Complete Mitochondrial Genome and a Set of 10 Novel Kompetitive Allele-Specific PCR Markers in Ginseng (Panax ginseng C. A. Mey.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, DNA Extraction, and Sequencing
2.2. Mitogenome Assembly and Annotation
2.3. Comparative Analysis
2.4. KASP Marker Development
2.5. KASP Marker Application
3. Results
3.1. Complete Mitogenome Sequence of P. ginseng
3.2. Structure and Variation in Ginseng Mitogenome
3.3. Development and Validation of KASP Markers
3.4. Genetic Diversity in Ginseng
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Yun, T.K. Brief introduction of Panax ginseng C. A. Meyer. J. Korean Med. Sci. 2001, 16 (Suppl. S3–S5). [Google Scholar] [CrossRef]
- Baeg, I.H.; So, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng. Res. 2013, 37, 1–7. [Google Scholar] [CrossRef]
- Quan, F.S.; Compans, R.W.; Cho, Y.-K.; Kang, S.-M. Ginseng and Salviae herbs play a role as immune activators and modulate immune responses during influenza virus infection. Vaccine 2007, 25, 272–282. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Park, K.-K.; Lee, J.-M.; Chun, K.-S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surh, Y.-J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000, 150, 41–48. [Google Scholar] [CrossRef]
- Lee, H.-S.; Kim, M.-R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: In vitro and animal study. J. Med. Food 2012, 15, 1015–1023. [Google Scholar] [CrossRef]
- Jimenez-Perez, Z.E.; Singh, P.; Kim, Y.J.; Mathiyalagan, R.; Kim, D.H.; Lee, M.H.; Yang, D.C. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. Ginseng. Res. 2018, 42, 327–333. [Google Scholar] [CrossRef]
- Tamamoto, L.C.; Schmidt, S.J.; Lee, S.Y. Sensory profile of a model energy drink with varying levels of functional ingredients-caffeine, ginseng, and taurine. J. Food Sci. 2010, 75, S271–S278. [Google Scholar] [CrossRef]
- Choi, K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C. A. Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef]
- Mackenzie, S.; McIntosh, L. Higher plant mitochondria. Plant Cell 1999, 11, 571–586. [Google Scholar] [CrossRef]
- Birky, C.W., Jr. The inheritance of genes in mitochondria and chloroplasts: Laws, mechanisms, and models. Ann. Rev. Genet. 2001, 35, 125–148. [Google Scholar] [CrossRef]
- Ward, B.L.; Anderson, R.S.; Bendich, A.J. The mitochondrial genome is large and variable in a family of plants (Cucurbitaceae). Cell 1981, 25, 793–803. [Google Scholar] [CrossRef]
- Mower, J.; Sloan, D.; Alverson, A. Plant mitochondrial diversity–the genomics revolution. In Plant Genome Diversity; Wendel, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sugiyama, Y.; Watase, Y.; Nagase, M.; Makita, N.; Yagura, S.; Hirai, A.; Sugiura, M. The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: Comparative analysis of mitochondrial genomes in higher plants. Mol. Genet. Genom. 2005, 272, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Varre, J.S.; D’Agostino, N.; Touzet, P.; Gallina, S.; Tamburino, R.; Cantarella, C.; Ubrig, E.; Cardi, T.; Drouard, L.; Gualberto, J.M.; et al. Complete Sequence, Multichromosomal Architecture and Transcriptome Analysis of the Solanum tuberosum Mitochondrial Genome. Int. J. Mol. Sci. 2019, 20, 4788. [Google Scholar] [CrossRef] [PubMed]
- Alverson, A.J.; Rice, D.W.; Dickinson, S.; Barry, K.; Palmer, J.D. Origins and recombination of the bacterial-sized multichromosomal mitochondrial genome of cucumber. Plant Cell 2011, 23, 2499–2513. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Li, W.-H.; Sharp, P.M. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 9054–9058. [Google Scholar] [CrossRef]
- Hiesel, R.; von Haeseler, A.; Brennicke, A. Plant mitochondrial nucleic acid sequences as a tool for phylogenetic analysis. Proc. Natl. Acad. Sci. USA 1994, 91, 634–638. [Google Scholar] [CrossRef]
- Bi, C.; Paterson, A.H.; Wang, X.; Xu, Y.; Wu, D.; Qu, Y.; Jiang, A.; Ye, Q.; Ye, N. Analysis of the complete mitochondrial genome sequence of the diploid cotton Gossypium raimondii by comparative genomics approaches. BioMed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Kim, N.H.; Jayakodi, M.; Lee, S.C.; Choi, B.S.; Jang, W.; Lee, J.; Kim, H.H.; Waminal, N.E.; Lakshmanan, M.; van Nguyen, B.; et al. Genome and evolution of the shade-requiring medicinal herb Panax ginseng. Plant Biotechnol. J. 2018, 16, 1904–1917. [Google Scholar] [CrossRef]
- Jayakodi, M.; Choi, B.S.; Lee, S.C.; Kim, N.H.; Park, J.Y.; Jang, W.; Lakshmanan, M.; Mohan, S.V.G.; Lee, D.Y.; Yang, T.J. Ginseng Genome Database: An open-access platform for genomics of Panax ginseng. BMC Plant Biol. 2018, 18, 62. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, H.L. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004, 11, 247–261. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.-C.; Lee, J.; Lee, H.O.; Joh, H.J.; Kim, N.-H.; Park, H.-S.; Yang, T.-J. Comprehensive survey of genetic diversity in chloroplast genomes and 45S nrDNAs within Panax ginseng species. PLoS ONE 2015, 10, e0117159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, J.; Guo, H.; Zhang, Y.; Xiao, W.; Sun, C.; Wu, J.; Qu, X.; Yu, J.; Wang, X. The complete chloroplast genome provides insight into the evolution and polymorphism of Panax ginseng. Front. Plant Sci. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Nair, S.; Bhagwat, A.; Krishna, T.; Yano, M.; Bhatia, C.; Sasaki, T. Genome mapping, molecular markers and marker-assisted selection in crop plants. Mol. Breed. 1997, 3, 87–103. [Google Scholar] [CrossRef]
- Jang, W.; Jang, Y.; Kim, N.-H.; Waminal, N.E.; Kim, Y.C.; Lee, J.W.; Yang, T.-J. Genetic diversity among cultivated and wild Panax ginseng populations revealed by high-resolution microsatellite markers. J. Ginseng. Res. 2020, 44, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Linh Giang, V.N.; Waminal, N.E.; Park, H.S.; Kim, N.H.; Jang, W.; Lee, J.; Yang, T.J. Comprehensive comparative analysis of chloroplast genomes from seven Panax species and development of an authentication system based on species-unique single nucleotide polymorphism markers. J. Ginseng. Res. 2020, 44, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Semagn, K.; Babu, R.; Hearne, S.; Olsen, M. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): Overview of the technology and its application in crop improvement. Mol. Breed. 2014, 33, 1–14. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef]
- Schwartz, S.; Kent, W.J.; Smit, A.; Zhang, Z.; Baertsch, R.; Hardison, R.C.; Haussler, D.; Miller, W. Human-mouse alignments with BLASTZ. Genom. Res. 2003, 13, 103–107. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Munch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-sites: Rapid efficient extraction of SNPs from multi-FASTA alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. II. Gene frequency data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a problem in classification. Evolution 1957, 11, 130–162. [Google Scholar] [CrossRef]
- Sandhya, S.; Srivastava, H.; Kaila, T.; Tyagi, A.; Gaikwad, K. Methods and Tools for Plant Organelle Genome Sequencing, Assembly, and Downstream Analysis. In Legume Genomics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 49–98. [Google Scholar]
- Unseld, M.; Marienfeld, J.R.; Brandt, P.; Brennicke, A. The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366,924 nucleotides. Nat. Genet. 1997, 15, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Notsu, Y.; Masood, S.; Nishikawa, T.; Kubo, N.; Akiduki, G.; Nakazono, M.; Hirai, A.; Kadowaki, K. The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: Frequent DNA sequence acquisition and loss during the evolution of flowering plants. Genet. Genom. 2002, 268, 434–445. [Google Scholar] [CrossRef]
- Clifton, S.W.; Minx, P.; Fauron, C.M.-R.; Gibson, M.; Allen, J.O.; Sun, H.; Thompson, M.; Barbazuk, W.B.; Kanuganti, S.; Tayloe, C. Sequence and comparative analysis of the maize NB mitochondrial genome. Plant Physiol. 2004, 136, 3486–3503. [Google Scholar] [CrossRef]
- Bena, I.; Warner, N.P. One ring to rule them all and in the darkness bind them? Adv. Theor. Math. Phys. 2005, 9, 667–701. [Google Scholar] [CrossRef]
- Sloan, D.B.; Alverson, A.J.; Chuckalovcak, J.P.; Wu, M.; McCauley, D.E.; Palmer, J.D.; Taylor, D.R. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol. 2012, 10, e1001241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Shi, F.-X.; Li, M.-R.; Liu, B.; Wen, J.; Xiao, H.-X.; Li, L.-F. Positive selection driving cytoplasmic genome evolution of the medicinally important ginseng plant genus Panax. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.R.; Keeling, P.J. Twenty-fold difference in evolutionary rates between the mitochondrial and plastid genomes of species with secondary red plastids. J. Eukaryot. Microbiol. 2012, 59, 181–184. [Google Scholar] [CrossRef]
- Zhu, A.; Guo, W.; Jain, K.; Mower, J.P. Unprecedented heterogeneity in the synonymous substitution rate within a plant genome. Mol. Biol. Evol. 2014, 31, 1228–1236. [Google Scholar] [CrossRef]
- Drouin, G.; Daoud, H.; Xia, J. Relative rates of synonymous substitutions in the mitochondrial, chloroplast and nuclear genomes of seed plants. Mol. Phylogenet. Evol. 2008, 49, 827–831. [Google Scholar] [CrossRef]
- Smith, D.R. Mutation rates in plastid genomes: They are lower than you might think. Genom. Biol. Evol. 2015, 7, 1227–1234. [Google Scholar] [CrossRef]
- Davila, J.I.; Arrieta-Montiel, M.P.; Wamboldt, Y.; Cao, J.; Hagmann, J.; Shedge, V.; Xu, Y.-Z.; Weigel, D.; Mackenzie, S.A. Double-strand break repair processes drive evolution of the mitochondrial genome in Arabidopsis. BMC Biol. 2011, 9, 1–14. [Google Scholar] [CrossRef]
- Christensen, A.C. Plant mitochondrial genome evolution can be explained by DNA repair mechanisms. Genom. Biol. Evol. 2013, 5, 1079–1086. [Google Scholar] [CrossRef]
- Soria-Hernanz, D.F.; Braverman, J.M.; Hamilton, M.B. Parallel rate heterogeneity in chloroplast and mitochondrial genomes of Brazil nut trees (Lecythidaceae) is consistent with lineage effects. Mol. Biol. Evol. 2008, 25, 1282–1296. [Google Scholar] [CrossRef][Green Version]
- Choi, H.I.; Waminal, N.E.; Park, H.M.; Kim, N.H.; Choi, B.S.; Park, M.; Choi, D.; Lim, Y.P.; Kwon, S.J.; Park, B.S. Major repeat components covering one-third of the ginseng (Panax ginseng C. A. Meyer) genome and evidence for allotetraploidy. Plant J. 2014, 77, 906–916. [Google Scholar] [CrossRef]
- Jang, W.; Kim, N.-H.; Lee, J.; Waminal, N.E.; Lee, S.-C.; Jayakodi, M.; Choi, H.-I.; Park, J.Y.; Lee, J.-E.; Yang, T.-J. A glimpse of Panax ginseng genome structure revealed from ten BAC clone sequences obtained by SMRT sequencing platform. Plant Breed Biotechnol. 2017, 5, 25–35. [Google Scholar] [CrossRef]
- Choi, H.-I.; Kim, N.-H.; Lee, J.; Choi, B.S.; Do Kim, K.; Park, J.Y.; Lee, S.-C.; Yang, T.-J. Evolutionary relationship of Panax ginseng and P. quinquefolius inferred from sequencing and comparative analysis of expressed sequence tags. Genet. Resour. Crop. Evol. 2013, 60, 1377–1387. [Google Scholar] [CrossRef]
- Kim, N.-H.; Choi, H.-I.; Kim, K.H.; Jang, W.; Yang, T.-J. Evidence of genome duplication revealed by sequence analysis of multi-loci expressed sequence tag–simple sequence repeat bands in Panax ginseng Meyer. J. Ginseng. Res. 2014, 38, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Jayakodi, M.; Lee, S.H.; Jeon, J.-H.; Lee, H.-O.; Park, J.Y.; Moon, B.C.; Kim, C.-K.; Wing, R.A.; Newmaster, S.G. Mitochondrial plastid DNA can cause DNA barcoding paradox in plants. Sci. Rep. 2020, 10, 6112. [Google Scholar] [CrossRef]
- Kim, N.-H.; Choi, H.-I.; Ahn, I.-O.; Yang, T.-J. EST-SSR marker sets for practical authentication of all nine registered ginseng cultivars in Korea. J. Ginseng. Res. 2012, 36, 298–307. [Google Scholar] [CrossRef]
- Choi, H.I.; Kim, N.H.; Kim, J.H.; Choi, B.S.; Ahn, I.O.; Lee, J.S.; Yang, T.J. Development of Reproducible EST-derived SSR Markers and Assessment of Genetic Diversity in Panax ginseng Cultivars and Related Species. J. Ginseng. Res. 2011, 35, 399–412. [Google Scholar] [CrossRef]
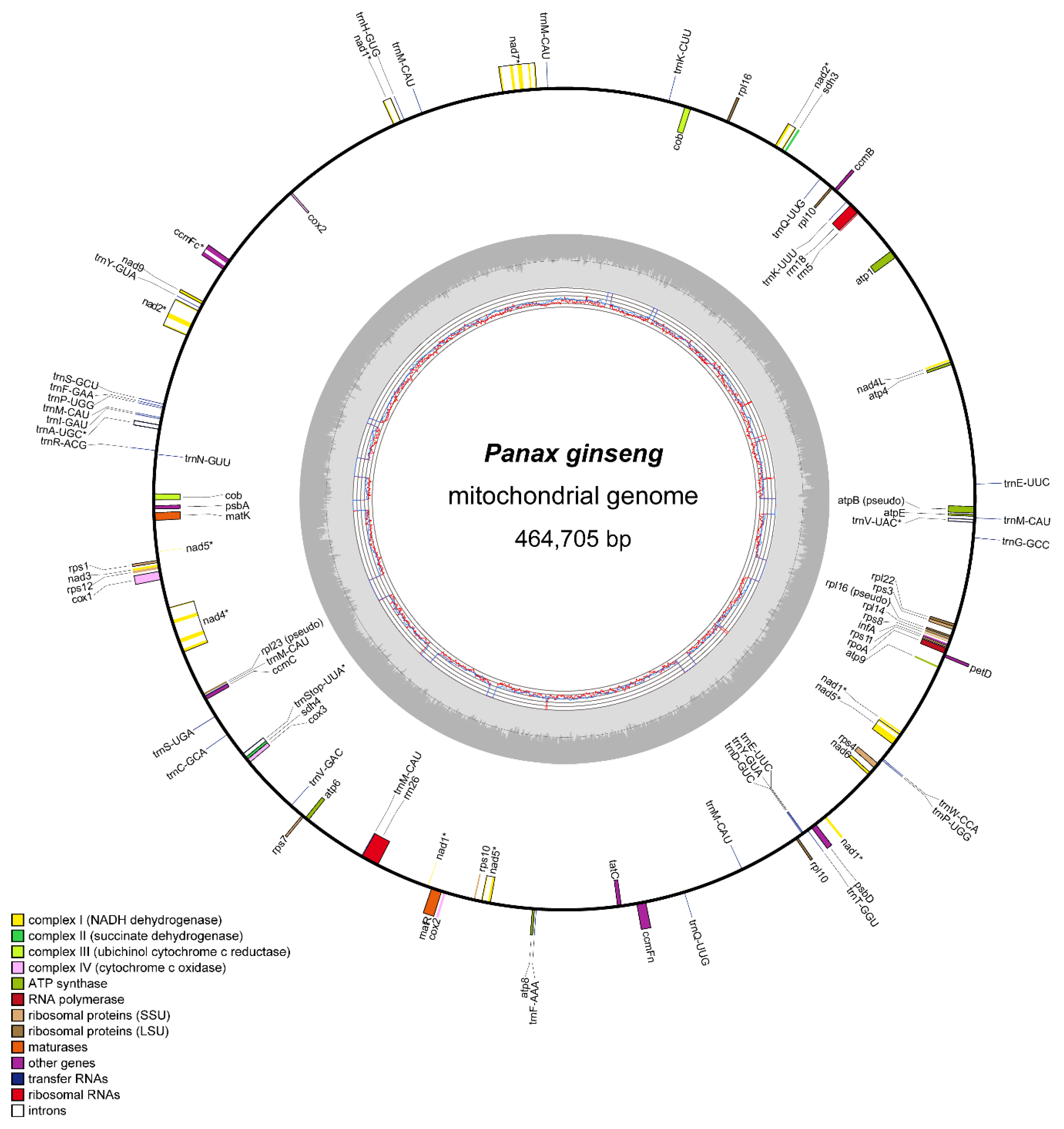
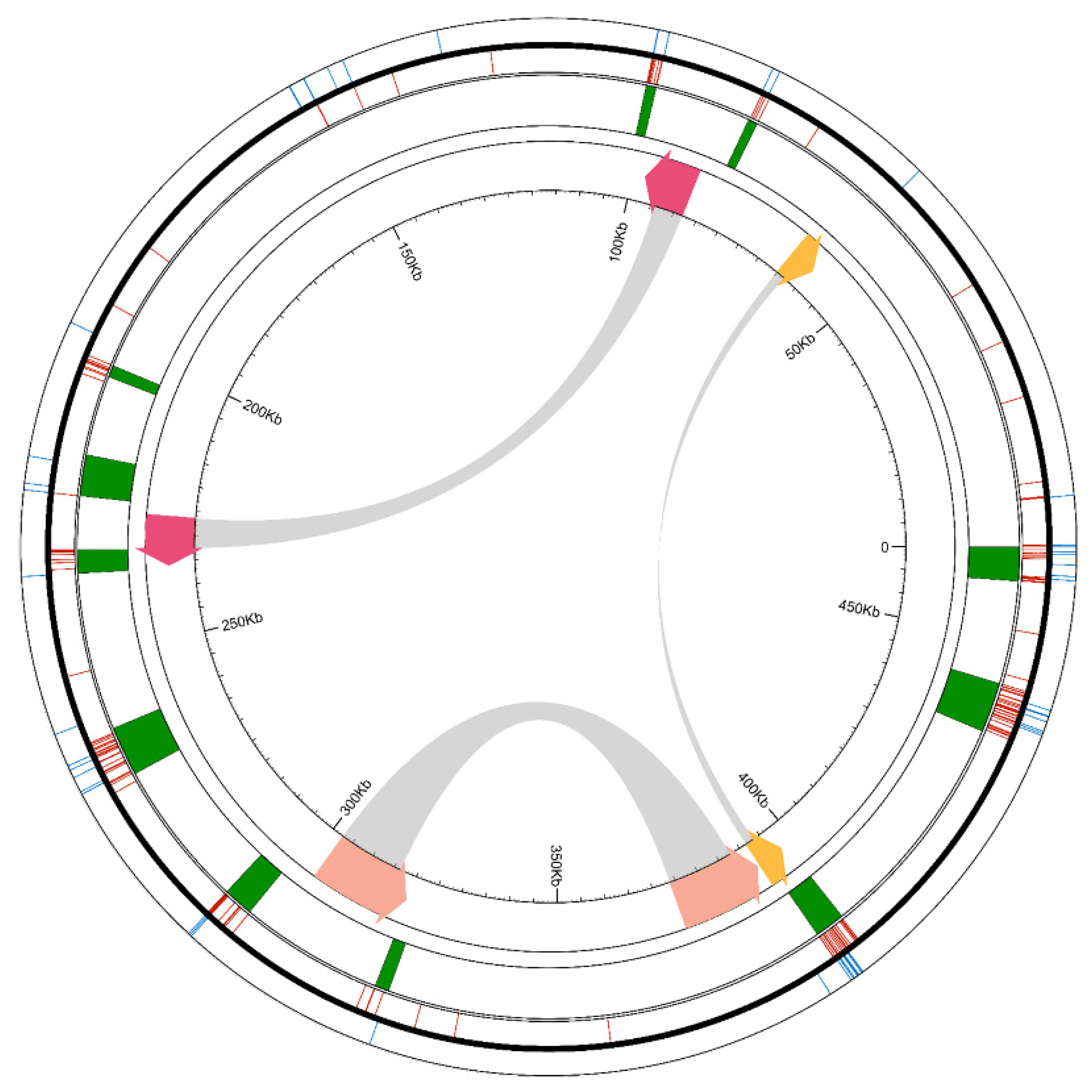
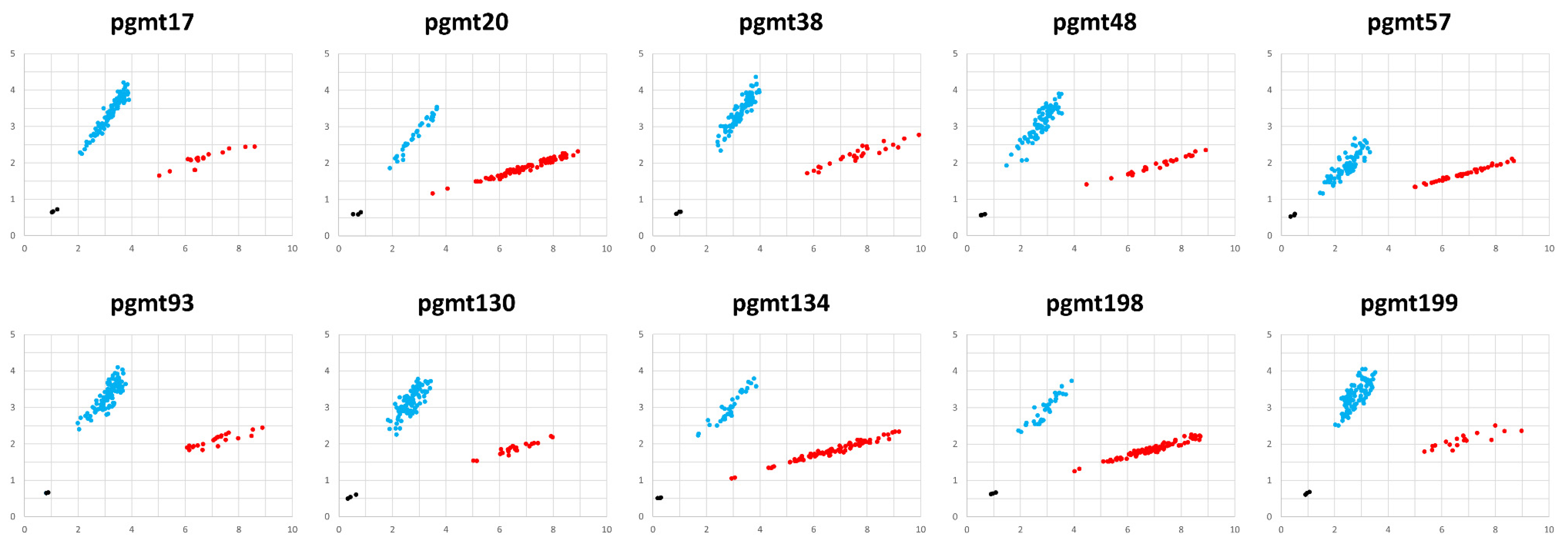
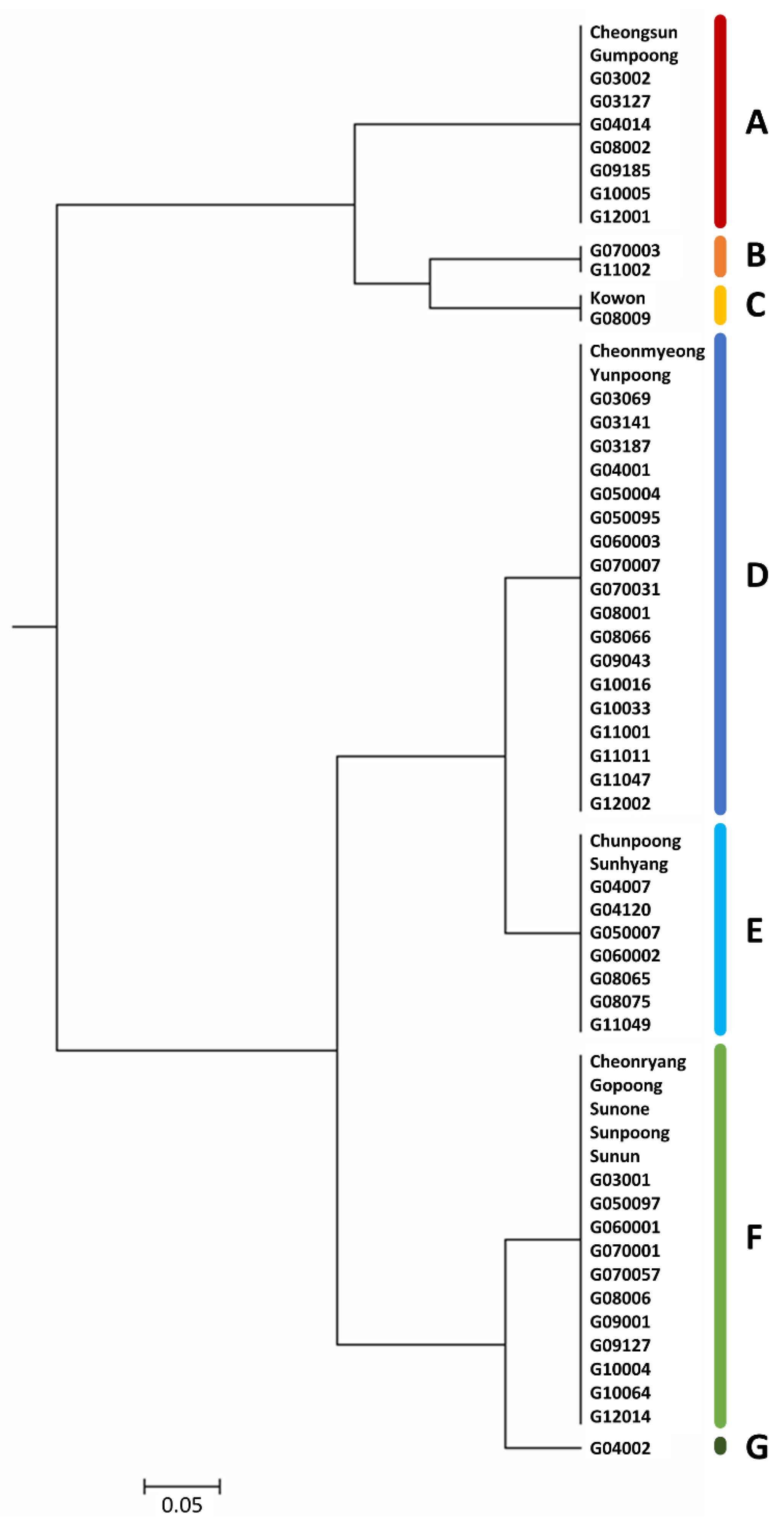
| Function | Genes |
|---|---|
| Complex I (NADH dehydrogenase) | nad1 *,T, nad2 *,T, nad3, nad4 *, nad4L, nad5 *,T, nad6, nad7 *, nad9 |
| Complex II (succinate dehydrogenase) | sdh3, sdh4 |
| Complex III (ubiquinol-cytochrome c reductase) | cob |
| Complex IV (cytochrome c oxidase) | cox1, cox2 *,T, cox3 |
| Complex V (ATP synthase) | atp1, atp4, atp6, atp8, atp9, atpE |
| Cytochrome c biogenesis | ccmB, ccmC, ccmFc *, ccmFn |
| Large subunit ribosomal proteins | rpl10, rpl14, rpl16, rpl22 |
| Small subunit ribosomal proteins | rps1, rps10, rps11, rps12, rps3, rps4, rps7, rps8 |
| Maturase | matK, matR |
| Ribosomal RNAs | rrn18, rrn26, rrn5 |
| Transfer RNAs | trnA-UGC*, trnC-GCA, trnD-GUC, trnE-UUC, trnF-AAA, trnF-GAA, trnG-GCC, trnH-GUG, trnI-GAU, trnK-CUU, trnK-UUU, trnM-CAU, trnN-GUU, trnP-UGG, trnQ-UUG, trnR-ACG, trnS-GCU, trnS-UGA, trnStop-UUA *, trnT-GGU, trnV-GAC, trnV-UAC *, trnW-CCA, trnY-GUA |
| Other genes | infA, petD, psbA, psbD, rpoA, tatC |
| No | Marker Name | Region | Nucleotide Position | Allele | Allele-Specific Primer Sequence (5′->3′) | Common Primer Sequence (5′->3′) | |
|---|---|---|---|---|---|---|---|
| 1 | pgmt17 | tRNA-Glu(TTC)~atp4 | 9736 | FAM | G | GAGTCGCCCCACTCACTTGAG | TAGGCAAGTGGGAAACAAGGAATTGAATT |
| HEX | T | AGAGTCGCCCCACTCACTTGAT | |||||
| 2 | pgmt20 | atp4~rrn5 | 40,917 | FAM | A | CGTTGATATGCAAAACAGAGGAAAAGAT | GAGTGCGCGAAGGTACAATCCTAAA |
| HEX | C | GTTGATATGCAAAACAGAGGAAAAGAG | |||||
| 3 | pgmt38 | orf109~tRNA-Met(CAT) | 124,782 | FAM | G | TGAGGCTTTCTTTCCCTTATTAGTC | GAACGTCCCGCGCCCCCTT |
| HEX | T | GTTGAGGCTTTCTTTCCCTTATTAGTA | |||||
| 4 | pgmt48 | ccmFc~nad9 | 194,878 | FAM | C | AGTCGATAATAAGGTCAGCTACCTG | ATGCGAAGGCTCACCTCATTGGAAA |
| HEX | A | AAGTCGATAATAAGGTCAGCTACCTT | |||||
| 5 | pgmt57 | orf214~tRNA-Ser(GCT) | 206,035 | FAM | T | GCCCGTTACTTCATCAAGATAGACTT | TTGCACTTGGTGGTATCCCGACTTT |
| HEX | G | CCCGTTACTTCATCAAGATAGACTG | |||||
| 6 | pgmt93 | ccmC~tRNA-Ser(TGA) | 270,424 | FAM | G | ATCTCGTCTAATAAGAAGAGCGCTC | CGAGACTAGCGTGATGTAAGACAGAA |
| HEX | T | AAATCTCGTCTAATAAGAAGAGCGCTA | |||||
| 7 | pgmt130 | rps7~matR | 319,240 | FAM | C | GAGATAAGGAGCTAGCCTTTTAGAAG | AGGTACTAGTGACTTCTTGCATCTCAAA |
| HEX | A | AGAGATAAGGAGCTAGCCTTTTAGAAT | |||||
| 8 | pgmt134 | matR~atp8 | 328,368 | FAM | A | AGGCTTTTTTTATAGATTAAGAGGTGAGTA | AGTTGGGGCTCTTTGGTCTTCCATT |
| HEX | C | GGCTTTTTTTATAGATTAAGAGGTGAGTC | |||||
| 9 | pgmt198 | rpl22~tRNA-Gly(GCC) | 444,629 | FAM | A | ATAGAACTCCTAGCTCTGGAGCT | GCGAAGCCTTTCGGTAGCTGTTAAA |
| HEX | C | AGAACTCCTAGCTCTGGAGCG | |||||
| 10 | pgmt199 | rpl22~tRNA-Gly(GCC) | 451,679 | FAM | C | TGGTTTTTCTCTTTCTACGTTCTTCC | CTTCTAGCAACTAAGCACTCGGACAA |
| HEX | A | GTTTGGTTTTTCTCTTTCTACGTTCTTCA | |||||
| Marker | NA | MAF | GD | PIC |
|---|---|---|---|---|
| Pgmt17 | 2 | 0.8475 | 0.2585 | 0.2251 |
| Pgmt20 | 2 | 0.7119 | 0.4102 | 0.3261 |
| Pgmt38 | 2 | 0.7797 | 0.3436 | 0.2846 |
| Pgmt48 | 2 | 0.7797 | 0.3436 | 0.2846 |
| Pgmt57 | 2 | 0.6271 | 0.4677 | 0.3583 |
| Pgmt93 | 2 | 0.8136 | 0.3034 | 0.2573 |
| Pgmt130 | 2 | 0.8136 | 0.3034 | 0.2573 |
| Pgmt134 | 2 | 0.7119 | 0.4102 | 0.3261 |
| Pgmt198 | 2 | 0.7288 | 0.3953 | 0.3172 |
| Pgmt199 | 2 | 0.8475 | 0.2585 | 0.2251 |
| Mean | 2 | 0.7661 | 0.3494 | 0.2862 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Lee, H.O.; Kim, J.-U.; Lee, J.-W.; Hong, C.-E.; Bang, K.-H.; Chung, J.-W.; Jo, I.-H. Complete Mitochondrial Genome and a Set of 10 Novel Kompetitive Allele-Specific PCR Markers in Ginseng (Panax ginseng C. A. Mey.). Agronomy 2020, 10, 1868. https://doi.org/10.3390/agronomy10121868
Jang W, Lee HO, Kim J-U, Lee J-W, Hong C-E, Bang K-H, Chung J-W, Jo I-H. Complete Mitochondrial Genome and a Set of 10 Novel Kompetitive Allele-Specific PCR Markers in Ginseng (Panax ginseng C. A. Mey.). Agronomy. 2020; 10(12):1868. https://doi.org/10.3390/agronomy10121868
Chicago/Turabian StyleJang, Woojong, Hyun Oh Lee, Jang-Uk Kim, Jung-Woo Lee, Chi-Eun Hong, Kyong-Hwan Bang, Jong-Wook Chung, and Ick-Hyun Jo. 2020. "Complete Mitochondrial Genome and a Set of 10 Novel Kompetitive Allele-Specific PCR Markers in Ginseng (Panax ginseng C. A. Mey.)" Agronomy 10, no. 12: 1868. https://doi.org/10.3390/agronomy10121868
APA StyleJang, W., Lee, H. O., Kim, J.-U., Lee, J.-W., Hong, C.-E., Bang, K.-H., Chung, J.-W., & Jo, I.-H. (2020). Complete Mitochondrial Genome and a Set of 10 Novel Kompetitive Allele-Specific PCR Markers in Ginseng (Panax ginseng C. A. Mey.). Agronomy, 10(12), 1868. https://doi.org/10.3390/agronomy10121868







