A Global Screening Assay to Select for Maize Phenotypes with a High Tolerance or Resistance to Fusarium verticillioides (Sacc.) Nirenberg Rots
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Decontamination of Seeds
2.3. Conidia Suspensions and Inoculation of Maize Seeds by Fv
2.4. Fv Effect on the Post-Germination of Maize Emergencia (VE Stage)
2.5. Fv Effect on Maize Seedlings at the Second Leaf Collar (V2 Stage)
2.6. Fv Effect on Maize Plantlet at the Fourth Leaf Collar (v4 Stage) in Greenhouse
2.7. Fusarium Effect in Stalks at the Silking (R1 Stage)
2.8. Fv Effect on Ears at the Maturity (R6 Stage)
2.9. Statistics Analysis
3. Results
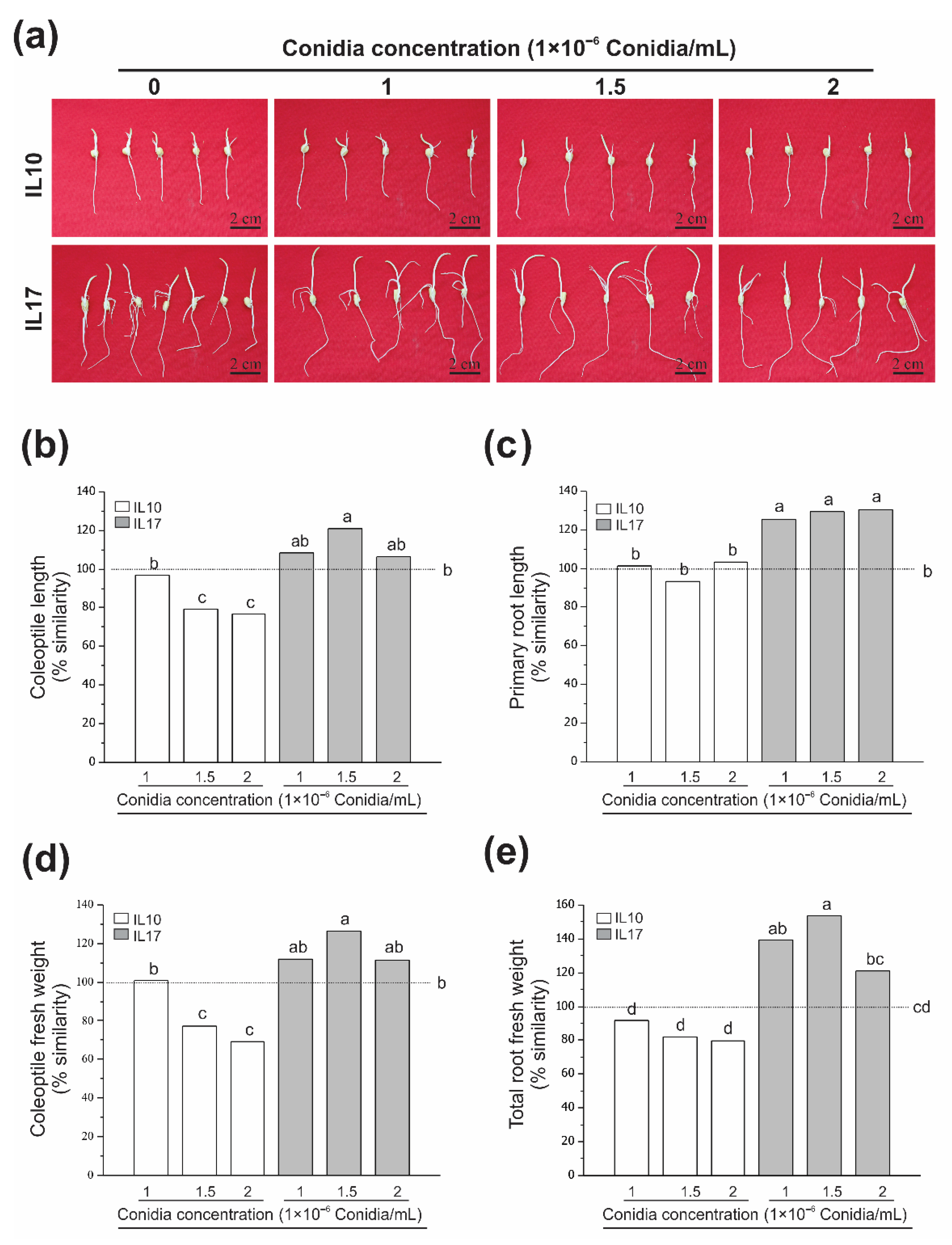
3.1. Fv Infection Does Not Diminish Growth at the Post-Germinative Stage (Seed Rot)
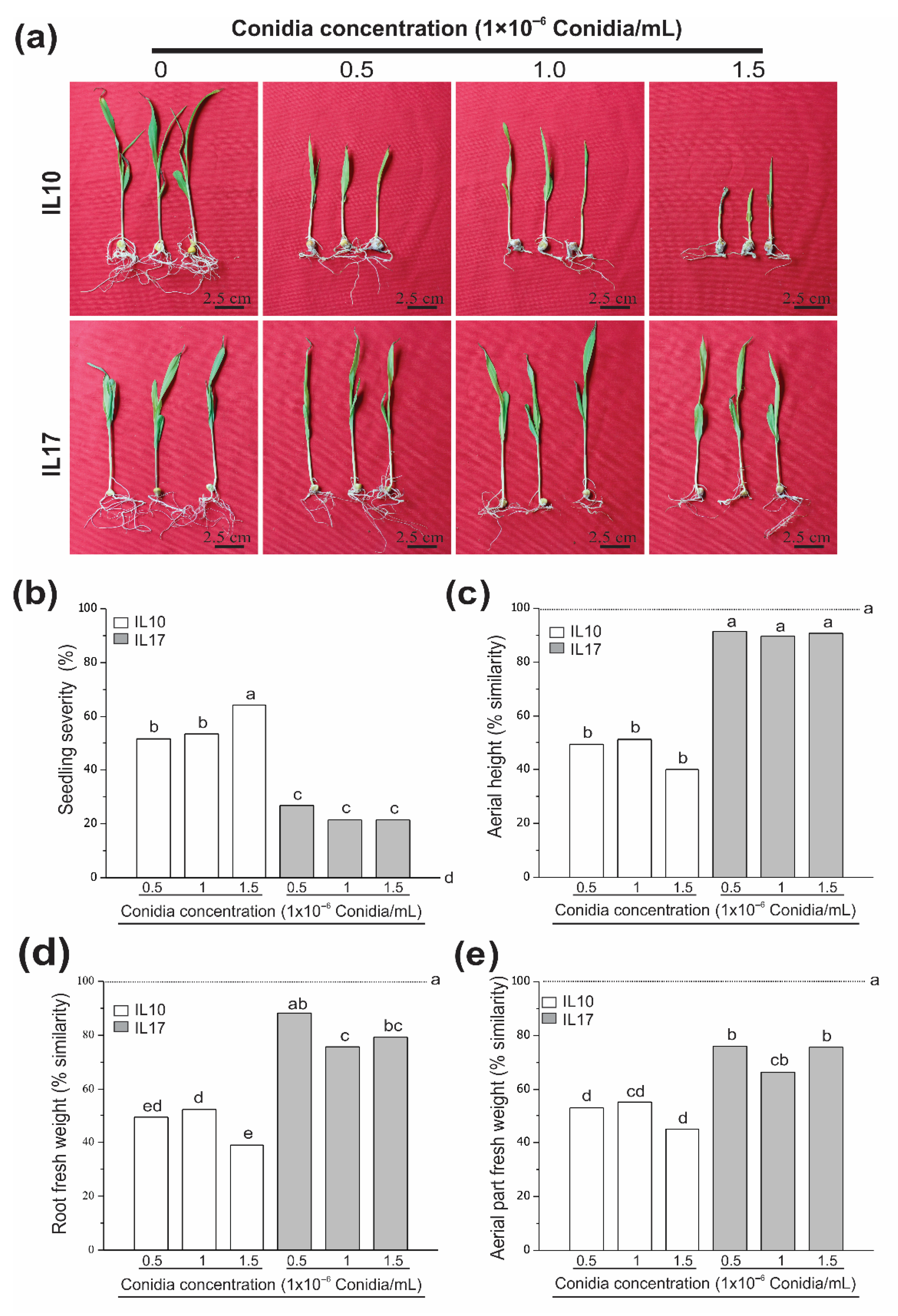
3.2. Fv-Infected Seeds Were Differentially Affected at the V2 Stage (Seedling Root Rot)
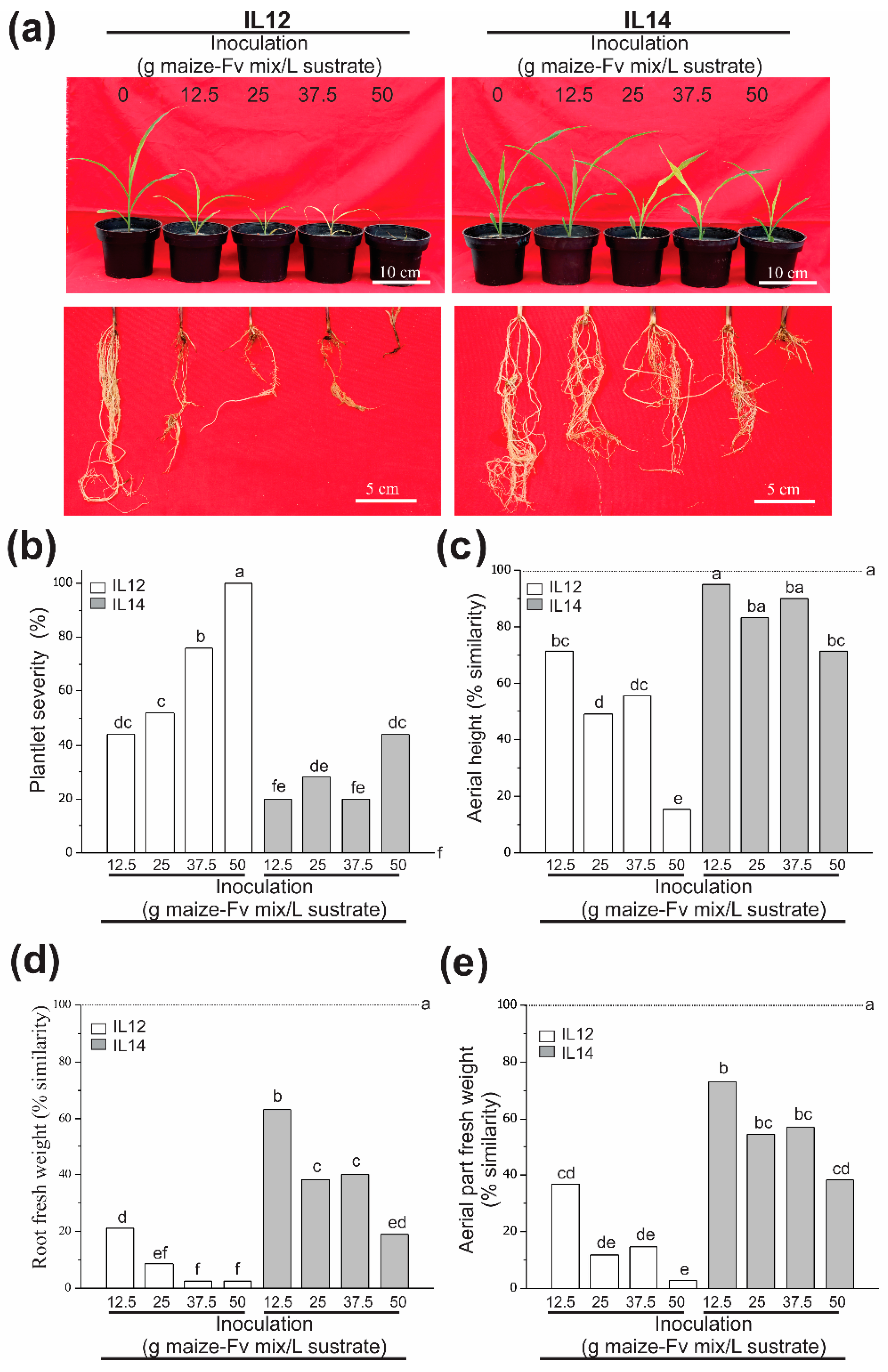
3.3. Fv Drastically Affects Susceptible Genotypes at the V4 Stage (Plantlet Root Rot)
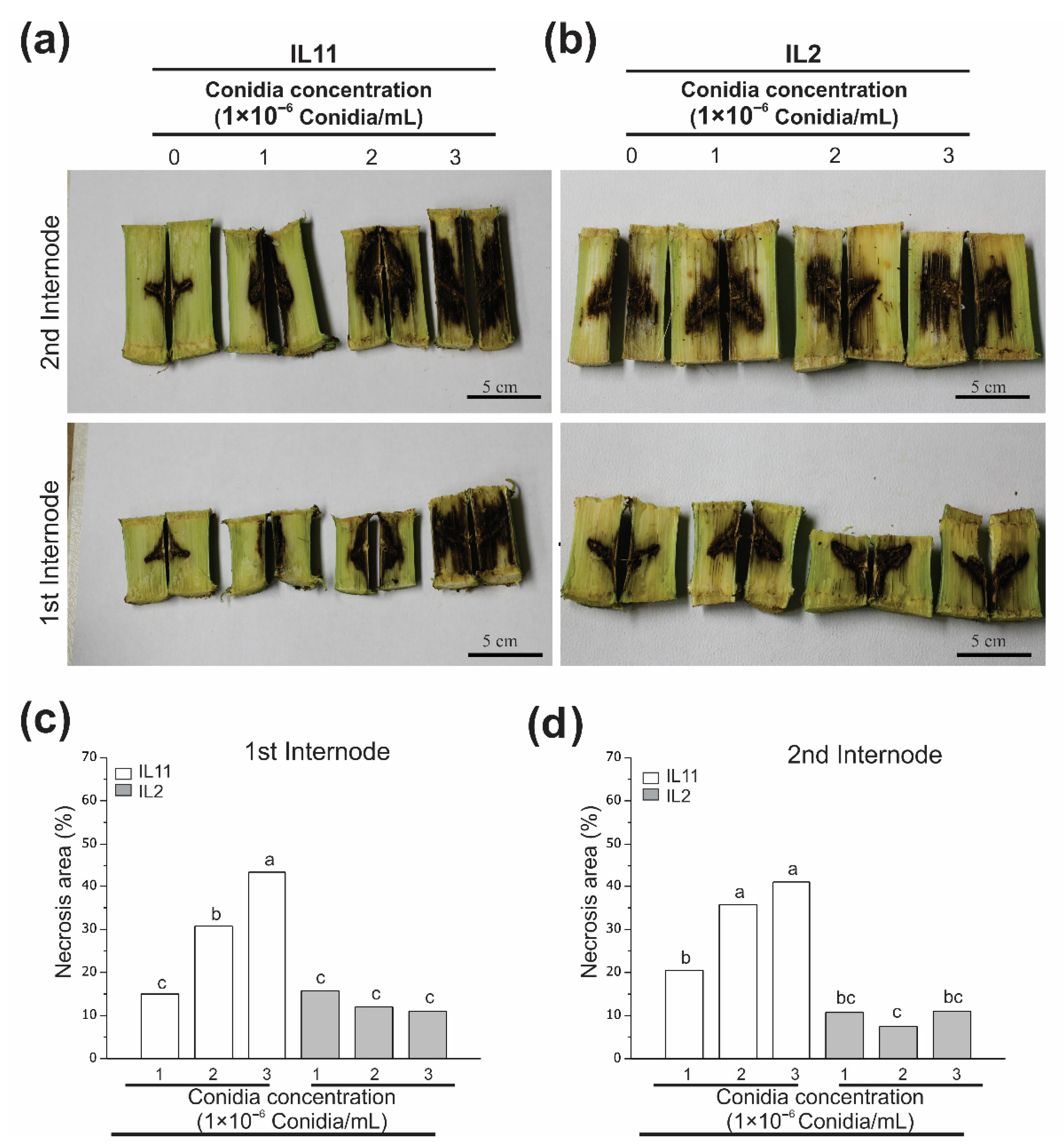
3.4. Fv Stalk Rot Affects Stalk Internodes at a Similar Level on the Same Genotype
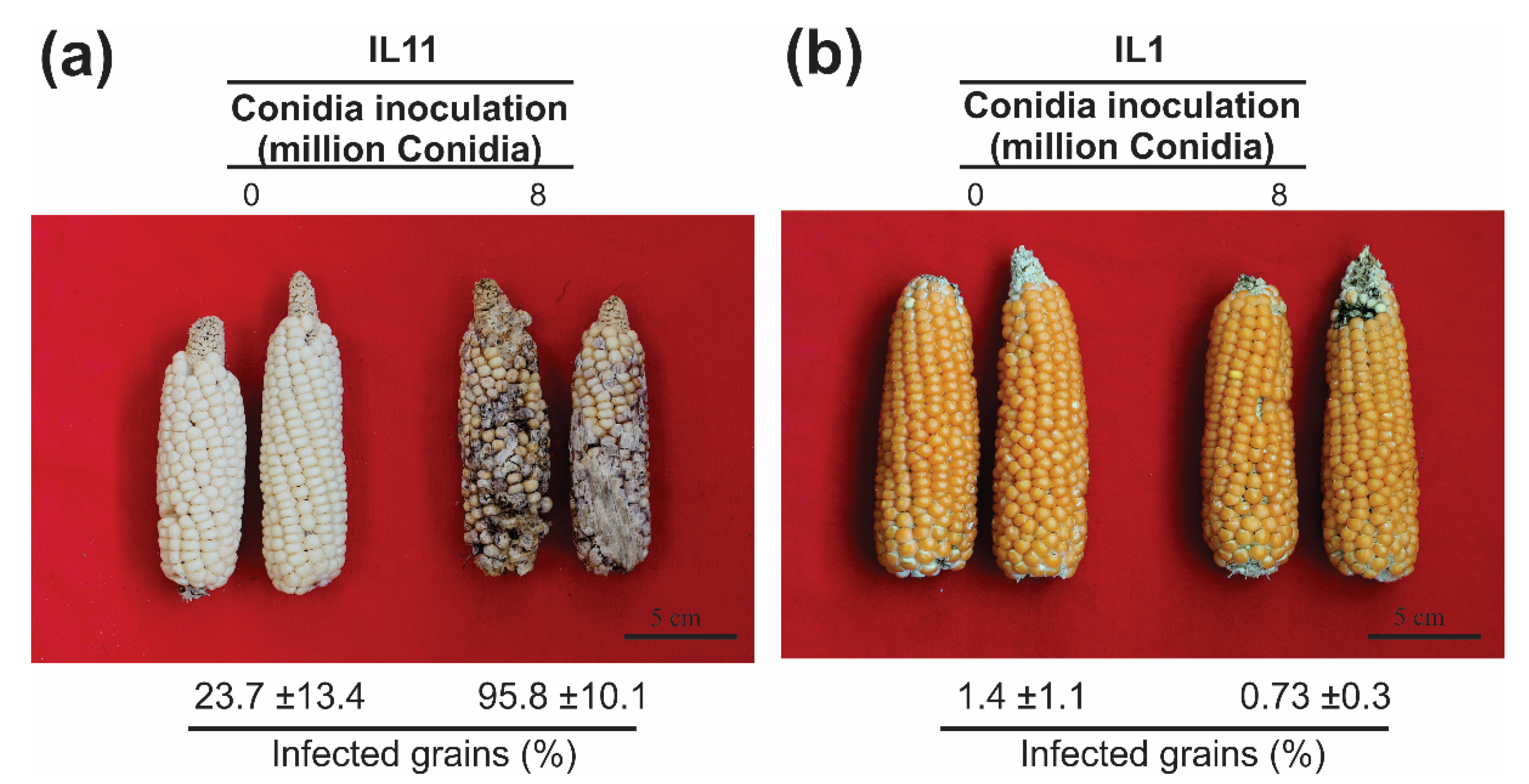
3.5. Fv Infection with Eight Million Conidia Was Effective for Screening Resistant Genotypes (Ear Rot)
3.6. Behavior of IL throughout the Maize Life Cycle-Fv Rots
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranum, P.; Pena-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Koohafkan, P.; Stewart, B.A. Cereal Production in Drylands; Koohafkan, P., Stewart, B.A., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; pp. 17–23. [Google Scholar]
- Power, A.G. Plant community diversity, herbivore movement, and an insect-transmitted disease of maize. Ecology 1987, 68, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Goodman, M.; Muse, S.; Smith, J.S.; Buckler, E.; Doebley, J. Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 2003, 165, 2117–2128. [Google Scholar] [PubMed]
- Holbert, J.R.; Burlison, W.L.; Koehler, B.; Woodworth, C.M.; Dungan, G.H. Corn Root, Stalk, and Ear Rot Diseases, and Their Control thru Seed Selection and Breeding; University of Illinios, Agricultural Experiment Station: Urbana, IL, USA, 1924; pp. 1–478. [Google Scholar]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Nagy, E.; Voichita, H.; Kadar, R. The influence of Fusarium ear infection on the maize yield and quality (Transylvania-Romania). Commun. Agric. Appl. Biol. Sci. 2006, 71, 1147–1150. [Google Scholar]
- Lizárraga-Sánchez, G.J.; Leyva-Madrigal, K.Y.; Sánchez-Peña, P.; Quiroz-Figueroa, F.R.; Maldonado-Mendoza, I.E. Bacillus cereus sensu lato strain B25 controls maize stalk and ear rot in Sinaloa, Mexico. Field Crop Res. 2015, 176, 11–21. [Google Scholar] [CrossRef]
- Nagy, E.; Haş, V.; Haş, I.; Suciu, A.; Florian, V. Fusarium ear infection on the maize yield and mycotoxin content (Transylvania-Romania). Breed. Seed Sci. 2012, 64, 10. [Google Scholar] [CrossRef]
- Rosiles, M.R.; Bautista, J.; Fuentes, V.O.; Ross, F. An outbreak of equine leukoencephalomalacia at Oaxaca, Mexico, associated with fumonisin B1. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 1998, 45, 299–302. [Google Scholar] [CrossRef]
- Ross, P.F.; Nelson, P.E.; Richard, J.L.; Osweiler, G.D.; Rice, L.G.; Plattner, R.D.; Wilson, T.M. Production of fumonisins by Fusarium moniliforme and Fusarium proliferatum isolates associated with equine leukoencephalomalacia and a pulmonary edema syndrome in swine. Appl. Environ. Microbiol. 1990, 56, 3225–3226. [Google Scholar] [CrossRef]
- Riley, R.T.; Voss, K.A.; Speer, M.; Stevens, V.L.; Waes, J.G.-V. Fumonisin inhibition of ceramide synthease: A possible risk factor for human nueral tube defects. In Sphingolipid Biology, 1st ed.; Hirabayashi, Y., Igarashi, Y., Merrill, A.H., Eds.; Springer: Tokyo, Japan; Berlin/Heidelberg, Germany; New York, NY, USA, 2006; pp. 345–361. [Google Scholar]
- Vanzinni Zago, V.; Manzano-Gayosso, P.; Hernández-Hernández, F. Queratomicosis en un centro de atención oftalmológica en la Ciudad de México. Rev. Iberoam. Micol. 2010, 27, 57–61. [Google Scholar] [CrossRef]
- Galletti, J.; Negri, M.; Grassi, F.L.; Kioshima-Cotica, E.S.; Svidzinski, T.I. Fusarium spp. is able to grow and invade healthy human nails as a single source of nutrients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Mena, R.; Carrasco, E.; Godoy-Martínez, P.; Stchigel, A.M.; Cano-Lira, J.F.; Zaror, L. Un caso de queratitis micótica por Fusarium solani en Valdivia, Chile. Rev. Iberoam. Micol. 2015, 32, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize—Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Román, S.G. Caracterización de Genotipos de Maíz (Zea mays L.) a la Infección de Fusarium Verticillioides en Diferentes Fases del Ciclo de Vida de la Planta y su Correlación con Marcadores Moleculares de Tipo SNPs; Instituto Politécnico Nacional: Guasave, Sinaloa, Mexico, 2017. [Google Scholar]
- Görtz, A.; Oerke, E.C.; Steiner, U.; Waalwijk, C.; de Vries, I.; Dehne, H.W. Biodiversity of Fusarium species causing ear rot of maize in Germany. Cereal Res. Commu. 2008, 36, 617–622. [Google Scholar] [CrossRef]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef]
- Leyva-Madrigal, K.Y.; Larralde-Corona, C.P.; Apodaca-Sánchez, M.A.; Quiroz-Figueroa, F.R.; Mexia-Bolaños, P.A.; Portillo-Valenzuela, S.; Ordaz-Ochoa, J.; Maldonado-Mendoza, I.E. Fusarium species from the Fusarium fujikuroi species complex involved in mixed infections of maize in northern Sinaloa, Mexico. J. Phytopathol. 2015, 163, 486–497. [Google Scholar] [CrossRef]
- Aguin, O.; Cao, A.; Pintos, C.; Santiago, R.; Mansilla, P.; Butron, A. Occurrence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathol. 2014, 63, 946–951. [Google Scholar] [CrossRef]
- Stumpf, R.; dos Santos, J.; Gomes, L.B.; Silva, C.N.; Tessmann, D.J.; Ferreira, F.D.; Machinski, M.; Del Ponte, E.M. Fusarium species and fumonisins associated with maize kernels produced in Rio Grande do Sul State for the 2008/09 and 2009/10 growing seasons. Braz. J. Microbiol. 2013, 44, 89–95. [Google Scholar] [CrossRef]
- Ilze, B.; Lindy, J.R.; Gordon, S.S.; Bradley, C.F.; Altus, V. Mycotoxigenic Fusarium species associated with grain crops in South Africa—A review. S. Afr. J. Sci. 2017, 113, 12. [Google Scholar] [CrossRef]
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bosley, D.B.; Bradley, C.A.; Broders, K.D.; Byamukama, E.; Chilvers, M.I.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada from 2012 to 2015. Plant Health Prog. 2016, 17, 12. [Google Scholar] [CrossRef]
- Ortiz, C.S.; Richards, C.; Terry, A.; Parra, J.; Shim, W.B. Genetic variability and geographical distribution of mycotoxigenic Fusarium verticillioides strains isolated from maize fields in Texas. Plant Pathol. J. 2015, 31, 203–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- SIAP, S. Servicio de Información Agroalimentaria y Pesquera. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 11 October 2020).
- Briones-Reyes, D.; Castillo-Gonz lez, F.; Ch vez-Servia, J.L.; Aguilar-Rincón, V.; García-de Alba, C.d.L.; Ramírez-Hernández, A. Respuesta de maíz nativo del Altiplano Mexicano a pudrición de Mazorca, bajo infección natural. Agron. Mesoam. 2015, 26, 73–85. [Google Scholar] [CrossRef][Green Version]
- González Huerta, A.; Vázquez García, L.M.; Sahagún Castellanos, J.; Rodríguez Pérez, J.E.; Pérez López, D.d.J. Rendimiento del maíz de temporal y su relación con la pudrición de mazorca. Agric. Téc. Méx. 2007, 33, 33–42. [Google Scholar]
- Quintero-Benítez, J.A.; Apodaca-Sánchez, M.A. Las pudriciones de tallos de maíz y su manejo en Sinaloa. In Manejo Sustentable del Maíz; Universidad Autónoma de Sinaloa; Fundación Produce Sinaloa; SAGARPA; Gobierno del Estado de Sinaloa: Culiacán, Mexico, 2008; pp. 67–70. (In Spanish) [Google Scholar]
- Apodaca-Sánchez, M.A.; Quintero-Benítez, J.A. Pudrición de mazorca. In Manejo Sustentable del Maíz; Universidad Auntónoma de Sinaloa; Fundación Produce Sinaloa; SAGARPA; Gobierno del Estado de Sinaloa: Culiacán, Mexico, 2008; pp. 71–78. [Google Scholar]
- García Pérez, R.D.; Velarde Félix, S.; Garzón Tiznado, J.A.; Ureta Tellez, J. Distribución geográfica y caracterización molecular de Fusarium spp. en el centro-sur de Sinaloa y respuesta de variedades de maíz al ataque de este patógeno. In Avances de Investigación 2011 del CEVACU; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Mexico City, Mexico, 2012; Volume 15, pp. 15–19. [Google Scholar]
- Duncan, K.E.; Howard, R.J. Biology of maize kernel infection by Fusarium verticillioides. Mol. Plant Microbe Interact. 2010, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Dong, H.; Wang, S.; Liu, B.; Zhang, Z.; Li, X.; Gao, Z. Infection cycle of maize stalk rot and ear rot caused by Fusarium verticillioides. PLoS ONE 2018, 13, e0201588. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; McGee, D.C.; Carlton, W.M. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 1997, 87, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J. Appl. Microbiol. 2011, 111, 693–706. [Google Scholar] [CrossRef]
- Zila, C.T.; Ogut, F.; Romay, M.C.; Gardner, C.A.; Buckler, E.S.; Holland, J.B. Genome-wide association study of Fusarium ear rot disease in the U.S.A. maize inbred line collection. BMC Plant Biol. 2014, 14, 372. [Google Scholar] [CrossRef]
- Stagnati, L.; Lanubile, A.; Samayoa, L.F.; Bragalanti, M.; Giorni, P.; Busconi, M.; Holland, J.B.; Marocco, A. A genome wide association study reveals markers and genes associated with resistance to Fusarium verticillioides infection of seedlings in a maize diversity panel. G3 Genes Genom. Genet. 2019, 9, 571–579. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Lemmens, M.; Reid, L.M. Breeding for resistance to ear rots caused by Fusarium spp. in maize—A review. Plant Breed. 2012, 131, 1–19. [Google Scholar]
- Warham, E.J.; Butler, L.D.; Sutton, B.C. Seed Testing of Maize and Wheat: A Laboratory Guide; CIMMYT: Mexico City, Mexico, 1996; pp. 1–84. [Google Scholar]
- Reid, L.M.; Zhu, X. Screening Corn for Resistance to Common Diseases in Canada; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2005; pp. 1–29. [Google Scholar]
- Al-Juboory, H.; Juber, K.S. Efficiency of some inoculation methods of Fusarium proliferatum and F.verticillioides on the systemic infection and seed transmission on maize under field conditions. Agric. Biol. J. N. Am. 2013, 4, 583–589. [Google Scholar] [CrossRef]
- Clements, M.J.; Kleinschmidt, C.E.; Maragos, C.M.; Pataky, J.K.; White, D.G. Evaluation of inoculation techniques for Fusarium ear rot and fumonisin contamination of corn. Plant Dis. 2003, 87, 147–153. [Google Scholar] [CrossRef]
- Mario, J.L.; Reis, E.M.; Juliatti, F.C. Three inoculation methods for screening corn germplasm to white ear rot resistance. Trop. Plant Pathol. 2011, 36, 362–366. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Ltd.: Hoboken, NJ, USA; Oxford, UK; Milton, Australia, 2007; Volume 2, pp. 1–369. [Google Scholar]
- García Espinoza, J.A. Asociación de Fusarium Verticillioides (Sacc.) Nir. a Semilla de Zea Mays L. en Sinaloa y su Control In Vitro Mediante Procloraz. Escuela Superior de Agricultura del Valle del Fuerte; Universidad Autónoma de Sinaloa: Juan José Ríos, Sinaloa, Mexico, 2009. [Google Scholar]
- Soonthornpoct, P.; Trevathan, L.E.; Gonzalez, M.S.; Tomaso-Peterson, M. Fungal occurrence, disease incidence and severity, and yield of maize symptomatic for seedling disease in Mississippi. Mycopathologia 2001, 150, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Intern. 2004, 11, 36–41. [Google Scholar]
- Munkvold, G.P.; Hellmich, R.L.; Showers, W.B. Reduced Fusarium ear rot and symptomless infection in kernels of maize genetically engineered for European corn borer resistance. Phytopathology 1997, 87, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.D.; Buddenhagen, I.W. Incidence and persistence of Fusarium moniliforme in symptomless maize kernels and seedlings in Nigeria. Mycologia 1980, 72, 882–887. [Google Scholar] [CrossRef]
- Quesada-Ocampo, L.M.; Al-Haddad, J.; Scruggs, A.C.; Buell, C.R.; Trail, F. Susceptibility of maize to stalk rot caused by Fusarium graminearum deoxynivalenol and zearalenone mutants. Phytopathology 2016, 106, 920–927. [Google Scholar] [CrossRef]
- Borah, S.N.; Deka, S.; Sarma, H.K. First Report of Fusarium verticillioides causing stalk rot of maize in Assam, India. Plant Dis. 2016, 100, 1501. [Google Scholar] [CrossRef]
- Shin, J.H.; Han, J.H.; Lee, J.K.; Kim, K.S. Characterization of the maize stalk rot pathogens Fusarium subglutinans and F. temperatum and the effect of fungicides on their mycelial growth and colony formation. Plant Pathol. J. 2014, 30, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to Fusarium ear rot in maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.; Butron, A. Genetic factors involved in fumonisin accumulation in maize kernels and their implications in maize agronomic management and breeding. Toxins 2015, 7, 3267–3296. [Google Scholar] [CrossRef]
- Yates, I.E.; Bacon, C.W.; Hinton, D.M. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 1997, 81, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.A.; Fessel, S.A.; Panizzi, R.C. Effect of Fusarium graminearum and infection index on germination and vigor of maize seeds. Fitopatol. Bras. 2005, 30, 5. [Google Scholar] [CrossRef][Green Version]
- Troncoso, C.; Gonzalez, X.; Bomke, C.; Tudzynski, B.; Gong, F.; Hedden, P.; Rojas, M.C. Gibberellin biosynthesis and gibberellin oxidase activities in Fusarium sacchari, Fusarium konzum and Fusarium subglutinans strains. Phytochemistry 2010, 71, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Kaufman, P.B. The role of gibberellins in plant cell elongation. Crit. Rev. Plant Sci. 1983, 1, 23–47. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Catalysts of plant cell wall loosening. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Quiroz-Figueroa, F.R.; Quiroz-Chávez, J.; García-Pérez, L.M.; Roman, S.G. Mejoramiento genético del maíz el caso de la fusariosis. Cienc. Y Desarro. 2016, 281, 41–49. [Google Scholar]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Kenganal, M.; Patil, M.B.; Nimbaragi, Y. Management of stalk rot of maize caused by Fusarium moniliforme (Sheldon). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 7. [Google Scholar]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize—Present status and future prospects. Maydica 2014, 59, 16. [Google Scholar]
- Christensen, J.J.; Wilcoxson, R.D. Stalk Rot of Corn; The American Phytopathlogical Society: Saint Paul, MN, USA, 1966; Volume 3, pp. 1–59. [Google Scholar]
- Gopala; Gogoi, R.; Hooda, K.S.; Rai, S.N.; Kumar, A.; Hossain, F. Rapid screening technique for evaluation of maize genotypes against stalk rot complex caused by Macrophomina phaseolina and Fusarium verticilloides. Indian J. Agr. Sci. 2016, 86, 1024–1030. [Google Scholar]
- Yang, D.E.; Zhang, C.L.; Zhang, D.S.; Jin, D.M.; Weng, M.L.; Chen, S.J.; Nguyen, H.; Wang, B. Genetic analysis and molecular mapping of maize (Zea mays L.) stalk rot resistant gene Rfg1. Appl. Genet. 2004, 108, 706–711. [Google Scholar] [CrossRef]
- Yang, D.E.; Jin, D.M.; Wang, B.; Zhang, D.S.; Nguyen, H.T.; Zhang, C.L.; Chen, S.J. Characterization and mapping of Rpi1, a gene that confers dominant resistance to stalk rot in maize. Mol. Genet. Genom. 2005, 274, 229–234. [Google Scholar] [CrossRef]
- Yang, Q.; Yin, G.; Guo, Y.; Zhang, D.; Chen, S.; Xu, M. A major QTL for resistance to Gibberella stalk rot in maize. Appl. Genet. 2010, 121, 673–687. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Du, W.P.; Xu, L.Y.; Jiang, Y.; Zhang, J.; Xiang, X.L.; Yu, G.R. Identification, mapping, and molecular marker development for Rgsr8.1: A new quantitative trait locus conferring resistance to gibberella stalk rot in maize (Zea mays L.). Front Plant Sci. 2017, 8, 1355. [Google Scholar] [CrossRef]
- Ma, C.Y.; Ma, X.N.; Yao, L.S.; Liu, Y.J.; Du, F.L.; Yang, X.H.; Xu, M.L. Qrfg3, a novel quantitative resistance locus against Gibberella stalk rot in maize. Appl. Genet. 2017, 130, 1723–1734. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.; Wang, W.; Li, Y.; Guo, Y.; Zhang, D.; Ma, X.; Song, W.; Zhao, J.; Xu, M. A transposon-directed epigenetic change in ZmCCT underlies quantitative resistance to Gibberella stalk rot in maize. New Phytol. 2017, 215, 1503–1515. [Google Scholar] [CrossRef]
- Mendoza Elos, M.; Andrio Enríquez, E.; López Benítez, A.; Rodríguez Guerra, R.; Latournerie Moreno, L.; Rodríguez Herrera, S.A. Tasa de infección de la pudrición del tallo en maíz causada por Fusarium moniliforme. Agron. Mesoam. 2006, 17, 6. [Google Scholar] [CrossRef]
- Presello, D.A.; Iglesias, J.; Botta, G.; Reid, L.M.; Lori, G.A.; Eyhérabide, G.H. Stability of maize resistance to the ear rots caused by Fusarium graminearum and F. verticillioides in Argentinian and Canadian environments. Euphytica 2006, 147, 403–407. [Google Scholar] [CrossRef]
- Ding, J.Q.; Wang, X.M.; Chander, S.; Yan, J.B.; Li, J.S. QTL mapping of resistance to Fusarium ear rot using a RIL population in maize. Mol. Breed. 2008, 22, 395–403. [Google Scholar] [CrossRef]
- Robertson-Hoyt, L.A.; Jines, M.P.; Balint-Kurti, P.J.; Kleinschmidt, C.E.; White, D.G.; Payne, G.A.; Maragos, C.M.; Molnr, T.L.; Holland, J.B. QTL mapping for Fusarium ear rot and fumonisin contamination resistance in two maize populations. Crop Sci. 2006, 46, 1734–1743. [Google Scholar] [CrossRef]
- Ono, E.Y.S.; Biazon, L.; da Silva, M.; Vizoni, E.; Sugiura, Y.; Ueno, Y.; Hirooka, E.Y. Fumonisins in corn: Correlation with Fusarium sp. count, damaged kernels, protein and lipid content. Braz. Arch. Biol. Techn. 2006, 49, 63–71. [Google Scholar] [CrossRef]
- Hoenisch, R.W.; Davis, R.M. Relationship between Kernel Pericarp Thickness and Susceptibility to Fusarium Ear Rot in-Field Corn. Plant Dis. 1994, 78, 517–519. [Google Scholar] [CrossRef]
- Sen, A.; Bergvinson, D.; Miller, S.S.; Atkinson, J.; Fulcher, R.G.; Arnason, J.T. Distribution and microchemical detection of phenolic-acids, flavonoids, and phenolic-acid amides in maize kernels. J. Agric. Food Chem. 1994, 42, 1879–1883. [Google Scholar] [CrossRef]
- Bily, A.C.; Reid, L.M.; Taylor, J.H.; Johnston, D.; Malouin, C.; Burt, A.J.; Bakan, B.; Regnault-Roger, C.; Pauls, K.P.; Arnason, J.T.; et al. Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: Resistance factors to Fusarium graminearum. Phytopathology 2003, 93, 712–719. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalan, C.A.N. Phenylpropanoids from maize pericarp: Resistance factors to kernel infection and fumonisin accumulation by Fusarium verticillioides. Eur. J. Plant Pathol. 2013, 135, 105–113. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic profile and susceptibility to Fusarium infection of pigmented maize cultivars. Front. Plant. Sci. 2018, 9, 1189. [Google Scholar] [CrossRef]
- Rosas Jáuregui, M.G. Evaluación de Parentrales de Maíz (Zea mays L.) a la Infección por el Fitopatógeno Fusarium Nygamai Bajo Condiciones de Invernadero; Instituto Tecnológico del Vaye del Yaqui: San Ignacio Río Muerto, Sonora, Mexico, 2016. [Google Scholar]
| Stage of Development | ||||
|---|---|---|---|---|
| Genotypes | V2 (Seedling) | V4 (Plantlet) | R1 (Stalk) | R6 (Ears) |
| IL1 | Tolerant | Tolerant | Resistant | Resistant |
| IL2 | Highly tolerant | Tolerant | Resistant | Resistant |
| IL10 | Highly susceptible | Susceptible | Susceptible | Resistant |
| IL11 | Susceptible | Highly susceptible | Susceptible | Susceptible |
| IL12 | Susceptible | Susceptible | Susceptible | Susceptible |
| IL14 | Highly tolerant | Tolerant | Resistant | Resistant |
| IL17 | Highly tolerant | Tolerant | Resistant | Resistant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román, S.G.; Quiroz-Chávez, J.; Villalobos, M.; Urías-Gutiérrez, V.; Nava-Pérez, E.; Ruíz-May, E.; Singh, R.K.; Sharma, L.; Quiroz-Figueroa, F.R. A Global Screening Assay to Select for Maize Phenotypes with a High Tolerance or Resistance to Fusarium verticillioides (Sacc.) Nirenberg Rots. Agronomy 2020, 10, 1990. https://doi.org/10.3390/agronomy10121990
Román SG, Quiroz-Chávez J, Villalobos M, Urías-Gutiérrez V, Nava-Pérez E, Ruíz-May E, Singh RK, Sharma L, Quiroz-Figueroa FR. A Global Screening Assay to Select for Maize Phenotypes with a High Tolerance or Resistance to Fusarium verticillioides (Sacc.) Nirenberg Rots. Agronomy. 2020; 10(12):1990. https://doi.org/10.3390/agronomy10121990
Chicago/Turabian StyleRomán, Shamir Gabriel, Jesús Quiroz-Chávez, Miguel Villalobos, Vianey Urías-Gutiérrez, Eusebio Nava-Pérez, Eliel Ruíz-May, Rupesh Kumar Singh, Lav Sharma, and Francisco Roberto Quiroz-Figueroa. 2020. "A Global Screening Assay to Select for Maize Phenotypes with a High Tolerance or Resistance to Fusarium verticillioides (Sacc.) Nirenberg Rots" Agronomy 10, no. 12: 1990. https://doi.org/10.3390/agronomy10121990
APA StyleRomán, S. G., Quiroz-Chávez, J., Villalobos, M., Urías-Gutiérrez, V., Nava-Pérez, E., Ruíz-May, E., Singh, R. K., Sharma, L., & Quiroz-Figueroa, F. R. (2020). A Global Screening Assay to Select for Maize Phenotypes with a High Tolerance or Resistance to Fusarium verticillioides (Sacc.) Nirenberg Rots. Agronomy, 10(12), 1990. https://doi.org/10.3390/agronomy10121990







