Long-Term Effects of Organic Amendments on Soil Organic Matter Quantity and Quality in Conventional Cropping Systems in Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Characteristics and Agronomic Practices
2.2. Experimental Design and Soil Sampling
2.3. Chemical Characterization of Organic Amendments
2.4. Characterization of Bulk SOM and Soil Particle-Size Fractions
2.5. Enzyme Assay
2.6. Statistical Analysis
3. Results
3.1. Chemical Characterization of Organic Amendments
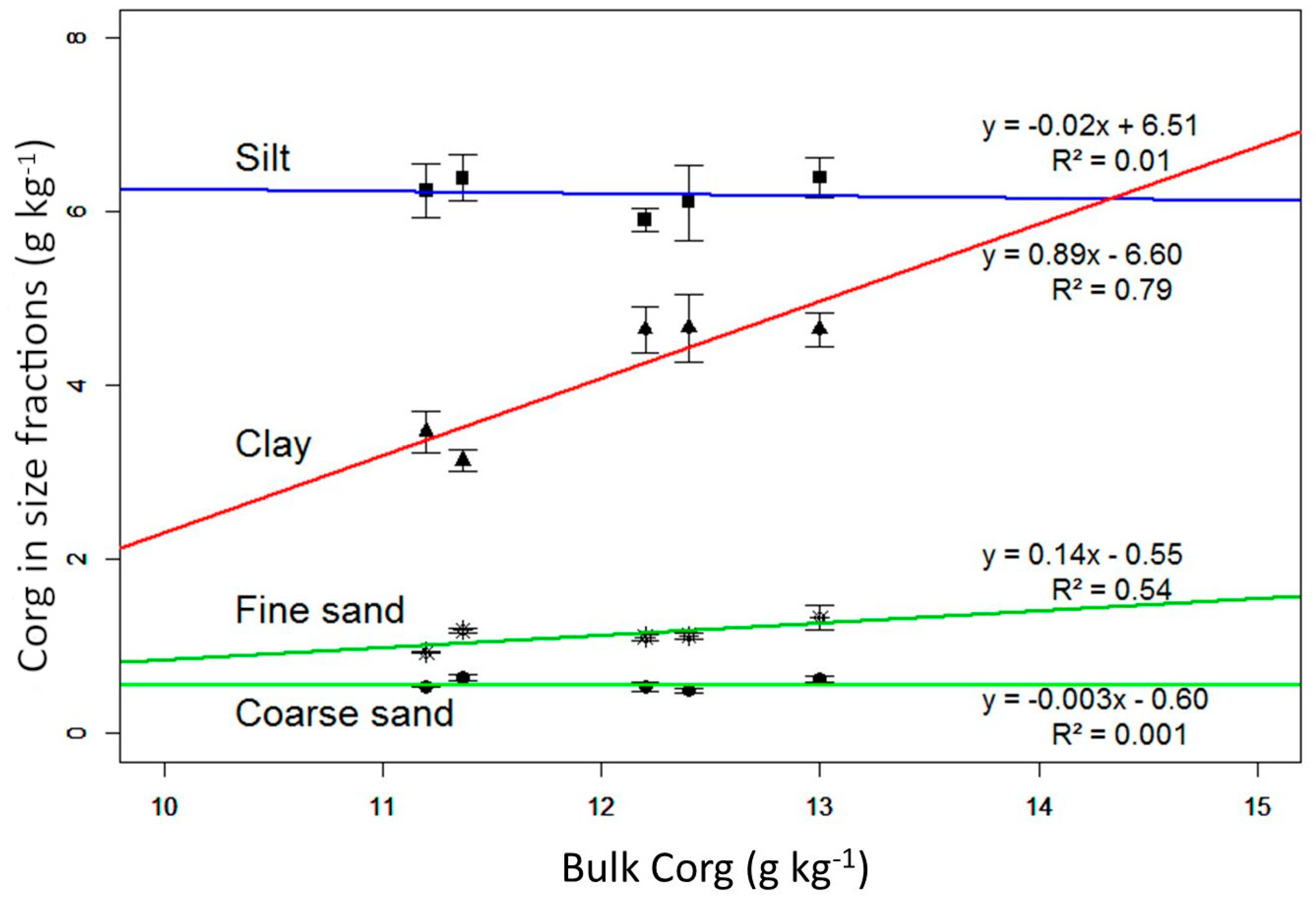
3.2. Organic Carbon Content and Partitioning into Soil Particle-Size Fractions
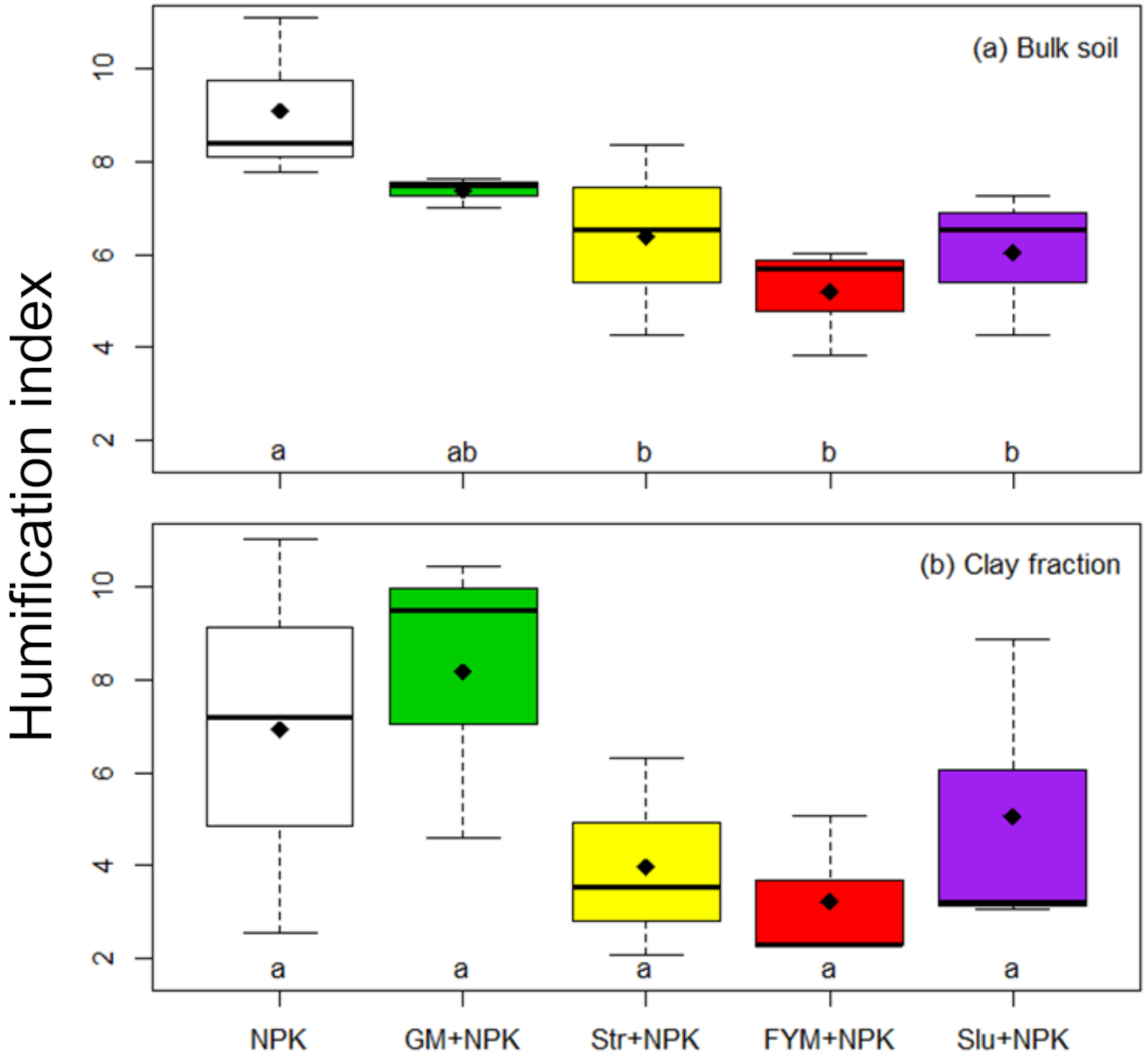
3.3. Soil Enzymatic Activity and Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Digging deeper: A holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Amelung, W.; Bossio, D.; Vries, W. De Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 1–10. [Google Scholar] [CrossRef]
- Keel, S.G.; Anken, T.; Büchi, L.; Chervet, A.; Fliessbach, A.; Flisch, R.; Huguenin-Elie, O.; Mäder, P.; Mayer, J.; Sinaj, S.; et al. Loss of soil organic carbon in Swiss long-term agricultural experiments over a wide range of management practices. Agric. Ecosyst. Environ. 2019, 286, 106654. [Google Scholar] [CrossRef]
- Guillaume, T.; Bragazza, L.; Levasseur, C.; Libohova, Z.; Sinaj, S. Long-term soil organic carbon dynamics in temperate cropland-grassland systems. Agric. Ecosyst. Environ. 2021, 305. [Google Scholar] [CrossRef]
- Fließbach, A.; Oberholzer, H.R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Benbi, D.K.; Toor, A.S.; Kumar, S. Management of organic amendments in rice-wheat cropping system determines the pool where carbon is sequestered. Plant Soil 2012, 360, 145–162. [Google Scholar] [CrossRef]
- Blanchet, G.; Gavazov, K.; Bragazza, L.; Sinaj, S. Responses of soil properties and crop yields to different inorganic and organic amendments in a Swiss conventional farming system. Agric. Ecosyst. Environ. 2016, 230, 116–126. [Google Scholar] [CrossRef]
- Lal, R. Challenges and opportunities in soil organic matter research. Eur. J. Soil Sci. 2009, 60, 158–169. [Google Scholar] [CrossRef]
- Maltas, A.; Kebli, H.; Oberholzer, H.R.; Weisskopf, P.; Sinaj, S. The effects of organic and mineral fertilizers on carbon sequestration, soil properties, and crop yields from a long-term field experiment under a Swiss conventional farming system. Land Degrad. Dev. 2018, 29, 926–938. [Google Scholar] [CrossRef]
- Šimon, T.; Mikanová, O.; Cerhanová, D. Long-term effect of straw and farmyard manure on soil organic matter in field experiment in the Czech Republic. Arch. Agron. Soil Sci. 2013, 59, 1193–1205. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019. [Google Scholar] [CrossRef]
- Bai, X.; Huang, Y.; Ren, W.; Coyne, M.; Jacinthe, P.A.; Tao, B.; Hui, D.; Yang, J.; Matocha, C. Responses of soil carbon sequestration to climate-smart agriculture practices: A meta-analysis. Glob. Chang. Biol. 2019, 25, 2591–2606. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 130. [Google Scholar]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2019, 261–273. [Google Scholar] [CrossRef]
- Haddix, M.L.; Gregorich, E.G.; Helgason, B.L.; Janzen, H.; Ellert, B.H.; Francesca Cotrufo, M. Climate, carbon content, and soil texture control the independent formation and persistence of particulate and mineral-associated organic matter in soil. Geoderma 2020, 363, 114160. [Google Scholar] [CrossRef]
- Six, J.; Feller, C.; Denef, K.; Ogle, S.M.; de Morales Sa, J.C.; Albrect, A. Soil organic matter, biota and aggregation in temperate and tropical soils—Effects of no-tillage. Agronomie 2002, 22, 755–775. [Google Scholar] [CrossRef]
- von Lutzow, M.; Kogel-Knabner, I.; Ekschmittb, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.F.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018. [Google Scholar] [CrossRef]
- Giannetta, B.; Plaza, C.; Vischetti, C.; Cotrufo, M.F.; Zaccone, C. Distribution and thermal stability of physically and chemically protected organic matter fractions in soils across different ecosystems. Biol. Fertil. Soils 2018, 54, 671–681. [Google Scholar] [CrossRef]
- Courtier-Murias, D.; Simpson, A.J.; Marzadori, C.; Baldoni, G.; Ciavatta, C.; Fernández, J.M.; López-de-Sá, E.G.; Plaza, C. Unraveling the long-term stabilization mechanisms of organic materials in soils by physical fractionation and NMR spectroscopy. Agric. Ecosyst. Environ. 2013, 171, 9–18. [Google Scholar] [CrossRef]
- Hassink, J. A Model of the Physical Protection of Organic Matter in Soils The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Ranalli, M.G.; Haddix, M.L.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Dexter, A.R.; Richard, G.; Arrouays, D.; Czyz, E.A.; Jolivet, C.; Duval, O. Complexed organic matter controls soil physical properties. Geoderma 2008, 144, 620–627. [Google Scholar] [CrossRef]
- Keller, T.; Dexter, A.R. Plastic limits of agricultural soils as functions of soil texture and organic matter content. Soil Res. 2012, 50, 7–17. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Johannes, A.; Matter, A.; Schulin, R.; Weisskopf, P.; Baveye, P.C.; Boivin, P. Optimal organic carbon values for soil structure quality of arable soils. Does clay content matter? Geoderma 2017, 302, 14–21. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Chang. Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Xu, X.; Hu, Y.; Blagodatskaya, E.; Liu, Y.; Schaefer, D.; Kuzyakov, Y. Carbon and nitrogen additions induce distinct priming effects along an organic-matter decay continuum. Sci. Rep. 2016, 6, 19865. [Google Scholar] [CrossRef] [PubMed]
- Dippold, M.; Biryukov, M.; Kuzyakov, Y. Sorption affects amino acid pathways in soil: Implications from position-specific labeling of alanine. Soil Biol. Biochem. 2014, 72, 180–192. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Ding, G.; Novak, J.M.; Amarasiriwardena, D.; Hunt, P.G.; Xing, B. Soil Organic Matter Characteristics as Affected by Tillage Management. Soil Sci. Soc. Am. J. 2002, 66, 421–429. [Google Scholar] [CrossRef]
- Veum, K.S.; Goyne, K.W.; Kremer, R.J.; Miles, R.J.; Sudduth, K.A. Biological indicators of soil quality and soil organic matter characteristics in an agricultural management continuum. Biogeochemistry 2014, 117, 81–99. [Google Scholar] [CrossRef]
- Margenot, A.J.; Calderón, F.J.; Bowles, T.M.; Parikh, S.J.; Jackson, L.E. Soil Organic Matter Functional Group Composition in Relation to Organic Carbon, Nitrogen, and Phosphorus Fractions in Organically Managed Tomato Fields. Soil Sci. Soc. Am. J. 2015, 79, 772. [Google Scholar] [CrossRef]
- Maillard, É.; Angers, D.A. Animal manure application and soil organic carbon stocks: A meta-analysis. Glob. Chang. Biol. 2014, 20, 666–679. [Google Scholar] [CrossRef]
- Samson, M.É.; Chantigny, M.H.; Vanasse, A.; Menasseri-Aubry, S.; Angers, D.A. Coarse mineral-associated organic matter is a pivotal fraction for SOM formation and is sensitive to the quality of organic inputs. Soil Biol. Biochem. 2020, 149. [Google Scholar] [CrossRef]
- Jilling, A.; Kane, D.; Williams, A.; Yannarell, A.C.; Davis, A.; Jordan, N.R.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; et al. Rapid and distinct responses of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma 2020, 359, 114001. [Google Scholar] [CrossRef]
- Häni, F.J.; Sarthou, V. Protection des Plantes en Production Intégrée: Grandes Cultures: Maladies, Ravageurs, Auxiliaires; LmZ—Centrale des moyens d’enseignement agricole: Zollikofen, Switzerland, 2004. [Google Scholar]
- Sinaj, S.; Richner, W. Principes de fertilisation des cultures agricoles en Suisse (PRIF 2017). Rech. Agron. Suisse Publ. Spéciale 2017, 8, 276. [Google Scholar]
- von Haden, A.C.; Yang, W.H.; DeLucia, E.H. Soils’ dirty little secret: Depth-based comparisons can be inadequate for quantifying changes in soil organic carbon and other mineral soil properties. Glob. Chang. Biol. 2020, 26, 3759–3770. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, M.; Gerzabek, M.H.; Kandeler, E. Organic matter and enzyme activity in particle-size fractions of soils obtained after low-energy sonication. Soil Biol. Biochem. 1998, 30, 9–17. [Google Scholar] [CrossRef]
- Antil, R.S.; Gerzabek, M.H.; Haberhauer, G.; Eder, G. Long-term effects of cropped vs. fallow and fertilizer amendments on soil organic matter. I. Organic carbon. J. Plant Nutr. Soil Sci. 2005, 168, 108–116. [Google Scholar] [CrossRef]
- Poppe, L.J.; Fredericks, J.J.; Hathaway, J.C. A computer program to calculate centrifugation parameters for sedimentation analyses. Comput. Geosci. 1988, 14, 541–545. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Müller, T.; Cadisch, G. Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Reeves, J.B. Near- versus mid-infrared diffuse reflectance spectroscopy for soil analysis emphasizing carbon and laboratory versus on-site analysis: Where are we and what needs to be done? Geoderma 2010, 158, 3–14. [Google Scholar] [CrossRef]
- González Pérez, M.; Martin-Neto, L.; Saab, S.C.; Novotny, E.H.; Milori, D.M.B.P.; Bagnato, V.S.; Colnago, L.A.; Melo, W.J.; Knicker, H. Characterization of humic acids from a Brazilian Oxisol under different tillage systems by EPR, 13C NMR, FTIR and fluorescence spectroscopy. Geoderma 2004, 118, 181–190. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Shahbaz, M.; Menichetti, L.; Kätterer, T.; Börjesson, G. Impact of long-term N fertilisation on CO2 evolution from old and young SOM pools measured during the maize cropping season. Sci. Total Environ. 2019, 658, 1539–1548. [Google Scholar] [CrossRef]
- Lemke, R.L.; VandenBygaart, A.J.; Campbell, C.A.; Lafond, G.P.; Grant, B. Crop residue removal and fertilizer N: Effects on soil organic carbon in a long-term crop rotation experiment on a Udic Boroll. Agric. Ecosyst. Environ. 2010, 135, 42–51. [Google Scholar] [CrossRef]
- Taghizadeh-Toosi, A.; Olesen, J.E. Modelling soil organic carbon in Danish agricultural soils suggests low potential for future carbon sequestration. Agric. Syst. 2016, 145, 83–89. [Google Scholar] [CrossRef]
- Emmel, C.; Winkler, A.; Hörtnagl, L.; Revill, A.; Ammann, C.; D’Odorico, P.; Buchmann, N.; Eugster, W. Integrated management of a Swiss cropland is not sufficient to preserve its soil carbon pool in the long term. Biogeosciences 2018, 15, 5377–5393. [Google Scholar] [CrossRef]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Johannes, A.; Weisskopf, P.; Schulin, R.; Boivin, P. Soil structure quality indicators and their limit values. Ecol. Indic. 2019. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Garcia Cardenas, M.; Kaonga, M.; Koutika, L.S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 2020, 49, 350–360. [Google Scholar] [CrossRef]
- Marschner, B.; Kalbitz, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L.; García-Martínez, A.M.; Parrado, J. Application of a green manure and green manure composted with beet vinasse on soil restoration: Effects on soil properties. Bioresour. Technol. 2008, 99, 4949–4957. [Google Scholar] [CrossRef]
- Bertrand, I.; Viaud, V.; Daufresne, T.; Pellerin, S.; Recous, S. Stoichiometry constraints challenge the potential of agroecological practices for the soil C storage. A review. Agron. Sustain. Dev. 2019, 39. [Google Scholar] [CrossRef]
- Rüegg, J.; Quezada, J.C.; Santonja, M.; Ghazoul, J.; Kuzyakov, Y.; Buttler, A.; Guillaume, T. Drivers of soil carbon stabilization in oil palm plantations. Land Degrad. Dev. 2019, 1–12. [Google Scholar] [CrossRef]
- Finn, D.; Page, K.; Catton, K.; Kienzle, M.; Robertson, F.; Armstrong, R.; Dalal, R. Ecological stoichiometry controls the transformation and retention of plant-derived organic matter to humus in response to nitrogen fertilisation. Soil Biol. Biochem. 2016, 99, 117–127. [Google Scholar] [CrossRef]
- Loeppmann, S.; Blagodatskaya, E.; Pausch, J.; Kuzyakov, Y. Enzyme properties down the soil profile—A matter of substrate quality in rhizosphere and detritusphere. Soil Biol. Biochem. 2016, 103, 274–283. [Google Scholar] [CrossRef]
- He, H.; Zhang, W.; Zhang, X.; Xie, H.; Zhuang, J. Temporal responses of soil microorganisms to substrate addition as indicated by amino sugar differentiation. Soil Biol. Biochem. 2011, 43, 1155–1161. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Chen, J.; Dippold, M.A.; Seven, J.; Zilla, T.; Blagodatskaya, E.; Kuzyakov, Y. Microbial C:N:P stoichiometry and turnover depend on nutrients availability in soil: A 14C, 15N and 33P triple labelling study. Soil Biol. Biochem. 2019. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, Z.; Xu, X.; Liu, S.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.; Ge, T. Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]




| Treatment | Organic Amendments | Mineral Fertilization (kg·ha−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type of Amendments | Application Frequency | Chemical Properties (g·kg−1 DM) | Total C Input since 1976 (t·ha−1) | Crop Type | ||||||
| C-org | N-tot | P-tot | Wheat | Barley/ Spring Oat | Rapeseed | Maize | ||||
| NPK | - | - | - | - | - | - | 110 N 30.5 P 99.6 K | 70 N 30.5 P 99.6 K | 110 N 30.5 P 99.6 K | 110 N 30.5 P 99.6 K |
| GM + NPK | Mustard (Brassica juncea) incorporation, typically cultivated between two cropping seasons | Every 2 years | 446.3 | 16.7 | 3.4 | 20.4 | 110 N 30.5 P 99.6 K | 70 N 30.5 P 99.6 K | 110 N 30.5 P 99.6 K | 110 N 30.5 P 99.6 K |
| Str + NPK | Cereal straw restitution (wheat, barley and oat) | Years with cereal | 450.7 | 4.5 | 1.1 | 53.0 | 110 N 26.2 P 66.4 K | 70 N 26.2 P 66.4 K | 110 N 26.2 P 66.4 K | 110 N 26.2 P 66.4 K |
| FYM + NPK | Farmyard manure (35 t/ha) | Every 3 years | 424.0 * | 19.6 | 12.6 | 37.0 | 110 N 21.8 P 66.4 K | 70 N 21.8 P 66.4 K | 110 N 0 P 0 K | 110 N 0 P 0 K |
| Slu + NPK | Cattle slurry (60 m3/ha) | 1976–1993: every year 1994-present: every 3 years | 410.0 * | 26.0 | 19.1 | 20.4 | 110 N 30.5 P 58.1 K | 70 N 30.5 P 58.1 K | 110 N 0 P 0 P | 110 N 0 P 0 K |
| Treatment | Corg | C stocks | C/N | pH | CEC | K-tot | Mg-tot | P-tot | K-EDTA | Mg-EDTA | P-EDTA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g·kg−1 | Mg·ha−1 | H2O | cmol + kg−1 | g·kg−1 | Mg·kg−1 | |||||||||||||||||
| NPK | 11.20 a | ±0.09 | 32.93 a | ±0.26 | 9.84 a | ±0.07 | 6.93 a | ±0.68 | 7.97 a | ±0.54 | 15.03 a | ±0.47 | 6.67 a | ±0.59 | 0.82 a | ±0.02 | 183.00 a | ±14.73 | 63.27 a | ±12.45 | 51.39 a | ±19.32 |
| GM + NPK | 11.37 a | ±0.27 | 33.43 a | ±0.79 | 9.89 a | ±0.07 | 6.50 a | ±0.17 | 7.85 a | ±1.30 | 15.23 a | ±1.55 | 6.70 a | ±1.33 | 0.77 a | ±0.02 | 191.33 a | ±15.50 | 70.53 a | ±14.08 | 31.20 ab | ±3.98 |
| Str + NPK | 12.20 a | ±0.40 | 35.87 a | ±1.18 | 10.03 a | ±0.02 | 6.90 a | ±0.10 | 7.85 a | ±0.88 | 14.77 a | ±0.51 | 6.37 a | ±0.58 | 0.81 a | ±0.02 | 177.00 a | ±21.07 | 69.07 a | ±6.04 | 44.78 ab | ±1.94 |
| FYM + NPK | 13.00 a | ±0.53 | 38.22 a | ±1.56 | 10.10 a | ±0.10 | 7.00 a | ±0.56 | 8.19 a | ±1.19 | 15.03 a | ±1.00 | 6.57 a | ±0.94 | 0.82 a | ±0.03 | 221.67 a | ±49.65 | 83.63 a | ±26.34 | 50.63 ab | ±15.89 |
| Slu + NPK | 12.40 a | ±0.60 | 36.46 a | ±1.76 | 9.71 a | ±0.06 | 6.90 a | ±0.66 | 8.58 a | ±2.09 | 15.60 a | ±1.47 | 7.10 a | ±1.44 | 0.79 a | ±0.02 | 190.67 a | ±26.41 | 83.17 a | ±13.32 | 29.46 b | ±6.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koishi, A.; Bragazza, L.; Maltas, A.; Guillaume, T.; Sinaj, S. Long-Term Effects of Organic Amendments on Soil Organic Matter Quantity and Quality in Conventional Cropping Systems in Switzerland. Agronomy 2020, 10, 1977. https://doi.org/10.3390/agronomy10121977
Koishi A, Bragazza L, Maltas A, Guillaume T, Sinaj S. Long-Term Effects of Organic Amendments on Soil Organic Matter Quantity and Quality in Conventional Cropping Systems in Switzerland. Agronomy. 2020; 10(12):1977. https://doi.org/10.3390/agronomy10121977
Chicago/Turabian StyleKoishi, Ayumi, Luca Bragazza, Alexandra Maltas, Thomas Guillaume, and Sokrat Sinaj. 2020. "Long-Term Effects of Organic Amendments on Soil Organic Matter Quantity and Quality in Conventional Cropping Systems in Switzerland" Agronomy 10, no. 12: 1977. https://doi.org/10.3390/agronomy10121977
APA StyleKoishi, A., Bragazza, L., Maltas, A., Guillaume, T., & Sinaj, S. (2020). Long-Term Effects of Organic Amendments on Soil Organic Matter Quantity and Quality in Conventional Cropping Systems in Switzerland. Agronomy, 10(12), 1977. https://doi.org/10.3390/agronomy10121977





