicity mostly co-exists together with some other abiotic stresses—e.g., salt and drought stress [34]. A high B toxicity reduces the plant growth and other attributes.
EI-Shazoly [25] conducted a study to describe the SA effects on B toxicity stress in wheat. The results of such a study were in agreement with those of the present study. The SA application also enhanced the root and shoot length, supporting the findings of the previous works [35]. It has been reported that a high level of B causes abnormal cell division in the root meristematic zone [35], hypodermis formation and suberin deposition [36], thus limiting plant growth and development. The excess of B also causes cytotoxic effects during mitosis, which in turn reduces the root and shoot biomass [37,38]. In the present study, 100 µM of SA significantly enhanced the plant biomass by mitigating the B toxicity. Sarafi et al. [39] reported that the B toxicity reduces the plant dry weight up to 48%, the number of leaves and the root dry weight in the pepper plant (Capsicum annuum). In this study, the applications of melatonin (MEL) and resveratrol (RES) were studied, where a treatment of 100 µM of RES and 1 µM of MEL effectively reversed the reductions in fresh and dry weights under B toxic effects, respectively. Eser and Aydemir [22] reported that kinetin application prevented the B-induced reductions in the plant fresh and dry weight of wheat plants under B stress. Moreover, the high B content (50 mg kg−1) in soil reduced the shoot fresh and dry weight of tomato plants [40]. It has been particularized that B toxicity causes the down-regulation of the photosystem biochemical components and the inhibition of the electron transport rate [41], thus lowering the activity of carbon fixation enzymes [41,42]. High levels of B can also cause the root growth inhibition, accompanied by a decrease in plant dry weight [43]. The reduction in root growth may be due to the intense lignification of cell wall [44]. However, it has been reported that lignification is not mainly responsible for root growth inhibition, but is rather a defensive attribute for reducing B uptake [36].
The high B level (30 mg kg−1) reduced the photosynthetic pigments biosynthesis. However, SA application reversed these negative effects, and the most effective treatment was 100 µM of SA. The findings of present study are in line with those of EI-Shazoly [25]. Plant growth and development are considerably dependent on photosynthetic pigments. It has been reported that the inhibition of plant growth by B stress is associated with reduced photosynthetic pigments. Indeed, the present study indicated that the biosynthesis of chlorophyll and carotenoid was negatively affected by B toxicity stress. Our results depicted a negative relationship between the biosynthesis of photosynthetic pigments and the increasing applied B stress regimes. This decline in photosynthetic pigments might be owing to H2O2 accumulation, which damages the photosynthetic reaction centers. Papadakis et al. [45] reported that one of the possible reasons for the reduction in photosynthetic activity in plants grown under excess of B was the structural damage of thylakoids. In general, SA, being a versatile molecule, interacts with other hormones to promote the induction of enzymes and antioxidants to alleviate the toxic effects of stress [46].
Regarding the mineral contents, Kaya and Ashraf [46] described that B toxicity significantly reduces the N, K and Ca contents in tomato. However, nitric oxide application induced the level of minerals and minimized the B toxicity effects. EI-Shazoly [25] described that a low level of boron (3 mg kg−1 soil) does not affect the K content in wheat plants, however a high level could decrease it. Moreover, the Ca level was reduced due to the B toxicity (3 mg kg−1 soil), but increased upon SA and thiamin application.
High levels of B increased the anthocyanin contents in sweet basil (Ocimum basilicum L.) plants, indicating possible stress responses or poor nutrient mobilization from the plant root [47]. We also found that B stress elevated the anthocyanin levels in the root and shoot of maize cultivar Gohar-19. The application of SA at higher levels reduced the stress level. Additionally, the reduction in anthocyanin content in plants treated with SA predicts a reduction in stress severity.
Ascorbic acid is an important antioxidant and scavenger of ROS [48,49,50]. The ASA content was significantly affected by B stress in the present study. Abiotic stresses result in a higher accumulation of ASA than that of other stress. Increased ROS scavenging enzymatic and nonenzymatic activity by excessive B concentrations has already been reported in barley, chickpea, tomato and grapes [51,52,53,54].
In general, plants up-regulate the synthesis of different osmolytes in cytosol and other organelles to cope with the deleterious effects of environmental stresses. Proline and GB are considered to be key osmolytes for the osmotic adjustment. Proline, being a secondary metabolite, plays a key role in stress tolerance as an antioxidant and osmoprotectant [55]. Stress-related genes are activated by GB to detoxify ROS and protect photosynthetic machinery under stressed conditions [56]. In the current study, SA applications triggered the accumulation of proline and GB to cope with the B toxicity effects through scavenging ROS and the activation of the antioxidant defense systems.
The PCA results depicted that the application of salicylic acid had a significant ameliorative effect for B toxicity on the studied parameters of maize plants. The same effects of SA have been reported in salt-stressed sunflower plants [57]. Overall, the applied B stress exerted hazardous effects on the growth and ecophysiological attributes of maize. These results were in accordance with the findings of previous reports which have reported decreases in the growth traits of various plant species grown under environmental stress conditions [58,59,60,61,62,63,64,65,66]. Based on the findings of the current study, we conclude that SA applications improved the growth of B-stressed maize plants at the seedling stage through increasing the biosynthesis of photosynthetic pigments, osmolytes and antioxidants. The high level of B deteriorates photosystem II centers, as the low levels of chlorophyll and carotenoids are linked with biomass reduction caused by B toxicity. High levels of osmoprotectants such as proline may act as signaling molecules for scavenging ROS, thus stabilizing the membrane structures as well as cascading the stress-tolerant gene expression. Further studies at the molecular level may elaborate the comprehensive understanding lying behind these modulations of SA against B toxicity in maize. The induction of B toxicity tolerance in maize plants after SA application is also associated with antioxidant defense system improvement.
4. Materials and Methods
4.1. Plant Material and Experimental Design
Seeds of maize (cultivar Gohar-19) were obtained from the Maize & Millets Research Institute, Yusafwala, Sahiwal, Pakistan, and the experiments were conducted at the Department of Botany, Government College University Faisalabad, Pakistan. The seeds were surface-sterilized using a 1% sodium hypochloride solution for 5 min. Surface-sterilized seeds were thoroughly washed with distilled water and air-dried for 12 h. These seeds were soaked in 0, 50, 100 and 150 µM of SA solution for 12 h. Plastic pots were filled with 1 kg of washed and air-dried sand at the botanical garden, Government College University Faisalabad, and a 100% field capacity was maintained in pots by adding boron-free water. The experiment was carried out in a completely randomized design (CRD) with three replicates. Ten seeds were sown in each pot. After 5 days of germination, 5 seedlings were selected based on their similarity in size and vigor. B stress was applied using Nable’s solution containing boric acid (H3BO3) by maintaining pH at 5.7. The final B concentrations of 0, 15 and 30 mg Kg−1 soil were maintained in each pot for one week. After one week of B stress application, the plants were harvested and stored in a freezer for further analysis.
4.2. Morphological Parameters and Plant Biomass
The root and shoot lengths were measured for individual plants using a meter scale. The root and shoot fresh and dry weight, after drying at 70 °C for 72 h, was calculated using the same weight balance.
4.3. Physiological and Biochemical Analysis
Different physio-biochemical analyses were carried out as described below.
4.3.1. Photosynthetic Pigments
Contents of chlorophyll a, b and carotenoids were determined using a 0.5 g fresh leaf sample. The Arnon [67] method with minor modifications was used for the determination of photosynthetic pigments. The collected sample was ground in 15 mL of 85% acetone and centrifuged at 10,000× g for 15 min. The absorbance of the supernatant was measured at 480, 645 and 663 nm using a spectrophotometer (Hitachi U-2001, Tokyo, Japan).
4.3.2. Anthocyanin Content
The anthocyanin content was measured as reported previously [68]. Fresh root and shoot samples (0.1 g each) were ground separately in 2 mL of 1% acidified methanol (1 mL HCL and 99 mL methanol), then the extract was heated up to 50 °C for one in a water bath. The anthocyanin content was then quantified using a spectrophotometer (Hitachi U-2001, Tokyo, Japan) at 535 nm.
4.3.3. Ascorbic Acid Content
The protocol of Mukherjee and Choudhuri [68] was followed to determine the ascorbic acid content. Root and shoot fresh samples (0.1 g) were taken and ground in 5 mL of trichloroacetic acid (TCA) using a pestle and mortar. The extract was filtered, and 4 mL of the homogenate sample was allowed to react with 2 mL of 2% dinitrophenyl hydrazine in an acidified medium. One drop of 10% thiourea (prepared in 70% ethanol) was added and the mixture was then allowed to boil at 100 °C for 15 min. The absorbance at 530 nm was recorded through UV-spectrophotometer (Hitachi U-2001, Tokyo, Japan) for calculating the ascorbic acid content.
4.3.4. H2O2 Content Determination
Shoot samples were extracted in a cold acetone for H2O2 content determination. One milliliter of extract was mixed with 1 mL of 0.1% titanium dioxide in 20% sulfuric acid and centrifuged at 8000 rpm for 15 min. The supernatant was then used to measure the absorbance at 415 nm. H2O2 content was calculated using a standard curve plotted in a range of 0.5–5 mM H2O2 and was expressed as mg g−1 FW.
4.3.5. Potassium Content
Dry samples of plant root and shoot (1 mg) were dissolved in 9 mL of distilled water. Flame photometer was used for the determination of potassium content, as reported previously [69].
4.3.6. Calcium Content
Dry samples of plant root and shoot (1 mg) were added to 9 mL of distilled water. Flame photometer method was used for the determination of the calcium content [69].
4.3.7. Nitrate Content
Dry root and shoot samples were dissolved in 1 mL of TCA (1.24 g TCA+ 500 mL H2SO4) and 1 mL of distilled water. For determining the nitrate oxide, the absorbance was recorded at 530 nm using a UV vis spectrophotometer (Hitachi U-2001, Tokyo, Japan).
5. Statistical Analysis
Collected data were subjected to an analysis of variance (ANOVA) using the statistical software Co-Stat version 6.2, Cohorts Software, 2003 (Monterey, CA, USA). The treatment means were equated by the least significant difference method (Fisher’s LSD) at p value of ≤0.05 level. Before applying ANOVA, the data were standardized using means of inverse or logarithmic transformations wherever necessary. The correlations and PCA of the mean values of all variables were found using XL-STAT 2010.
Author Contributions
Conceptualization, M.N., N.K., N.I., S.A., M.R., A.A.A. and M.N.A.; Data curation, M.N. and S.I.; Formal analysis, M.N. and S.I.; Funding acquisition, A.A.A. and M.N.A.; Investigation, S.I. and N.K.; Methodology, M.N., S.I. and N.I.; Project administration, M.N., N.I. and S.A.; Resources, M.N., H.I., S.A. and A.A.A.; Software, H.I., N.I., S.A., M.R. and M.N.A.; Supervision, M.N. and S.A.; Validation, H.I.; Visualization, H.I.; Writing–original draft, M.N., N.K., M.R. and A.A.A.; Writing–review & editing, N.K., S.A., M.R. and M.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Higher Education Commission (HEC) of Pakistan. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/236), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
This study was financially supported by the Higher Education Commission (HEC) of Pakistan. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/236), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaradat, A.; Simulated, A. Climate change deferentially impacts phenotypic plasticity and stoichiometric homeostasis in major food crops. Emir. J. Food Agr. 2018, 30, 429–442. [Google Scholar]
- Khan, N.; Ali, S.; Shahid, M.A.; Kharabian-Masouleh, A. Advances in detection of stress tolerance in plants through metabolomics approaches. Plant Omics 2017, 10, 153. [Google Scholar] [CrossRef]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Bakhsh, A. Engineering crop plants against abiotic stress: Current achievements and prospects. Emir. J. Food Agr. 2015, 27, 24–39. [Google Scholar] [CrossRef]
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221, 82–90. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef]
- Hussain, S.; Zhang, J.H.; Zhong, C.; Zhu, L.F.; Cao, X.C.; YU, S.M.; Jin, Q.Y. Effects of salt stress on rice growth, development characteristics and the regulating ways. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Liu, P.; Yang, Y.S.; Xu, G.D.; Fang, Y.H.; Yang, Y.A.; Kalin, R.M. The effect of molybdenum and boron in soil on the growth and photosynthesis of three soybean varieties. Plant Soil Environ. 2005, 51, 197–205. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Babar, M.A. The stimulatory effects of plant growth promoting rhizobacteria and plant growth regulators on wheat physiology grown in sandy soil. Arch. Microbiol. 2019, 201, 769–785. [Google Scholar] [CrossRef]
- Ayvaz, M.; Avcı, M.K.; Yamaner, C.; Koyuncu, M.; Güven, A.; Fagerstedt, K. Does excess boron affect the malondialdehyde levels of potato cultivars? Eurasia J. Biosci. 2013, 7, 47–53. [Google Scholar] [CrossRef]
- Stiles, A.R.; Liu, C.; Kayama, Y.; Wong, J.; Doner, H.; Funston, R.; Terry, N. Evaluation of the boron tolerant grass, Puccinellia distans, as an initial vegetative cover for the phytorestoration of a boron-contaminated mining site in southern California. Environ. Sci. Technol. 2011, 45, 8922–8927. [Google Scholar] [CrossRef] [PubMed]
- Princi, M.P.; Lupini, A.; Araniti, F.; Longo, C.; Mauceri, A.; Sunseri, F.; Abenavoli, M.R. Boron toxicity and tolerance in plants: Recent advances and future perspectives. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–147. [Google Scholar]
- Kayıhan, C.; Öz, M.T.; Eyidogan, F.; Yucel, M.; Oktem, H.A. Physiological, biochemical and transcriptomic responses to boron toxicity in leaf and root tissues of contrasting wheat cultivars. Plant Mol. Biol. Rep. 2017, 35, 97–109. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Ben-Gal, A.; Keren, R.; Reid, R.J. Combined effect of salinity and excess boron on plant growth and yield. Plant Soil 2008, 304, 73–87. [Google Scholar] [CrossRef]
- Diaz, F.J.; Grattan, S.R. Performance of tall wheatgrass (Thinopyrum ponticum cv. Jose) irrigated with saline-high boron drainage water: Implications on ruminant mineral nutrition. Agric. Ecosyst. Environ. 2009, 131, 128–136. [Google Scholar] [CrossRef]
- Zhang, B.; Chu, G.; Wei, C.; Ye, J.; Li, Z.; Liang, Y. The growth and antioxidant defense responses of wheat seedlings to omethoate stress. Pestic. Biochem. Phys. 2011, 100, 273–279. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan Shahid, M.; Yang, J. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef]
- Tavallali, V. Interactive effects of zinc and boron on growth, photosynthesis and water relations in pistachio. J. Plant Nutr. 2017, 40, 1588–1603. [Google Scholar] [CrossRef]
- Nasim, M.; Rengel, Z.; Aziz, T.; Regmi, B.D.; Saqib, M. Boron toxicity alleviation by zinc application in two barley cultivars differing in tolerance to boron toxicity. Pak. J. Agric. Sci. 2015, 52, 151–158. [Google Scholar]
- Samet, H.; Cikili, Y.; Dursun, S. The role of potassium in alleviating boron toxicity and combined effects on nutrient contents in pepper (Capsicum annuum L.). Bulg. J. Agric. Sci. 2015, 21, 64–70. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Ali, H.M.; Basalah, M.O.; Faisal, M.; Al-Amri, A.A. Calcium-induced amelioration of boron toxicity in radish. J. Plant Growth Regul. 2013, 32, 61–71. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mohamed, H.I.; Mahmoud, A.; Elkelish, A.; Misra, A.N.; Guy, K.M.; Kamran, M.; Ai, S.; Zhang, M. Salicylic Acid Stimulates Antioxidant Defense and Osmolyte Metabolism to Alleviate Oxidative Stress in Watermelons under Excess Boron. Plants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudia, K.; Arvinb, M.J. Salicylic acid and osmotic stress effects on seed germination and seedling growth of wheat (Triticum aestivum L.) cultivars. Plant Ecophysiol. 2010, 2, 7–11. [Google Scholar]
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.H. Salicylic acid or thiamin increases tolerance to boron toxicity stress in wheat. J. Plant Nutr. 2019, 42, 702–722. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Zhang, Q.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L. Maize Tolerance against Drought and Chilling Stresses Varied with Root Morphology and Antioxidative Defense System. Plants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, H.G.; Adamu, H.M. The potential of maize as phytoremediation tool of heavy metals. Eur. Sci. J. 2014, 6, 32–33. [Google Scholar]
- Wang, B.; Liu, C.; Zhang, D.; He, C.; Zhang, J.; Li, Z. Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol. 2019, 19, 335. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremediation 2018, 16, 405–414. [Google Scholar] [CrossRef]
- Brown, P.H.; Bellaloui, N.; Wimmer, M.A.; Bassil, E.S.; Ruiz, J.; Hu, H.; Pfeffer, H.; Dannel, F.; Römheld, V. Boron in plant biology. Plant Biol. 2002, 4, 205–223. [Google Scholar] [CrossRef]
- Parks, J.L.; Edwards, M. Boron in the environment. Crit. Rev. Environ. Sci. Technol. 2005, 35, 81–114. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Zafar-ul-Hye, M.; Munir, K.; Ahmad, M.; Imran, M. Influence of boron fertilization on growth and yield of wheat crop under salt stress environment. Soil Environ. 2016, 35, 181–186. [Google Scholar]
- Liu, D.; Jiang, W.; Zhang, L.; Li, L. Effects of boron ions on root growth and cell division of broad bean (Vicia Faba L.). Isr. J. Plant Sci. 2000, 48, 47–58. [Google Scholar] [CrossRef]
- Ghanati, F.; Morita, A.; Yokota, H. Deposition of suberin in roots of soybean induced by excess boron. Plant Sci. 2005, 168, 397–405. [Google Scholar] [CrossRef]
- Konuk, M.; Liman, R.; Cigerci, I.H. Determination of genotoxic effect of boron on Allium cepa root meristematic cells. Pak. J. Bot. 2007, 39, 73–79. [Google Scholar]
- Sarafi, E.; Tsouvaltzis, P.; Chatzissavvidis, C.; Siomos, A.; Therios, I. Melatonin and resveratrol reverse the toxic effect of high boron (B) and modulate biochemical parameters in pepper plants (Capsicum annuum L.). Plant Physiol. Biochem. 2017, 112, 173–182. [Google Scholar] [CrossRef]
- Eser, A.; Aydemir, T. The effect of kinetin on wheat seedlings exposed to boron. Plant Physiol. Biochem. 2016, 108, 158–164. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Chen, M.; Mishra, S.; Heckathorn, S.A.; Frantz, J.M.; Krause, C. Proteomic analysis of Arabidopsis thaliana leaves in response to acute boron deficiency and toxicity reveals effects on photosynthesis, carbohydrate metabolism and protein synthesis. J. Plant Physiol. 2013, 171, 235–242. [Google Scholar] [CrossRef]
- Turan, M.; Taban, N.; Taban, S. Effect of calcium on the alleviation of boron toxicity and localization of boron and calcium in cell wall of wheat. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 99–103. [Google Scholar]
- Ghanati, F.; Morita, A.; Yokota, H. Induction of suberin and increase of lignin content by excess boron in tobacco cells. J. Plant Nutr. Soil Sci. 2002, 48, 357–364. [Google Scholar] [CrossRef]
- Sharma, A.; Sidhu, G.P.S.; Araniti, F.; Bali, A.S.; Shahzad, B.; Tripathi, D.K.; Brestic, M.; Skalicky, M.; Landi, M. The Role of Salicylic Acid in Plants Exposed to Heavy Metals. Molecules 2020, 25, 540. [Google Scholar] [CrossRef] [PubMed]
- Papadakis, I.; Dimassi, K.; Bosabalidis, A.; Therios, I.; Patakas, A.; Giannakoula, A. Boron toxicity in ‘Clementine’ mandarin plants grafted on two root stocks. Plant Sci. 2004, 166, 539–547. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci. Hortic. 2015, 185, 43–47. [Google Scholar] [CrossRef]
- Pardossi, A.; Romani, M.; Carmassi, G.; Guidi, L.; Landi, M.; Incrocci, L.; Maggini, R.; Puccinelli, M.; Vacca, W.; Ziliani, M. Boron accumulation and tolerance in sweet basil (Ocimum basilicum L.) with green or purple leaves. Plant Soil 2015, 395, 375–389. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Qian, H.F.; Peng, X.F.; Han, X.; Ren, J.; Zhan, K.Y.; Zhu, M. The stress factor, exogenous ascorbic acid, affects plant growth and the antioxidant system in Arabidopsis thaliana. Russ. J. Plant Physiol. 2014, 61, 467–475. [Google Scholar] [CrossRef]
- Karabal, E.; Yucel, M.; Huseyin, A.O. Antioxidant responses of tolerant and sensitive barley cultivars to boron toxicity. Plant Sci. 2003, 164, 925–933. [Google Scholar] [CrossRef]
- Gunes, A.; Soylemezoglu, G.; Inal, A.; Bagci, E.G.; Coban, S.; Sahin, O. Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Sci. Hortic. 2006, 110, 279–284. [Google Scholar] [CrossRef]
- Cervilla, L.M.; Blasco, B.; Rıos, R.; Romero, L.; Ruiz, J. Oxidative stress and antioxidants in tomato (Solanum lycopericum) plants subjected to boron toxicity. Ann. Bot. 2007, 100, 747–756. [Google Scholar] [CrossRef]
- Ardic, M.; Sekmen, A.H.; Tokur, S.; Ozdemir, F.; Turkan, I. Antioxidant responses of chickpea plants subjected to boron toxicity. Plant Biol. 2009, 11, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Curá, J.A. Role of Beneficial Microorganisms and Salicylic Acid in Improving Rainfed Agriculture and Future Food Safety. Microorganisms 2020, 8, 1018. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Maboud, H.E.; Niknam, V. Effects of salicylic acid on hormonal cross talk, fatty acids profile, and ions homeostasis from salt-stressed safflower. J. Plant Interact. 2019, 14, 340–346. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hoque, M.A.; Burritt, D.J.; Fujita, M. Proline Protects Plants against Abiotic Oxidative Stress: Biochemical and Molecular Mechanisms. In Oxidative Damage to Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 477–522. [Google Scholar]
- El-Esawi, M.A.; Elkelish, A.; Soliman, M.; Elansary, H.O.; Zaid, A.; Wani, S.H. Serratia marcescens BM1 Enhances Cadmium Stress Tolerance and Phytoremediation Potential of Soybean Through Modulation of Osmolytes, Leaf Gas Exchange, Antioxidant Machinery, and Stress-Responsive Genes Expression. Antioxidants 2020, 9, 43. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alzahrani, S.M.; Ali, H.M.; Alayafi, A.A.; Ahmad, M. Serratia liquefaciens KM4 Improves Salt Stress Tolerance in Maize by Regulating Redox Potential, Ion Homeostasis, Leaf Gas Exchange and Stress-Related Gene Expression. Int. J. Mol. Sci. 2018, 19, 3310. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; Brtnicky, M.; Kintl, A.; Hussain, G.S.; El-Esawi, M.A. Effect of Cadmium-Tolerant Rhizobacteria on Growth Attributes and Chlorophyll Contents of Bitter Gourd under Cadmium Toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef]
- Naveed, M.; Bukhari, S.S.; Mustafa, A.; Ditta, A.; Alamri, S.; El-Esawi, M.A.; Rafique, M.; Ashraf, S.; Siddiqui, M.H. Mitigation of Nickel Toxicity and Growth Promotion in Sesame through the Application of a Bacterial Endophyte and Zeolite in Nickel Contaminated Soil. Int. J. Environ. Res. Public Health 2020, 17, 8859. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; El-Esawi, M.A.; Rana, M.S.; Saleem, M.H.; Riaz, M.; Ashraf, U.; Potcho, M.P.; Duan, M.; Rajput, I.A.; et al. Molybdenum Supply Alleviates the Cadmium Toxicity in Fragrant Rice by Modulating Oxidative Stress and Antioxidant Gene Expression. Biomolecules 2020, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Shahid, S.; Ali, S.; El-Esawi, M.A.; Hussain, A.I.; Perveen, R.; Iqbal, N.; Rizwan, M.; Nasser Alyemeni, M.; El-Serehy, H.A.; et al. Fertigation of Ajwain (Trachyspermum ammi L.) with Fe-Glutamate Confers Better Plant Performance and Drought Tolerance in Comparison with FeSO4. Sustainability 2020, 12, 7119. [Google Scholar] [CrossRef]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Stark, D.; Wray, V. Anthocyanins. In Methods in Plant Biology; Volume 1: Plant Phenolics; Harborne, J.B., Ed.; Academic Press: London, UK; Harcourt Brace Jovanovich: London, UK, 1989; pp. 325–356. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).