Application of Multi-Component Conditioner with Clinoptilolite and Ascophyllum nodosum Extract for Improving Soil Properties and Zea mays L. Growth and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Analysis of Soil Properties
2.3. Field and Laboratory Analysis of Maize
2.4. Statistical Analysis
3. Results
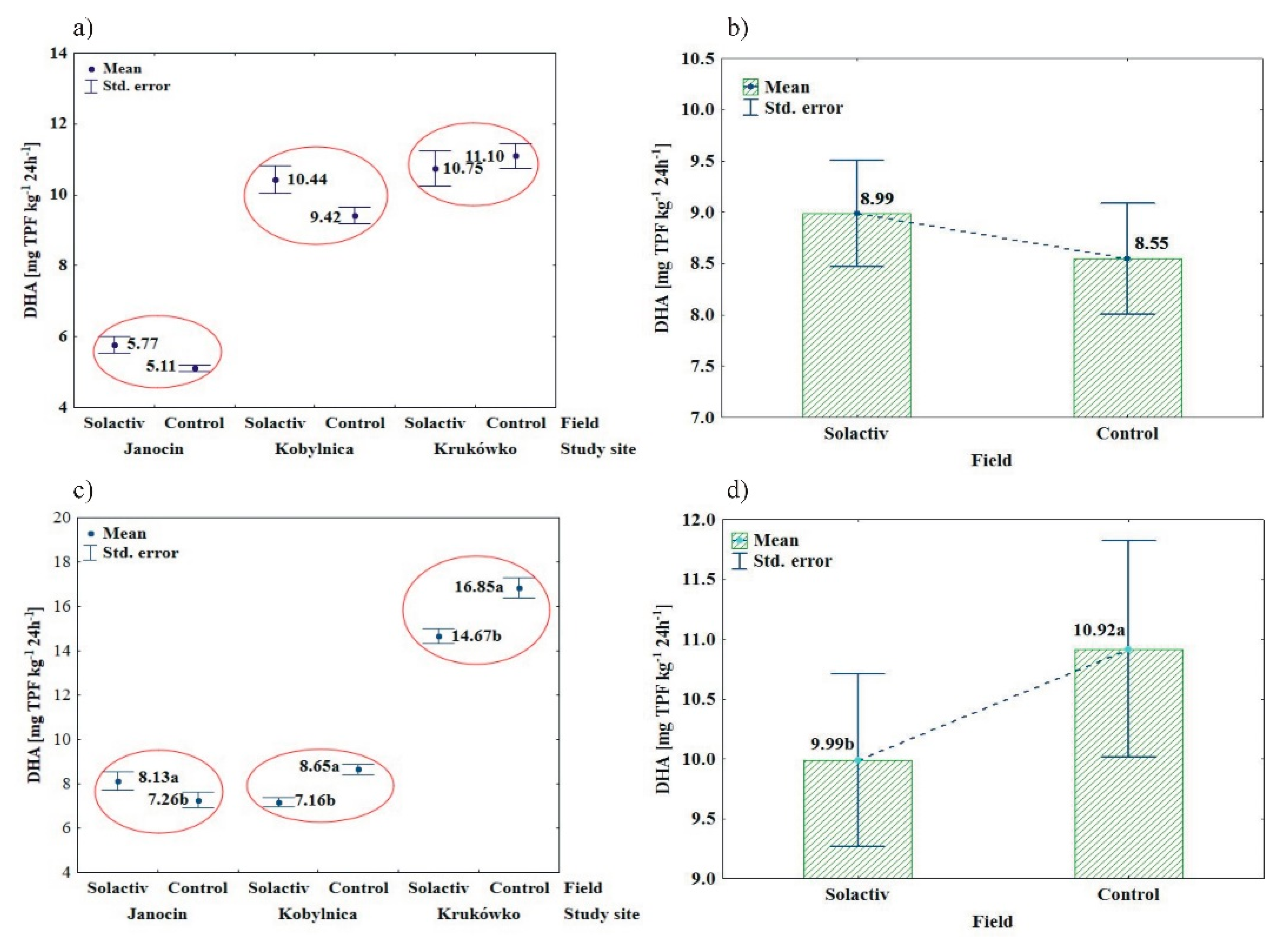
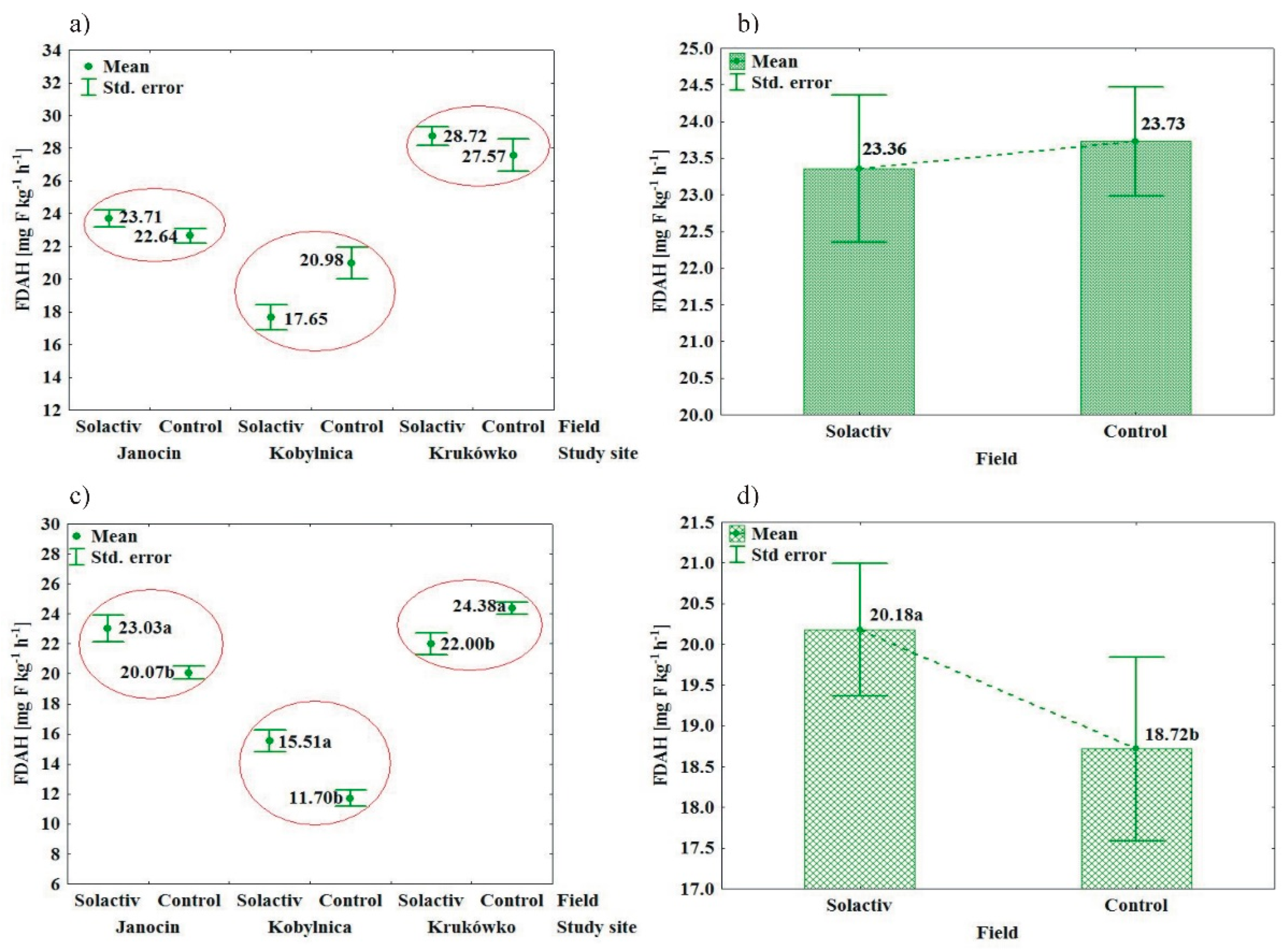
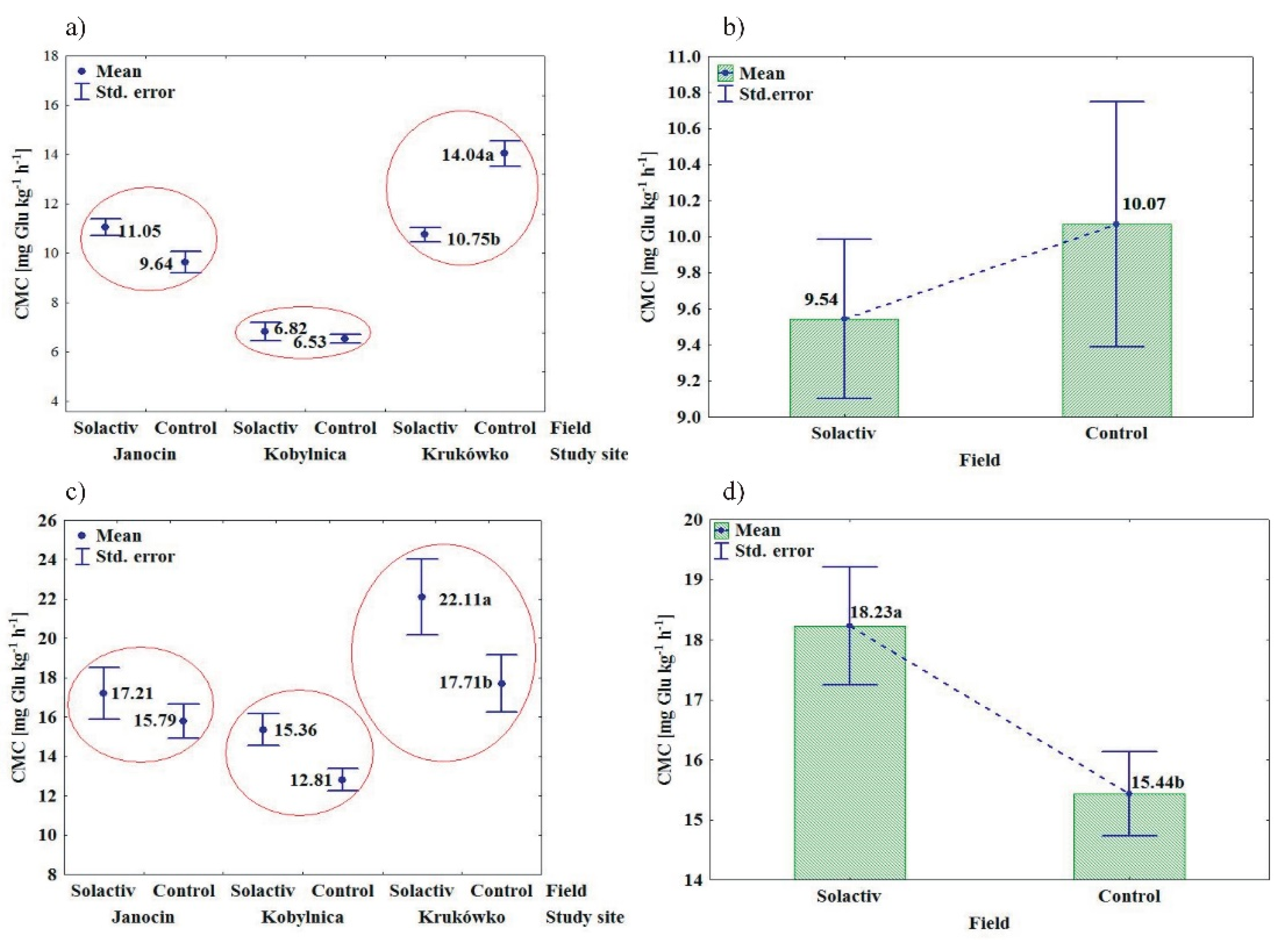
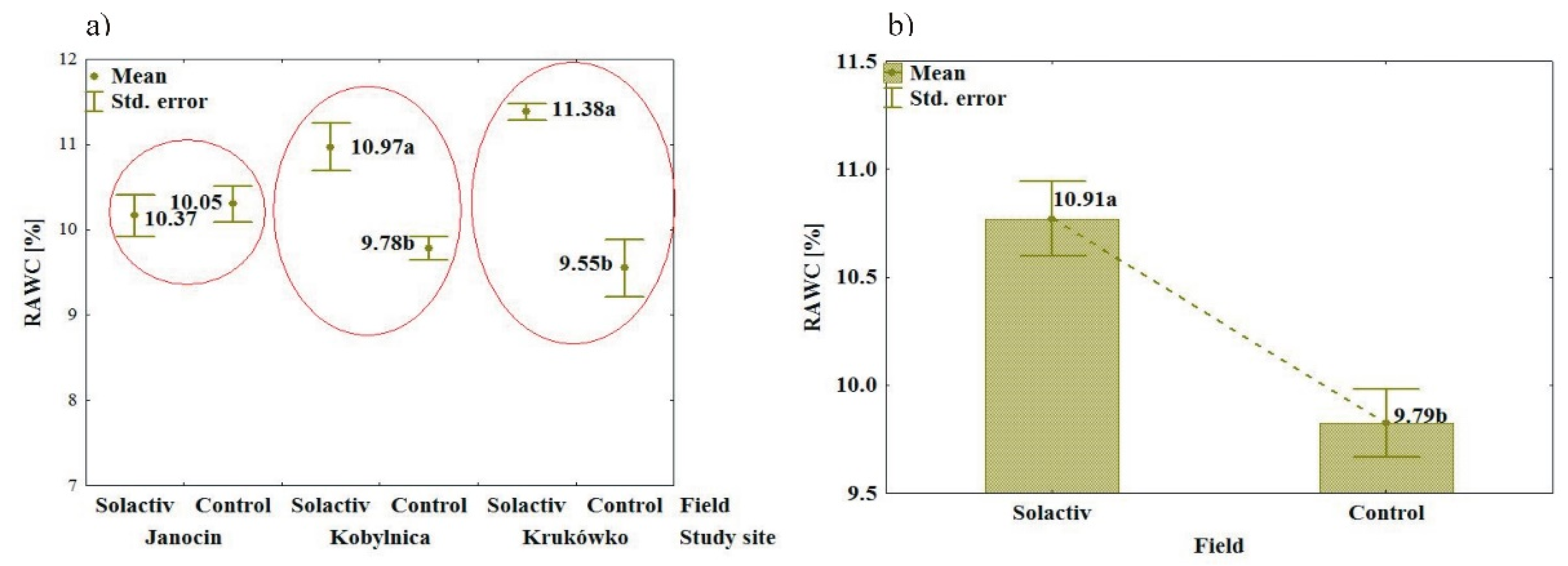
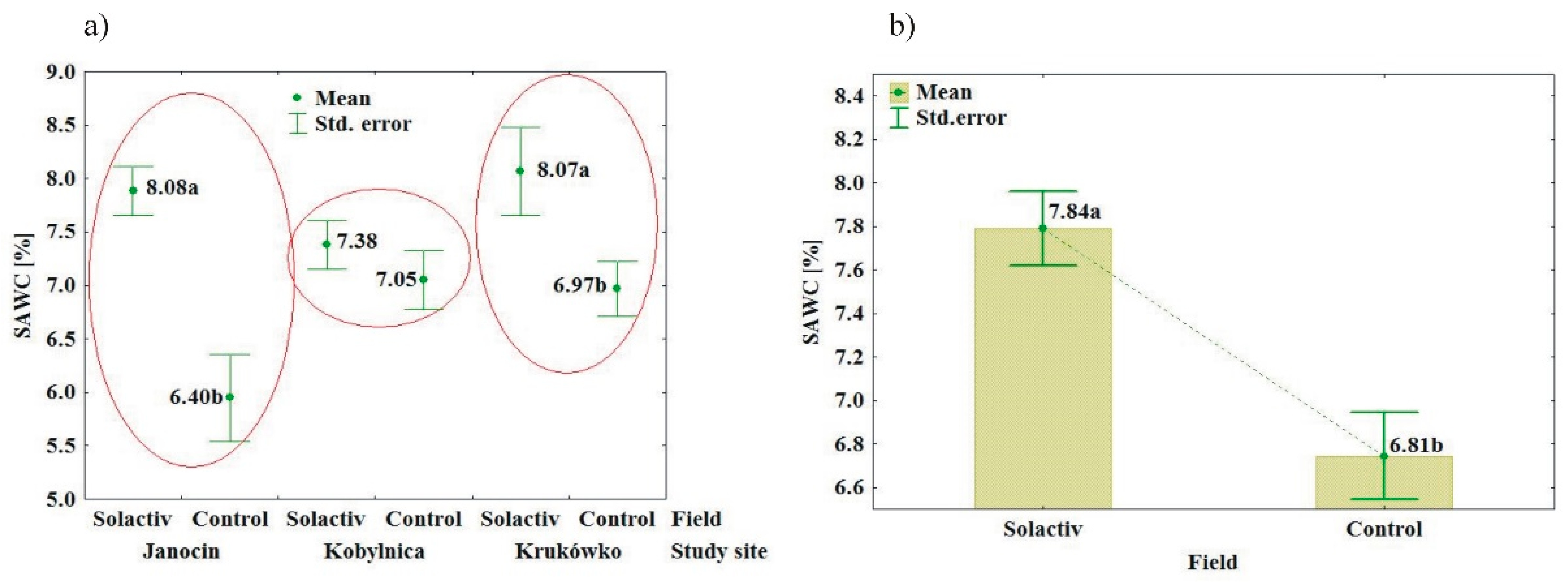
3.1. Soil Biochemical Properties
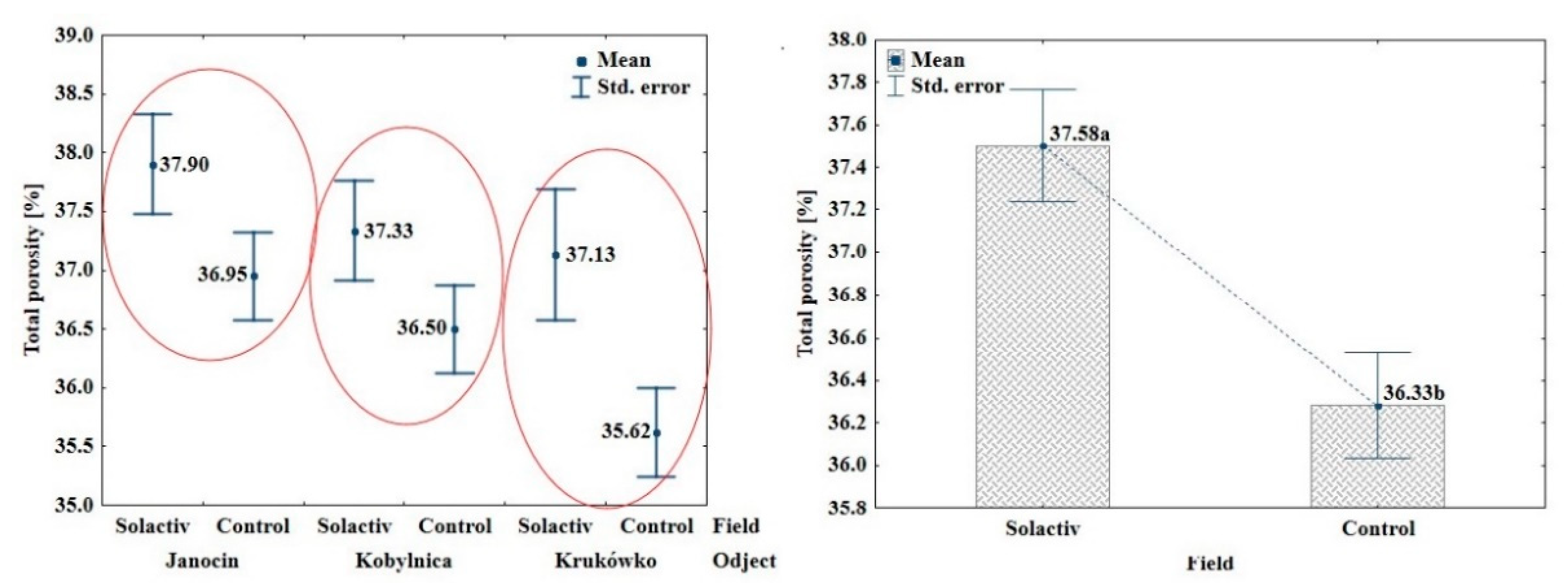
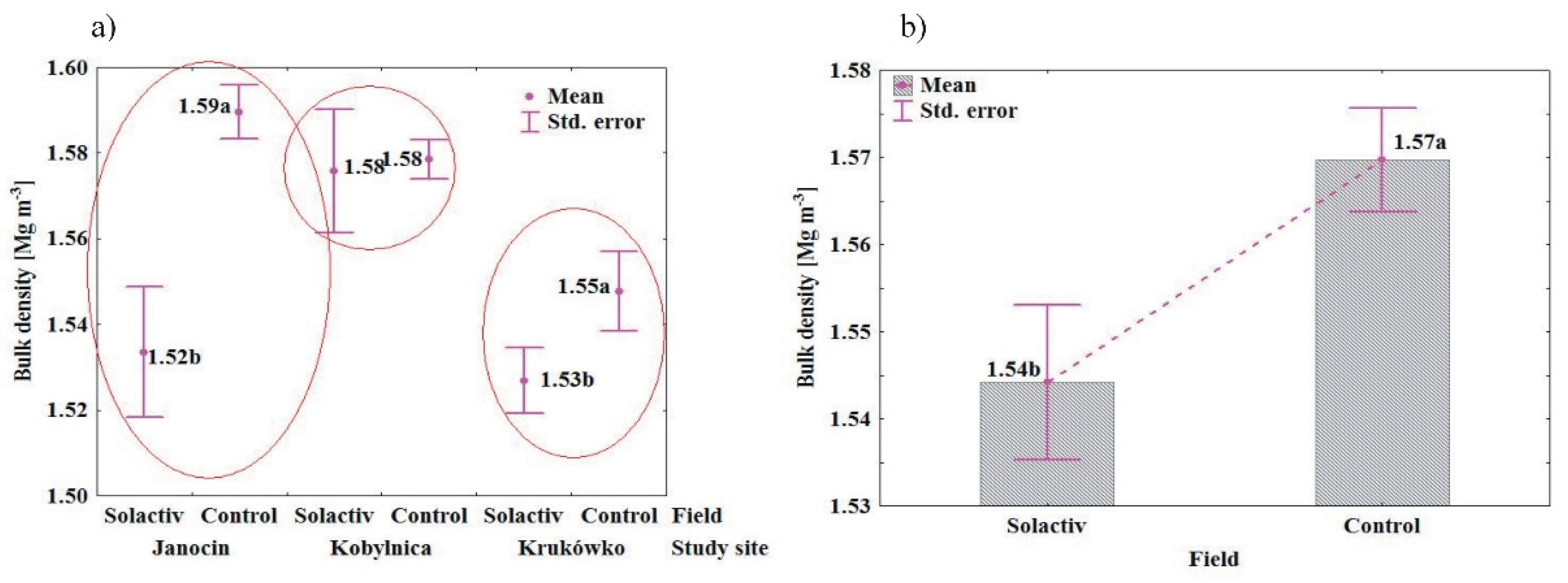
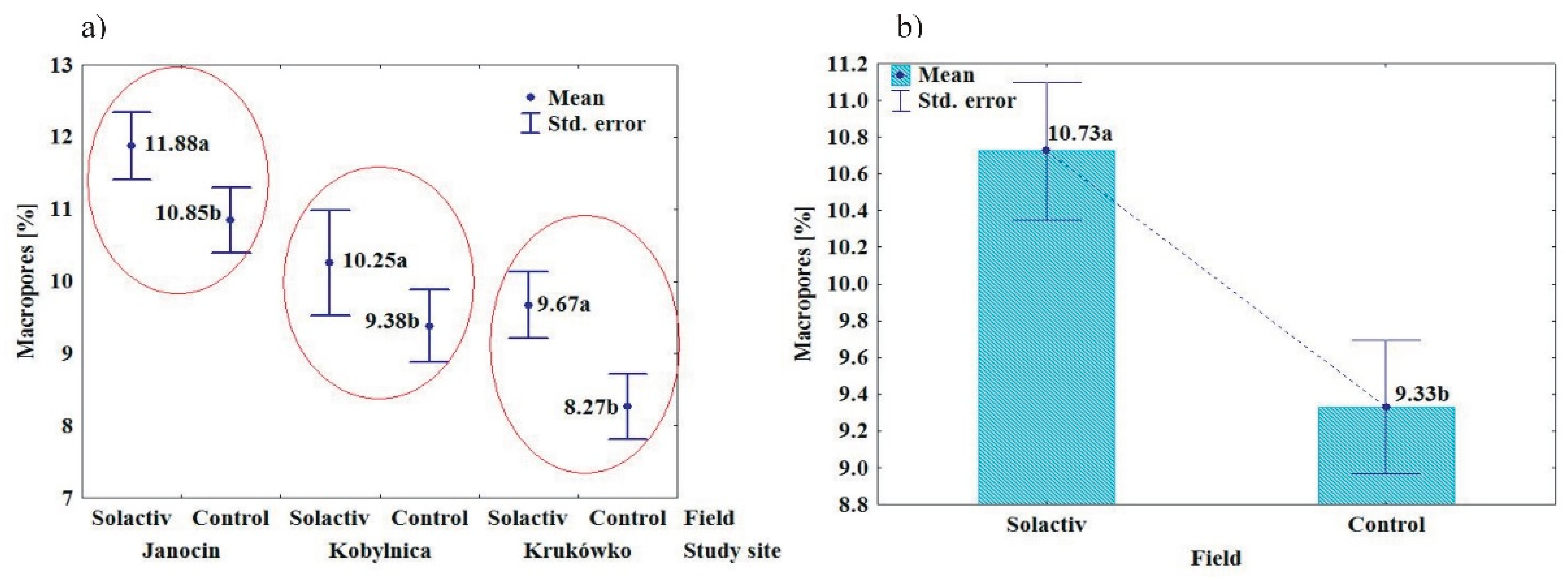
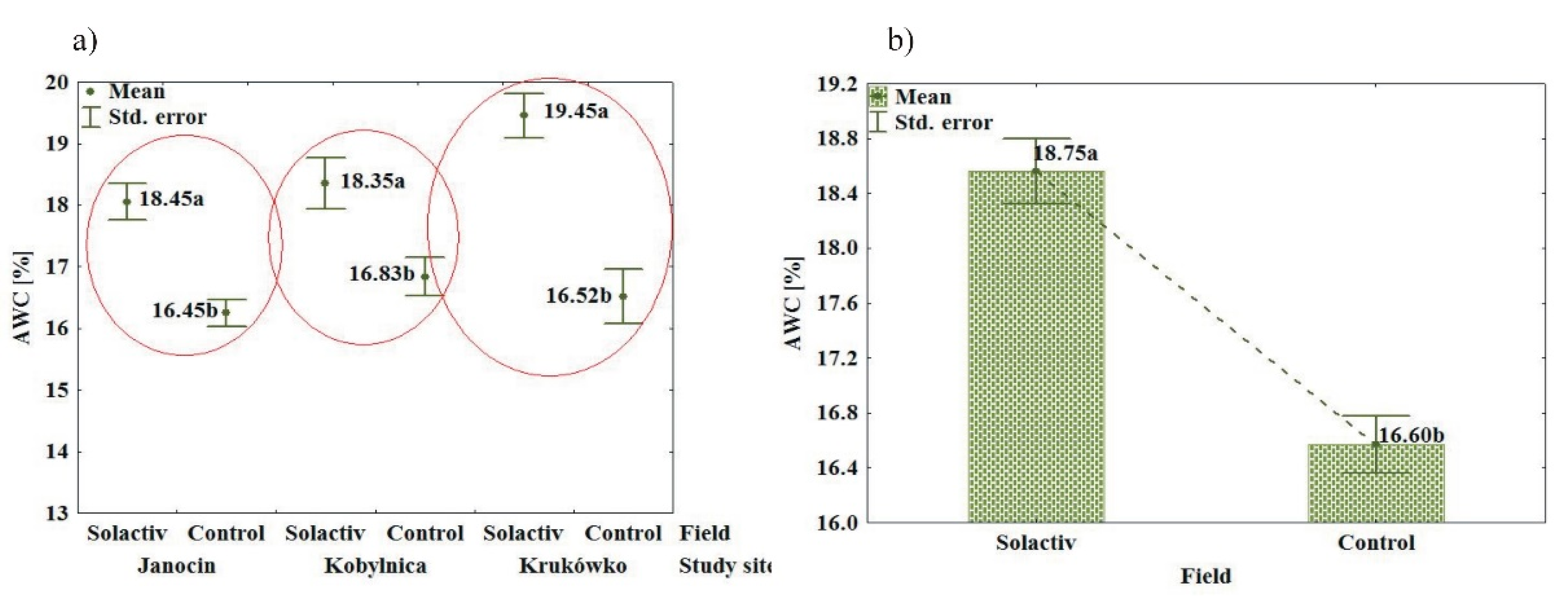
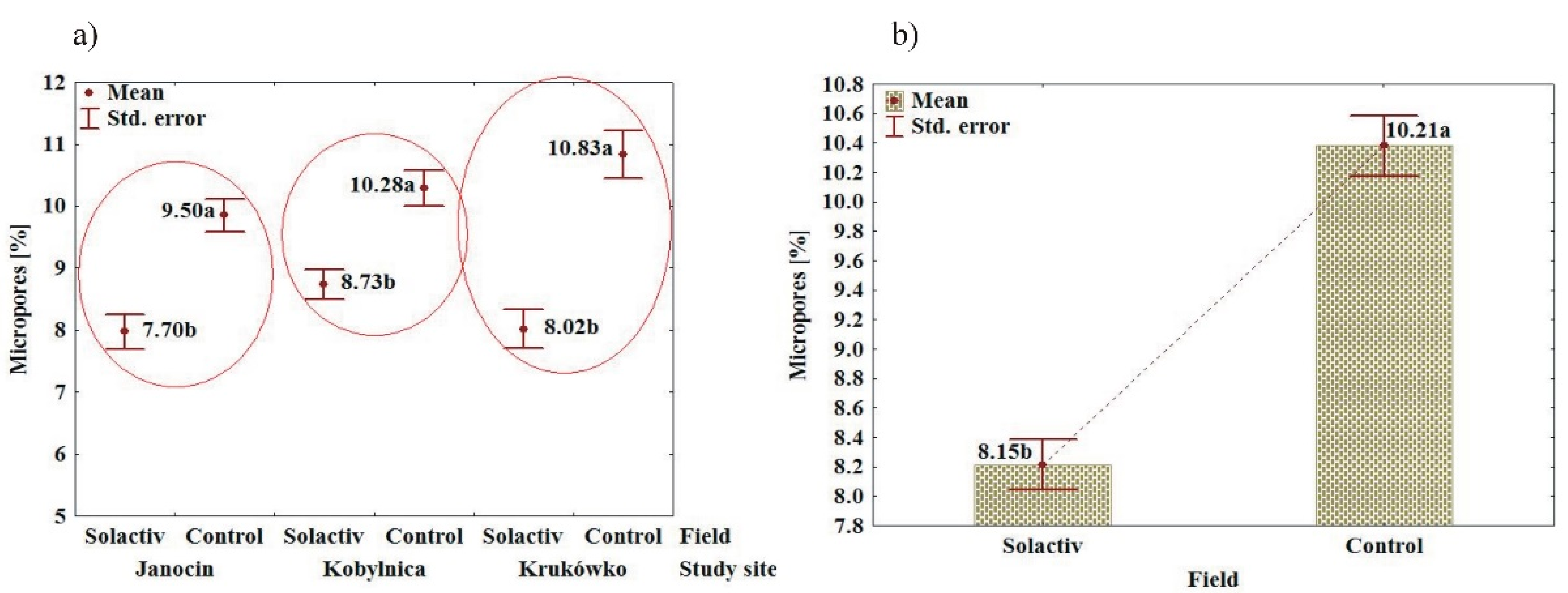
3.2. Soil Chemical and Physical Properties
3.3. Properties Related to the Growth and Yield of Maize
4. Discussion
4.1. Soil Biochemical Properties
4.2. Soil Chemical and Physical Properties
4.3. Plant Properties
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pavlik, P.; Vlckova, V.; Machar, I. Changes to Land Area Used for Grain Maize Production in Central Europe due to Predicted Climate Change. Int. J. Agron. 2019, 2019, 9168285. [Google Scholar] [CrossRef]
- Matyka, M.; Księżak, J. Yielding of selected plant species used to biogas production. Probl. Agric. Eng. 2012, 1, 69–75. [Google Scholar]
- Ureta, C.; González, E.J.; Espinosa, A.; Trueba, A.; Piñeyro-Nelson, A.; Álvarez-Buylla, E.R. Maize yield in Mexico under climate change. Agric. Syst. 2020, 177, 102697. [Google Scholar] [CrossRef]
- Zafar, S.; Iqbal, A.; Azar, A.T.; Atif, R.M.; Rana, I.A.; Rehman, H.M.; Nawaz, M.A.; Chung, G.; GM Maize for Abiotic Stresses. Potentials and Opportunities. In Recent Approaches in Omics for Plant Resilience to Climate, Change; Wani, S.H., Ed.; Springer: Switzerland, Cham, 2019; pp. 229–249. [Google Scholar]
- Gomiero, T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Krasowicz, S.; Oleszek, W.; Horabik, J.; Dębicki, R.; Jankowiak, J.; Stuczyński, T.; Jadczyszyn, J. Rational management of the soil environment in Poland. Pol. J. Agron. 2011, 7, 43–56. (In Polish) [Google Scholar]
- Ochal, P.; Jadczyszyn, T.; Jurga, B.; Kopiński, J.; Matyka, M.; Madej, A.; Rutkowska, A.; Smreczek, B.; Łysiak, M. Environmental aspects of soil acidity in Poland. In Studies and Reports of Institute of Soil Science and Plant Cultivation—State Research Institute; Report Prepared as a Part of the Task 2.2.; Institute of Soil Science and Plant Cultivation–State Research Institute: Poland, Puławy, 2017; p. 47. [Google Scholar]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Ouni, Y.; Ghnaya, T.; Montemurro, F.; Abdelly, C.; Lakhdar, A. The role of humic substances in mitigating the harmful effects of soil salinity and improve plant productivity. Int. J. Plant Prod. 2014, 8, 353–374. [Google Scholar]
- Wu, Q.; Chi, D.; Xia, G.; Chen, T.; Sun, Y.; Song, Y. Effects of Zeolite on Drought Resistance and Water–Nitrogen Use Efficiency in Paddy Rice. J. Irrig. Drain Eng. 2019, 145, 04019024. [Google Scholar] [CrossRef]
- Piotrowska, A.; Długosz, J.; Zamorski, R.; Bogdanowicz, P. Changes in some biological and chemical properties of an arable soil treated with the microbial biofertilizer UGmax. Pol. J. Environ. Stud. 2012, 21, 455–463. [Google Scholar]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.R. How effective are ‘Effective micro-organisms (EM)? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Doula, M.; Elaiopoulos, K.; Kavvadias, V.; Mavraganis, V. Use of clinoptilolite to improve and protect soil quality from the disposal olive oil mills wastes. J. Hazard. Mat. 2012, 207, 103–110. [Google Scholar] [CrossRef] [PubMed]
- De Silva Weeraddana, C. Extracts of the Brown Seaweed, Ascophyllum nodosum, effect Arabidopsis thaliana—Myzus persicae Interaction. Master’s Thesis, Dalhousie University Halifax, Halifax, NS, Canada, 2012. [Google Scholar]
- Palanivell, P.; Ahmed, O.H.; Susilawati, K.; Majid, N.M.A. Mitigating ammonia volatilization urea in waterlogged condition using clinoptilolite zeolite. Int. J. Agric. Biol. 2015, 17, 149–155. [Google Scholar]
- Gümüş, I.; Şeker, C. Influence of humic acid application on soil physicochemical properties. Solid Earth Discuss. 2015, 7, 2481–2500. [Google Scholar] [CrossRef]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Sen, A.; Srivastava, V.K.; Singh, R.K.; Singh, A.P.; Raha, P.; Ghosh, A.K.; De, N.; Rakshit, A.; Meena, R.N.; Kumar, A.; et al. Soil and Plant Responses to the Application of Ascophyllum nodosum Extract to No-Till Wheat (Triticum aestivum L.). Commun. Soil Sci. Plant Anal. 2015, 46, 123–136. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley: Hoboken, NJ, USA, 1994; Volume 13, pp. 236–256. [Google Scholar]
- Wang, X.J.; Wang, Z.Q.; Li, S.G. The effect of humic acids on the availability of phosphorus fertilizers in alkaline soils. Soil Use Manag. 1995, 11, 99–102. [Google Scholar] [CrossRef]
- Vallini, G.; Pera, A.; Agnolucci, M.; Valdrighi, M.M. Humic acids stimulate growth and activity of in vitro tested axenic cultures of soil autotrophic nitrifying bacteria. Biol. Fertil. Soils 1997, 24, 243–248. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World reference base for soil resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Gorbulewski, K.; Fronczak, J.; Leszczyńska, M. Specific surface area-basic parameter of reactive material’s characteristics. Sci. Rev. Eng. Environ. Sci. 2008, 17, 122–130. (In Polish) [Google Scholar]
- Soil Survy Staff. 2014 Soil Survey Staff, Keys to Soil Taxonomy, 11th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2010. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Studies concerning the chemical analysis of soils as background for soil nutrient assessment. II. Chemical extracting methods to determinate the phosphorous and potassium content of soil. Kungl. Lantbr. Ann. 1960, 26, 199–215. (In German) [Google Scholar]
- PN-ISO 11260:2018. Soil Quality—Determination of Effective Cationic Exchange Capacity and Base Saturation with Barium Chloride; PWN Press: Warsaw, Poland, 2018. [Google Scholar]
- PN-ISO 10390. Soil Quality. pH Determination; PWN Press: Warsaw, Poland, 1997. [Google Scholar]
- Walczak, R.; Ostrowski, J.; Witkowska-Walczak, B.; Sławiński, C. Spatial characteristics of water conductivityin the surface level of Polish arable soils. Int. Agrophys. 2002, 16, 239–247. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrodgenaseaktivitat im Boden mittels Triphenyltetrazolium-chlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinsen, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; SSSA: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Andronikashvili, T.; Urushadze, T.; Eprikashvili, L.; Gamisonia, M.; Nakaidze, E. Towards the biological activity of the natural zeolite—Clinoptilolite-containing tuff. Bull. Georg. Natl. Acad. Sci. 2008, 2, 99–107. [Google Scholar]
- Leggo, P.J.; Ledesert, B.; Christie, G. The role of clinoptilolite in organo-zeolitic-soil systems used for phytoremediation. Sci. Total Environ. 2006, 363, 1–10. [Google Scholar] [CrossRef]
- Karličić, V.; Živanoić, I.; Matijašević, D.; Raićević, V.; Nikšić, M.; Rac, W.; Simić, A. Stimulationof soil microbiological activity by clinoptilolite: The effect of plant growth. Rotar. Povrt. 2017, 54, 117–123. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Šimon, T. Effects of zeolite amendment on microbial biomass and respiratory activity in heavy metal contaminated soils. Plant Soil Environ. 2003, 49, 536–541. [Google Scholar] [CrossRef]
- Ramesh, K.; Reddy, D.D. Chapter four-zeolites and their potential uses in agriculture. Adv. Agron. 2011, 113, 219–241. [Google Scholar]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Li, Y.; Chen, L.; Liu, Z.; Wang, X.; Qin, S. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Canales López, B. Seaweed-enzymes: Possibilities for stimulating crop yield and improving soil quality. Terra Latinoam. 1999, 17, 271–276. (In Mexican) [Google Scholar]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of short-term application of seaweed fertilizer on bacterial diversity and community structure, soil nitrogen contents, and plant growth in maize rhizosphere soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babbohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- De Campos Bernardi, A.C.; Polidoro, J.C.; de Melo Monte, M.B.; Pereira, E.I.; Ribeiro, C.; Ramesh, K. Enhancing Nutrient Use Efficiency Using Zeolites Minerals—A Review. Adv. Chem. Eng. Sci. 2016, 6, 295–304. [Google Scholar] [CrossRef]
- Ahmed, O.H.; Azrumi, N.A.B.; Jalloh, M.B.; Jol, H. Using Clinoptilolite Zeolite for Enhancing Potassium Retention in Tropical Peat Soil. Adv. Trop. Soil Sci. 2015, 3, 112–127. [Google Scholar]
- Józefaciuk, G.; Szatniak-Kloc, A.; Ambrozewicz-Nita, A. The surface area of zeolite-amended soils exceeds the sum of the inherent surface areas of soil and zeolite. Eur. J. Soil Sci. 2018, 69, 787–790. [Google Scholar] [CrossRef]
- Filcheva, E.G.; Tsadilas, C.D. Influence of Clinoptilolite and compost on soil properties. Commun. Soil Sci. Plant Anal. 2002, 33, 95–607. [Google Scholar] [CrossRef]
- Sherrod, L.A.; Peterson, G.A.; Westfall, D.G.; Ahuja, L.R. Cropping intensity enhances soil organic carbon and nitrogen in a no-till agroecosystem. Soil Sci. Soc. Am. 2003, 67, 1533–1543. [Google Scholar] [CrossRef]
- Ravali, C.; Rao, K.J.; Anjaiah, T.; Suresh, K. Effect of zeolite on soil physical and physico-chemical properties. Int. Ref. Peer Rev. Ind. Quart. J. Sci. Agric. Eng. 2020, 10, 776–781. [Google Scholar]
- Abdel-Hassan, A.N.; Abdullah Radi, A.M. Effect of zeolite on some physical properties of wheat plant growth (Triticum aestivum L.). Plant Arch. 2017, 18, 2641–2648. [Google Scholar]
- Hassan, A.Z.A.; Mahmoud, A.W.M. The combined effect of bentonite and natural zeolite on sandy soil properties and productivity of some crops. Topcls. J. Agric. Res. 2013, 1, 22–28. [Google Scholar]
- Githinji, L.J.M.; Dane, J.H.; Walker, R.H. Physical and hydraulic properties of inorganic amendments and modelling their effects on water movement in sand-based root zones. Irrig. Sci. 2011, 29, 65–77. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Tarkalson, D.D.; Lehrsch, G.A. Zeolite soil application method affects inorganic nitrogen, moisture, and corn growth. Soil Sci. 2011, 176, 136–142. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Saberioon, M.; Borůvka, L.; Wayayok, A.; Mohd Soom, M.A. Leaf chlorophyll and nitrogen dynamics and their relationship to lowland rice yield for site-specific paddy management. Inf. Process. Agric. 2017, 4, 259–268. [Google Scholar] [CrossRef]
- Natywa, M.; Pociejowska, M.; Majchrzak, L.; Pudełko, K. Influence of irrigation and nitrogen fertilization on yield and leaf greenness index (spad) of maize. Acta Sci. Pol. 2014, 13, 39–50. [Google Scholar]
- Latique, S.; Chernane, H.; Mansori, M.; Kaoua, E. Seaweed liquid fertilizer effect on physiological and biochemical parameters of bean plant (Phaesolus vulgaris var Paulista) under hydroponic system. Eur. Sci. J. 2013, 9, 174–193. [Google Scholar]
- Blunden, G.; Jenkins, T.; Liu, Y. Enhanced leaf chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol. 1997, 8, 535–543. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased rootzone soil microbial activity. Can. J. Plant Sci. 2013, 94, 337–348. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Aainaa, H.; Ahmed, O.H.; Ab Majid, N.M. Effects of clinoptilolite zeolite on phosphorus dynamics and yield of Zea Mays, L. cultivated on an acid soil. PLoS ONE 2018, 13, e0204401. [Google Scholar]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.V.; Ascencio, J.; Ugarte, J.; Yzaguirre, L. Effect of Humusbol (double humate of potassium and phosphorus on the growth of corn in vegetative phase. Bioagro 2014, 26, 143–152. (In Spanish) [Google Scholar]
- Maruf, M.T.; Mam-Rasul, G.A. Effect of humic acid and sulfur fertilizer levels on some physiological traits of maize (Zea mays L.) on calcareous soil. Appl. Ecol. Environ. Res. 2019, 17, 13199–13217. [Google Scholar] [CrossRef]
- Hartz, T.K. Humic substances generally ineffective in improving vegetable crop nutrient uptake or productivity. Hortic. Sci. 2010, 45, 906–910. [Google Scholar] [CrossRef]
- Little, K.R.; Rose, M.T.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Do lignite-derived organic amendments improve early-stage pasture growth and key soil biological and physicochemical properties? Crop Pasture Sci. 2014, 65, 899–910. [Google Scholar] [CrossRef]
| Month | * Chrząstowo | ** Głębokie | ||
|---|---|---|---|---|
| Temperature (°C) | Precipitation (mm) | Temperature (°C) | Precipitation (mm) | |
| January | 0.7 | 56.8 | 0.7 | 37.5 |
| February | −1.0 | 2.3 | −3.1 | 4.9 |
| March | −0.2 | 31.8 | 0.4 | 24.6 |
| Aril | 11.8 | 48.8 | 12.8 | 22.4 |
| May | 17.1 | 5.1 | 16.6 | 29.6 |
| June | 18.2 | 44.5 | 18.4 | 42.8 |
| July | 20.5 | 120.0 | 20.8 | 122.0 |
| August | 20.1 | 14.3 | 21.1 | 23.0 |
| September | 15.5 | 31.9 | 16.2 | 24.6 |
| October | 9.7 | 24.8 | 10.6 | 26.7 |
| November | 4.2 | 8.7 | 4.8 | 17.5 |
| December | 2.0 | 52.2 | 2.2 | 39.3 |
| Property | Sampling Time | Solactive | Control | ||
|---|---|---|---|---|---|
| Mean (±SE) | Range | Mean (±SE) | Range | ||
| CORG (g∙kg−1) | I * | 8.08 (±0.39) | 5.99–11.4 | 9.07 (±0.33) | 7.04–11.9 |
| II ** | 7.94 (±0.39) | 5.47–11.4 | 8.54 (±0.37) | 6.67–11.2 | |
| NTOT (g∙kg−1) | I * | 1.08 (±0.05) | 0.81–1.46 | 1.19 (±0.04) | 0.95–1.58 |
| II ** | 0.98 (±0.04) | 0.68–1.25 | 1.05 (±0.04) | 0.84–1.34 | |
| DOC (mg∙kg−1) | I * | 92.1 (±2.53) | 71.8–110.3 | 96.6 (±2.63) | 79.1–131.7 |
| II ** | 76.0 (±2.81) | 55.2–97.2 | 80.8 (±2.09) | 65.0–93.0 | |
| DNt (mg∙kg−1) | I * | 27.4 (±1.56) | 18.6–42.0 | 31.4 (±1.56) | 20.6–44.3 |
| II ** | 20.7 (±1.45) | 12.8–36.8 | 25.7 (±2.66) | 14.4–48.8 | |
| DOC (%) | I * | 1.17 (±0.03) | 0.94–1.37 | 1.08 (±0.03) | 0.80–1.29 |
| II ** | 0.97 (±0.02) | 0.77–1.13 | 0.97 (±0.04) | 0.74–2.34 | |
| DNt (%) | I * | 2.54 (±0.08) | 1.98–3.09 | 2.65 (±0.10) | 1.89–3.51 |
| II ** | 2.07 (±0.07) | 1.56–2.94 | 2.33 (±0.16) | 1.48–3.76 | |
| MBC (mg∙kg−1) | I * | 118.6 (±4.27) | 104.5–128.9 | 103.7 (±5.07) | 78.4–135.7 |
| II ** | 104.7 (±5.25) | 78.9–139.7 | 93.2 (±4.66) | 65.5–132.2 | |
| MBN (mg∙kg−1) | I * | 18.9 (±1.17) | 16.1–20.6 | 17.5 (±2.25) | 13.4–22.2 |
| II ** | 16.8 (±2.55) | 12.9–22.3 | 15.3 (±1.88) | 11.2–21.7 | |
| Property | Sampling Time | Solactiv | Control | ||
|---|---|---|---|---|---|
| Mean (±SE) | Range | Mean (±SE) | Range | ||
| Mg-AVAIL (mg∙kg−1) | I * | 74.6 (±4.61) | 40.6–98.7 | 70.7 (±4.66) | 38.3–96.0 |
| II ** | 88.2 (±5.75)b | 45.4–132.7 | 93.6 (±6.78)a | 44.1–145.6 | |
| P-AVAIL (mg∙kg−1) | I * | 79.1 (±2.11)a | 63.0–97.0 | 64.5 (±1.70)b | 51.4–78.6 |
| II ** | 65.7 (±3.48)a | 34.4–96.5 | 51.7 (±2.82)b | 29.3–70.6 | |
| K-AVAIL (mg∙kg−1) | I * | 129.5 (±5.03) | 92.7–175.0 | 127.8 (±6.34) | 91.2–184.3 |
| II ** | 189.4 (±5.87)a | 145.7–256.6 | 167.7 (±12.4)b | 88.6–288.4 | |
| Mg-EXCHAN (cmol∙kg−1) | I * | 4.28 (±0.22) | 2.67–5.97 | 3.57 (±0.33) | 1.23–5.41 |
| II ** | 7.33 (±0.50) | 3.78–10.5 | 7.80 (±0.58) | 3.88–12.5 | |
| Ca-EXCHAN (cmol∙kg−1) | I * | 5.14 (±1.03)a | 4.23–5.99 | 4.51 (±2.40)b | 2.69–6.66 |
| II ** | 5.50 (±1.68)a | 3.98–6.91 | 5.10 (±1.84)b | 3.75–7.13 | |
| K-EXCHAN (cmol∙kg−1) | I * | 3.95 (±0.18) | 2.64–5.87 | 4.14 (±0.20) | 2.61–5.88 |
| II ** | 2.38 (±0.18) | 1.47–4.30 | 2.83 (±0.25) | 1.12–4.95 | |
| Na-EXCHAN (cmol∙kg−1) | I * | 2.89 (±0.16) | 1.73–3.79 | 2.71 (±0.17) | 1.45–3.98 |
| II ** | 1.94 (±0.19)b | 0.94–3.83 | 2.31 (±0.23)a | 0.72–3.83 | |
| pHKCl | I * | 6.04 (±0.14)a | 4.82–7.25 | 5.71 (±0.16)b | 4.14–6.78 |
| II ** | 6.03 (±0.12)a | 4.67–7.06 | 5.59 (±0.14)b | 4.32–6.92 | |
| Hh (cmol∙kg−1) | I * | 1.38 (±0.11)b | 0.37–2.30 | 1.83 (±0.15)a | 0.80–3.66 |
| II ** | 1.59 (±0.10)b | 0.66–2.62 | 2.13 (±0.15)a | 0.94–3.75 | |
| S (cmol∙kg−1) | I * | 6.25 (±0.11)a | 5.26–7.38 | 5.55 (±0.29)b | 3.33–7.68 |
| II ** | 6.66 (±0.18) | 5.18–8.03 | 6.39 (±0.27) | 4.40–9.19 | |
| CEC (cmol∙kg−1) | I * | 7.64 (±0.14) | 5.73–8.88 | 7.38 (±0.33) | 5.01–10.0 |
| II ** | 8.25 (±0.19) | 6.82–10.0 | 8.53 (±0.32) | 6.24–11.2 | |
| Basic saturation (%) | I * | 82.2 (±1.26)a | 71.9–94.8 | 75.0 (±1.78)b | 62.2–87.4 |
| II ** | 80.8 (±1.16)a | 67.5–91.6 | 75.1 (±1.41)b | 62.2–85.7 | |
| BBCH Growth Stage | Conditioner | Study Site | Mean ** | ||
|---|---|---|---|---|---|
| Janocin (I) | Kobylnica (II) | Krukówko (III) | |||
| BBCH 15–16 (three-leaf phase) | Solactiv | 322(±0.95) | 292(±3.51) | 282(±1.23) | 299 |
| Control | 323(±2.62) | 291(±5.01) | 279(±1.98) | 298 | |
| Mean * | 323 | 292 | 281 | 298 | |
| BBCH 33–34 (three-node phase) | Solactiv | 512(±2.20)a | 501(±1.27)a | 442(±2.76) | 485a |
| Control | 491(±3.36)b | 475(±3.31)b | 434(±1.39) | 466b | |
| Mean * | 501 | 488 | 438 | 476 | |
| BBCH 64–65 (panicle blooming phase) | Solactiv | 646(±2.55)a | 663(±4.69)a | 589(±1.97) | 633a |
| Control | 609(±3.39)b | 618(±5.23)b | 588(±2.03) | 605b | |
| Mean * | 627 | 641 | 588 | 619 | |
| Mean for the three measurements | Solactiv | 493(±1.18)a | 485(±2.27)a | 438(±1.49) | 472a |
| Control | 474(±2.60)b | 461(±4.31)b | 433(±1.40) | 456b | |
| Mean * | 484 | 473 | 436 | 464 | |
| Property | Field | Study Site | Mean ** | ||
|---|---|---|---|---|---|
| Janocin (I) | Kobylnica (II) | Krukówko (III) | |||
| Plant height (cm) before harvesting (BBCH 87–89) | Solactiv | 265.4(±31.73) | 275.4(±2.30) | 252.5(±1.53) | 264.4 |
| Control | 258.0(±1.55) | 267.8(±2.67) | 254.4(3.86) | 260.1 | |
| Mean * | 261.7 | 271.6 | 253.4 | 262.2 | |
| Fresh plant biomass (g) (BBCH 84–85) | Solactiv | 785.5(±14.8) | 745.6(±8.53) | 615.4(±11.1) | 715.5a |
| Control | 715.2(±7.52) | 720.1(±21.0) | 587.7(±14.9) | 674.3b | |
| Mean * | 750.3 | 732.9 | 601.5 | 694.9 | |
| Fresh cob biomass before harvesting (BBCH 87–89) | Solactiv | 311.4(±4.88) | 302.3(±2.94) | 212.6(±4.41) | 275.4a |
| Control | 292.1(±5.55) | 284.2(±12.4) | 202.0(±5.25) | 259.4b | |
| Mean * | 301.8 | 293.2 | 207.3 | 267.4 | |
| Grain yield (t∙ha−1) | Solactiv | 13.4(±0.51) | 9.82(±0.74) | 8.51(±0.34) | 10.6 |
| Control | 11.9(±0.76) | 9.62(±0.60) | 9.00(±0.41) | 10.2 | |
| Mean * | 12.6 | 9.7 | 8.7 | 10.4 | |
| Weight of 1000 seeds (g) | Solactiv | 308.4(±3.64)a | 211.4(±8.05) | 232.8(±6.43) | 250.8 |
| Control | 275.7(±12.9)b | 216.8(±5.45) | 243.5(±3.96) | 245.3 | |
| Mean * | 292.0 | 214.1 | 238.1 | 248.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Długosz, J.; Piotrowska-Długosz, A.; Kotwica, K.; Przybyszewska, E. Application of Multi-Component Conditioner with Clinoptilolite and Ascophyllum nodosum Extract for Improving Soil Properties and Zea mays L. Growth and Yield. Agronomy 2020, 10, 2005. https://doi.org/10.3390/agronomy10122005
Długosz J, Piotrowska-Długosz A, Kotwica K, Przybyszewska E. Application of Multi-Component Conditioner with Clinoptilolite and Ascophyllum nodosum Extract for Improving Soil Properties and Zea mays L. Growth and Yield. Agronomy. 2020; 10(12):2005. https://doi.org/10.3390/agronomy10122005
Chicago/Turabian StyleDługosz, Jacek, Anna Piotrowska-Długosz, Karol Kotwica, and Ewelina Przybyszewska. 2020. "Application of Multi-Component Conditioner with Clinoptilolite and Ascophyllum nodosum Extract for Improving Soil Properties and Zea mays L. Growth and Yield" Agronomy 10, no. 12: 2005. https://doi.org/10.3390/agronomy10122005
APA StyleDługosz, J., Piotrowska-Długosz, A., Kotwica, K., & Przybyszewska, E. (2020). Application of Multi-Component Conditioner with Clinoptilolite and Ascophyllum nodosum Extract for Improving Soil Properties and Zea mays L. Growth and Yield. Agronomy, 10(12), 2005. https://doi.org/10.3390/agronomy10122005






