Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China
Abstract
1. Introduction
2. Materials and Methods
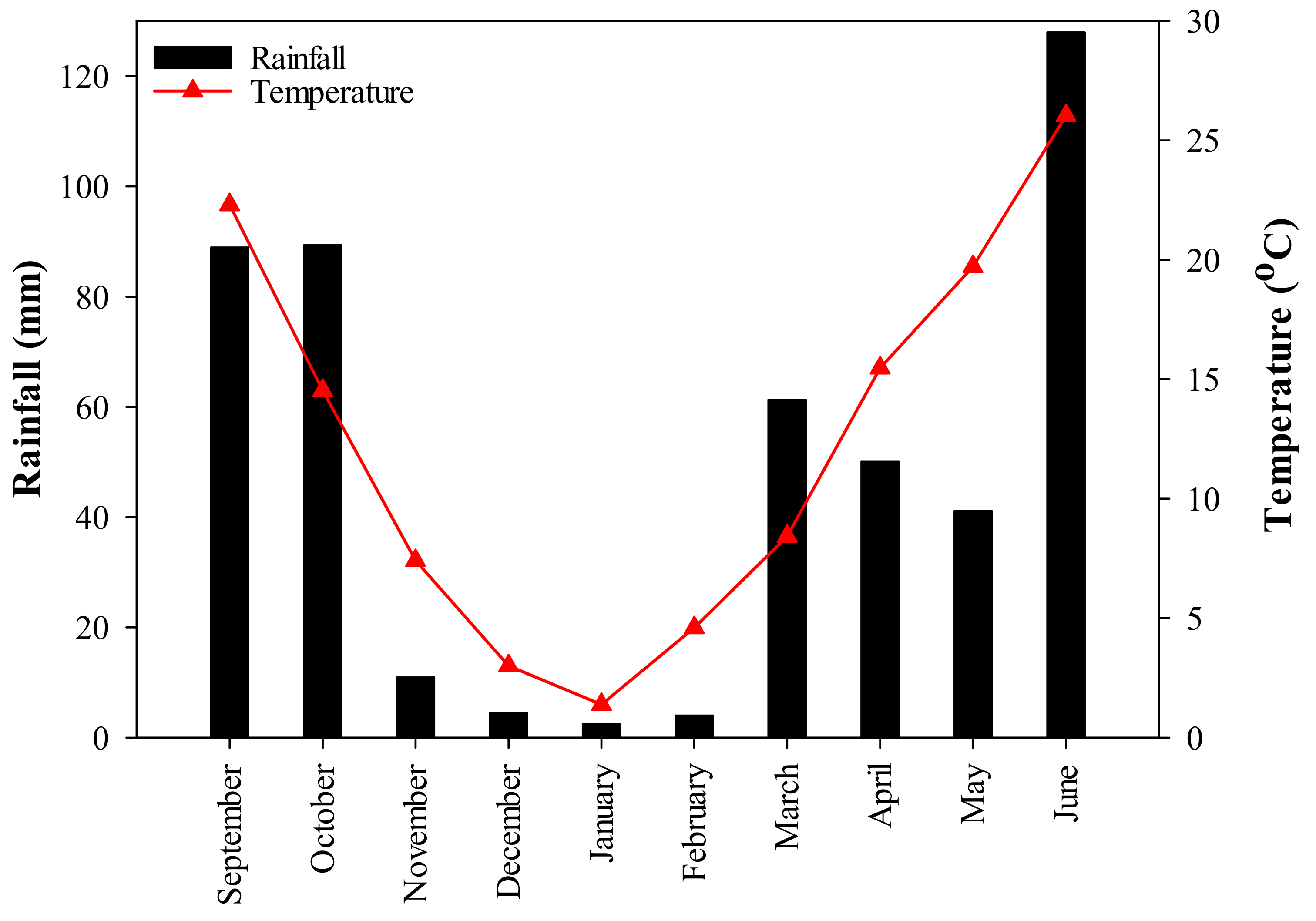
2.1. Study Site
2.2. Experimental Design and Treatments
2.3. Sample Collection and Analysis
2.3.1. Sampling and Analysis of Plants
2.3.2. Soil P Fractions Analysis
2.4. Data Analysis
3. Results
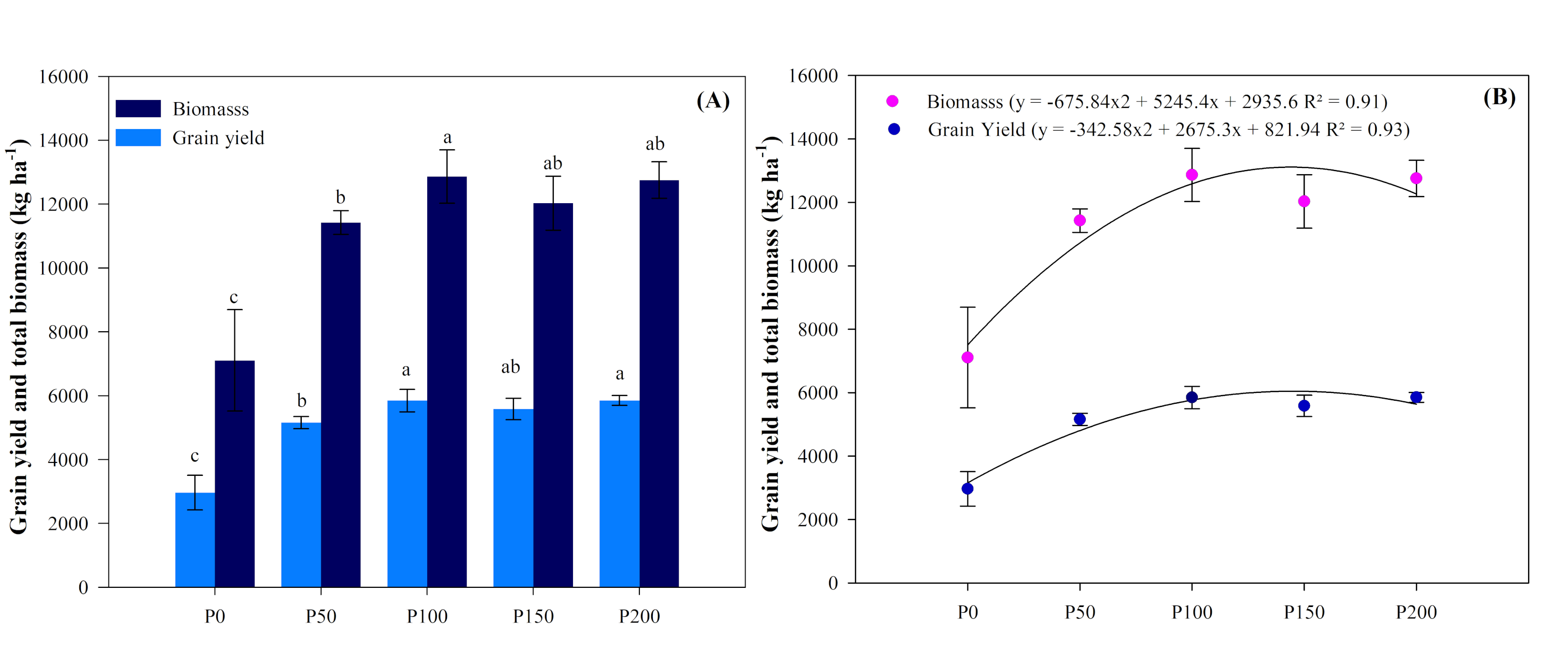
3.1. Grain Yield and Total Biomass
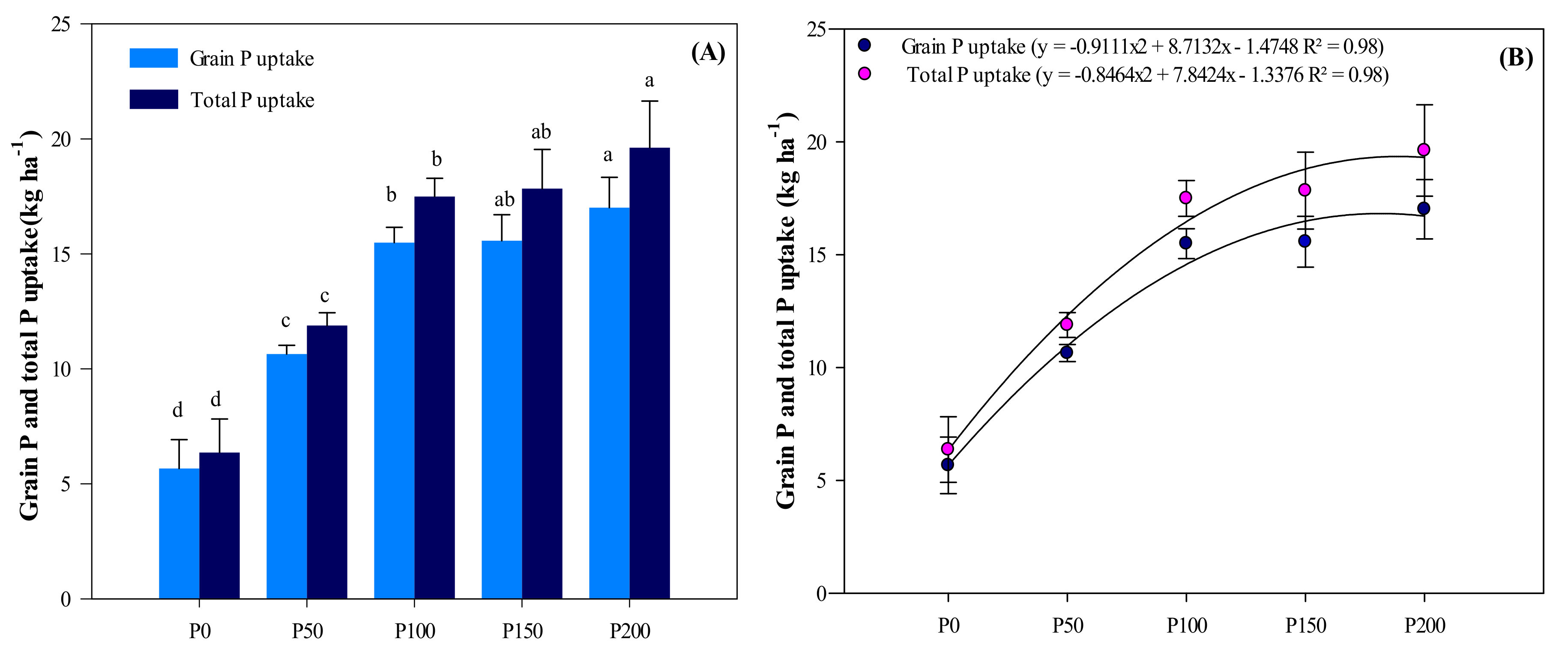
3.2. Grain P and Total P Uptake
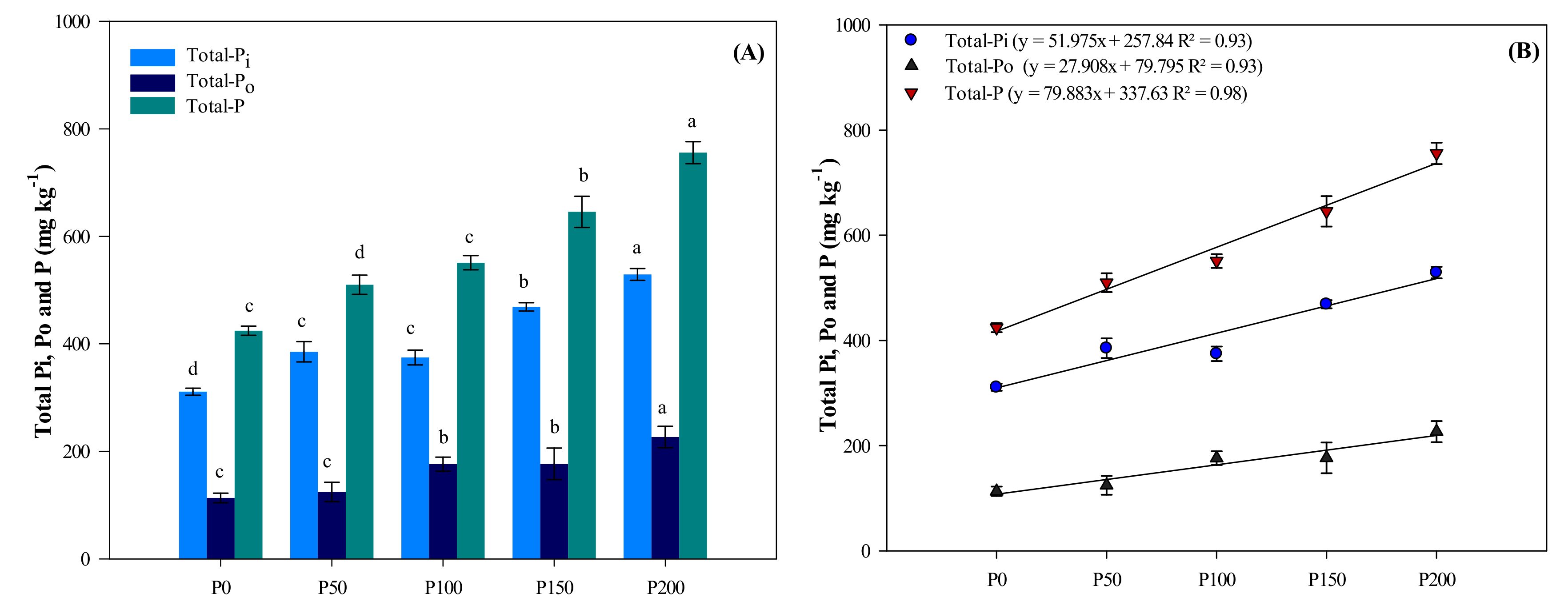
3.3. Total P (P), Organic P (Po) and Inorganic P (Pi) Concentrations in the Soil
3.4. Organic P (Po) Fractions
3.5. Inorganic P (Pi) Fractions
3.6. Residual P Fractions
3.7. Relations of Different Fractions to Yield and P Uptake
4. Discussion
4.1. Effect of Different P Rates on P Fractions
4.2. Relationship of Available P with Different P Fractions
4.3. Grain Yield, P Uptake and P Fractions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Elrys, A.S.; Desoky, E.M.; Ali, A.; Zhang, J.; Cai, Z.; Cheng, Y. Sub-Saharan Africa’s food nitrogen and phosphorus footprints: A scenario analysis for 2050. Sci. Total Environ. 2020, 752, 141964. [Google Scholar]
- Jiang, M.; Shen, M.X.; Shen, X.P.; Dai, Q.G. Effect of long-term fertilization pattern on weed community diversity in wheat field. Sci. Rep. 2018, 8, 1–7. [Google Scholar]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. FAO Fertil. Plant Nutr. Bull. 2008, 18, 108. [Google Scholar]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. USA 2012, 16, 6348–6353. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef]
- Oita, A.; Wirasenjaya, F.; Liu, J.; Webeck, E.; Matsubae, K. Trends in the food nitrogen and phosphorus footprints for Asia’s giants: China, India, and Japan. Resour. Conserv. Recycl. 2020, 157, 104752. [Google Scholar] [CrossRef]
- Yan, X.; Wei, Z.; Hong, Q.; Lu, Z.; Wu, J. Phosphorus fractions and sorption characteristics in a subtropical paddy soil as influenced by fertilizer sources. Geoderma 2017, 295, 80–85. [Google Scholar] [CrossRef]
- Vincent, A.G.; Turner, B.L.; Tanner, E.V.J. Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur. J. Soil Sci. 2010, 61, 48–57. [Google Scholar] [CrossRef]
- Elser, J.J. Phosphorus: A limiting nutrient for humanity? Curr. Opin. Biotechnol. 2012, 23, 833–838. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Mehmood, A.; Akhtar, M.; Khan, K.; Khalid, A.; Imran, M.; Rukh, S. Relationship of Phosphorus Uptake with Its Fractions in Different Soil Parent Materials. Int. J. Plant Soil Sci. 2015, 4, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Börjesson, G.; Hedlund, K. The effects of 55 years of different inorganic fertiliser regimes on soil properties and microbial community composition. Soil Biol. Biochem. 2013, 67, 41–46. [Google Scholar] [CrossRef]
- Malik, M.A.; Marschner, P.; Khan, K.S. Addition of organic and inorganic P sources to soil-Effects on P pools and microorganisms. Soil Biol. Biochem. 2012, 49, 106–113. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of Nitrogen with Other Nutrients and Water: Effect on Crop Yield and Quality, Nutrient Use Efficiency, Carbon Sequestration, and Environmental Pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- Cox, A.E.; Camberato, J.J.; Smith, B.R. Phosphate Availability and Inorganic Transformation in an Alum Sludge-Affected Soil. J. Environ. Qual. 1997, 26, 1393–1398. [Google Scholar] [CrossRef]
- Kuo, S.; Huang, B.; Bembenek, R. Effects of long-term phosphorus fertilization and winter cover cropping on soil phosphorus transformations in less weathered soil. Biol. Fertil. Soils 2005, 41, 116–123. [Google Scholar] [CrossRef]
- Solis, P.; Torrent, J. Phosphate Fractions in Calcareous Vertisols and Inceptisols of Spain. Soil Sci. Soc. Am. J. 1989, 53, 462–466. [Google Scholar] [CrossRef]
- Buehler, S.; Oberson, A.; Rao, I.M.; Friesen, D.K.; Frossard, E. Sequential Phosphorus Extraction of a P-Labeled Oxisol under Contrasting Agricultural Systems. Soil Sci. Soc. Am. J. 2014, 66, 868. [Google Scholar] [CrossRef]
- Kritzler, U.H.; Johnson, D. Mineralisation of carbon and plant uptake of phosphorus from microbially-derived organic matter in response to 19 years simulated nitrogen deposition. Plant Soil 2010, 326, 311–319. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Carreira, J.A.; García-Ruiz, R.; Liétor, J.; Harrison, A.F. Changes in soil phosphatase activity and P transformation rates induced by application of N- and S-containing acid-mist to a forest canopy. Soil Biol. Biochem. 2000, 32, 1357–1865. [Google Scholar] [CrossRef]
- Khan, K.S.; Joergensen, R.G. Changes in microbial biomass and P fractions in biogenic household waste compost amended with inorganic P fertilizers. Bioresour. Technol. 2009, 100, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.; Tiessen, H. Characterization of Available P by Sequential Extraction. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Khan, K.S.; Joergensen, R.G. Relationships between P fractions and the microbial biomass in soils under different land use management. Geoderma 2012, 173–174, 274–281. [Google Scholar] [CrossRef]
- Ahmed, W.; Jing, H.; Kaillou, L.; Qaswar, M.; Khan, M.N.; Jin, C.; Geng, S.; Qinghai, H.; Yiren, L.; Guangrong, L.; et al. Changes in phosphorus fractions associated with soil chemical properties under long-term organic and inorganic fertilization in paddy soils of southern China. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Ahmed, W.; Qaswar, M.; Jing, H.; Wenjun, D.; Geng, S.; Kailou, L.; Ying, M.; Ao, T.; Mei, S.; Chao, L.; et al. Tillage practices improve rice yield and soil phosphorus fractions in two typical paddy soils. J. Soils Sediments 2020, 20, 850–861. [Google Scholar] [CrossRef]
- Meason, D.F.; Idol, T.W.; Friday, J.B.; Scowcroft, P.G. Effects of fertilisation on phosphorus pools in the volcanic soil of a managed tropical forest. For. Ecol. Manag. 2009, 258, 2199–2206. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P-sorption properties in three alkaline soils after long-term mineral and manure applications in north-eastern Italy. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Relationship between soil test phosphorus and phosphorus release to solution in three soils after long-term mineral and manure application. Agric. Ecosyst. Environ. 2016, 233, 214–223. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Kabba, B.S.; Baddesha, H.S.; Bahl, G.S.; Gill, M.P.S. Crop yields and phosphorus fertilizer transformations after 25 years of applications to a subtropical soil under groundnut-based cropping systems. Field Crop. Res. 2003, 83, 283–296. [Google Scholar] [CrossRef]
- Zicker, T.; von Tucher, S.; Kavka, M.; Eichler-Löbermann, B. Soil test phosphorus as affected by phosphorus budgets in two long-term field experiments in Germany. Field Crop. Res. 2018, 218, 158–170. [Google Scholar] [CrossRef]
- Dobermann, A.; George, T.; Thevs, N. Phosphorus Fertilizer Effects on Soil Phosphorus Pools in Acid Upland Soils. Soil Sci. Soc. Am. J. 2002, 66, 652–660. [Google Scholar] [CrossRef]
- Delgado, A.; Madrid, A.; Kassem, S.; Andreu, L.; del Campillo, M.D.C. Phosphorus fertilizer recovery from calcareous soils amended with humic and fulvic acids. Plant Soil 2002, 245, 277–286. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Li, J.; Chen, Y.; Ying, X.; Liu, S. The effects of two organic manures on soil properties and crop yields on a temperate calcareous soil under a wheat-maize cropping system. Eur. J. Agron. 2009, 31, 36–42. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Zheng, Z.M.; Drury, C.F.; Hu, Q.C.; Tan, C.S. Legacy Phosphorus After 45 Years With Consistent Cropping Systems and Fertilization Compared to Native Soils. Front. Earth Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci. 1978, 125, 49–54. [Google Scholar] [CrossRef]
- Damodar Reddy, D.; Subba Rao, A.; Rupa, T.R. Effects of continuous use of cattle manure and fertilizer phosphorus on crop yields and soil organic phosphorus in a Vertisol. Bioresour. Technol. 2000, 75, 113–118. [Google Scholar] [CrossRef]
- Gupta, R.K.; Yadvinder-Singh; Ladha, J.K.; Bijay-Singh; Singh, J.; Singh, G.; Pathak, H. Yield and Phosphorus Transformations in a Rice-Wheat System with Crop Residue and Phosphorus Management. Soil Sci. Soc. Am. J. 2007, 71, 1500–1507. [Google Scholar] [CrossRef]
- Siddique, M.T.; Robinson, J.S. Phosphorus Sorption and Availability in Soils Amended with Animal Manures and Sewage Sludge. J. Environ. Qual. 2003, 32, 1114–1121. [Google Scholar] [CrossRef]
- Scherer, H.W.; Sharma, S.P. Phosphorus fractions and phosphorus delivery potential of a luvisol derived from loess amended with organic materials. Biol. Fertil. Soils 2002, 35, 414–419. [Google Scholar]
- Mao, X.; Xu, X.; Lu, K.; Gielen, G.; Luo, J.; He, L.; Donnison, A.; Xu, Z.; Xu, J.; Yang, W.; et al. Effect of 17 years of organic and inorganic fertilizer applications on soil phosphorus dynamics in a rice-wheat rotation cropping system in eastern China. J. Soils Sediments 2015, 15, 1889–1899. [Google Scholar] [CrossRef]
- Zhang, M.K.; He, Z.L.; Calvert, D.V.; Stoffella, P.J.; Yang, X.E.; Li, Y.C. Phosphorus and Heavy Metal Attachment and Release in Sandy Soil Aggregate Fractions. Soil Sci. Soc. Am. J. 2003, 67, 1158–1167. [Google Scholar] [CrossRef]
- Bravo, C.; Torrent, J.; Giráldez, J.V.; González, P.; Ordóñez, R. Long-term effect of tillage on phosphorus forms and sorption in a Vertisol of southern Spain. Eur. J. Agron. 2006, 25, 264–269. [Google Scholar] [CrossRef]
- Laboski, C.A.M.; Lamb, J.A. Impact of manure application on soil phosphorus sorption characteristics and subsequent water quality implications. Soil Sci. 2004, 169, 440–448. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Blake, L.; Mercik, S.; Koerschens, M.; Moskal, S.; Poulton, P.R.; Goulding, K.W.T.; Weigel, A.; Powlson, D.S. Phosphorus content in soil, uptake by plants and balance in three European long-term field experiments. Nutr. Cycl. Agroecosyst. 2000, 56, 263–275. [Google Scholar] [CrossRef]
- Redel, Y.; Staunton, S.; Durán, P.; Gianfreda, L.; Rumpel, C.; de la Luz Mora, M. Fertilizer P Uptake Determined by Soil P Fractionation and Phosphatase Activity. J. Soil Sci. Plant Nutr. 2019, 19, 166–174. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.; Strokal, M.; Ma, W.; Liu, X.; Kroeze, C. Hotspots for Nitrogen and Phosphorus Losses from Food Production in China: A County-Scale Analysis. Environ. Sci. Technol. 2018, 52, 5782–5791. [Google Scholar] [CrossRef]
- Ma, L.; Wang, F.; Zhang, W.; Ma, W.; Velthof, G.; Qin, W.; Oenema, O.; Zhang, F. Environmental assessment of management options for nutrient flows in the food chain in China. Environ. Sci. Technol. 2013, 52, 5782–5791. [Google Scholar] [CrossRef]
- Díez, J.A.; Hernaiz, P.; Muñoz, M.J.; de la Torre, A.; Vallejo, A. Impact of pig slurry on soil properties, water salinization, nitrate leaching and crop yield in a four-year experiment in Central Spain. Soil Use Manag. 2006, 20, 444–450. [Google Scholar] [CrossRef]
- Si, L.; Xie, Y.; Ma, Q.; Wu, L. The short-term effects of rice straw biochar, nitrogen and phosphorus fertilizer on rice yield and soil properties in a cold waterlogged paddy field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, nitrogen-use efficiency, and nitrogen management. AMBIO 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; Römheld, V.; Horlacher, D.; Schulz, R.; Böning-Zilkens, M.; Wang, P.; Claupein, W. Synchronizing N supply from soil and fertilizer and N demand of winter wheat by an improved Nmin method. Nutr. Cycl. Agroecosyst. 2006, 74, 91–98. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, X.; Chu, W.; Mao, J.; Yang, W.; Zhu, A.; Zhang, J.; Zhong, X. Characterization of fluvo-aquic soil phosphorus affected by long-term fertilization using solution 31P NMR spectroscopy. Sci. Total Environ. 2019, 692, 89–97. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. China Statistical Yearbook; National Bureau of Statistics of China: Beijing, China, 2018.
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Hui, X.; Luo, L.; Wang, S.; Cao, H.; Huang, M.; Shi, M.; Malhi, S.S.; Wang, Z. Critical concentration of available soil phosphorus for grain yield and zinc nutrition of winter wheat in a zinc-deficient calcareous soil. Plant Soil 2019, 444, 315–330. [Google Scholar] [CrossRef]
- Bouain, N.; Shahzad, Z.; Rouached, A.; Khan, G.A.; Berthomieu, P.; Abdelly, C.; Poirier, Y.; Rouached, H. Phosphate and zinc transport and signalling in plants: Toward a better understanding of their homeostasis interaction. J. Exp. Bot. 2014, 65, 5725–5741. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Qin, S.; Zhang, J.; Zhu, A.; Yang, W.; Zhang, X. Yield, phosphorus use efficiency and balance response to substituting long-term chemical fertilizer use with organic manure in a wheat-maize system. Field Crop. Res. 2017, 208, 27–33. [Google Scholar] [CrossRef]
- Schröder, J. Revisiting the agronomic benefits of manure: A correct assessment and exploitation of its fertilizer value spares the environment. Bioresour. Technol. 2005, 96, 253–261. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Li, X.Y.; Li, X.P.; Shi, X.J.; Huang, S.M.; Wang, B.R.; Zhu, P.; Yang, X.Y.; Liu, H.; Chen, Y.; et al. Long-term fertilizer experiment network in china: Crop yields and soil nutrient trends. Agron. J. 2010, 102, 216–230. [Google Scholar] [CrossRef]
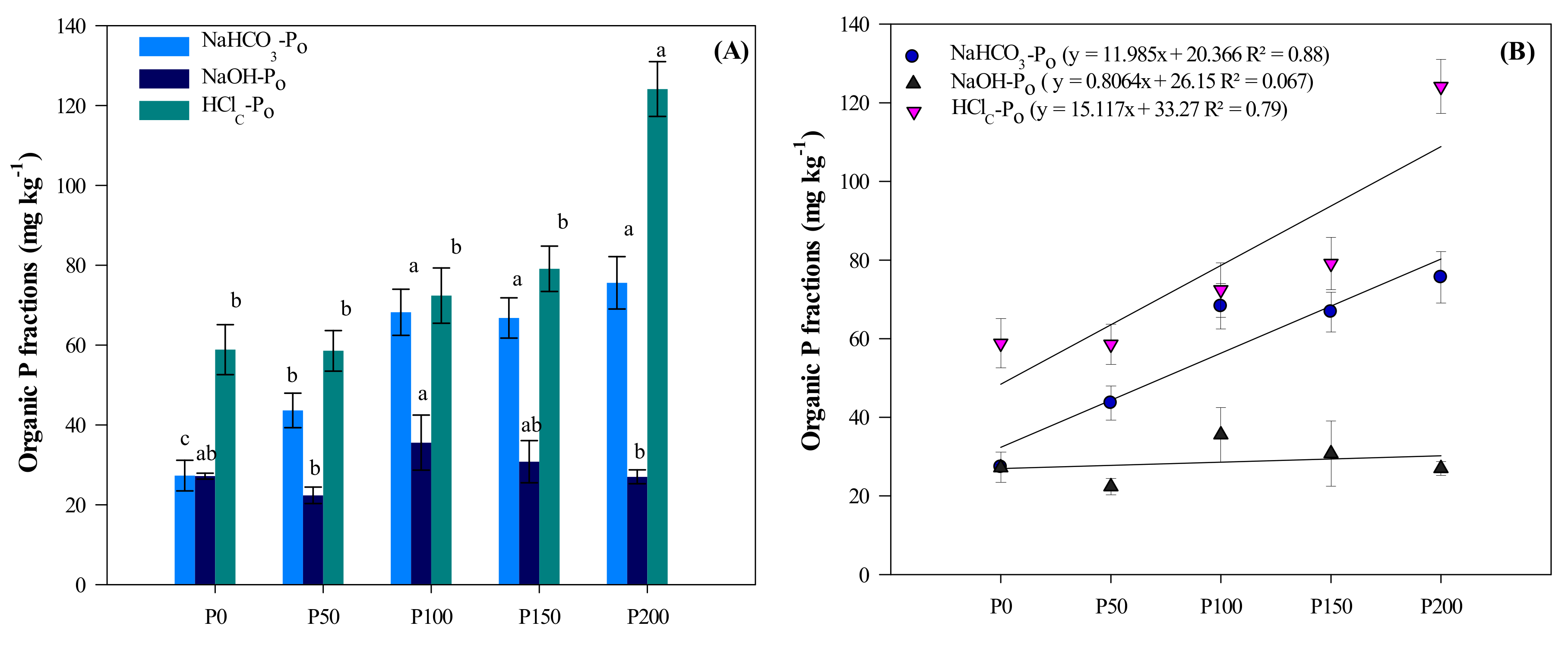

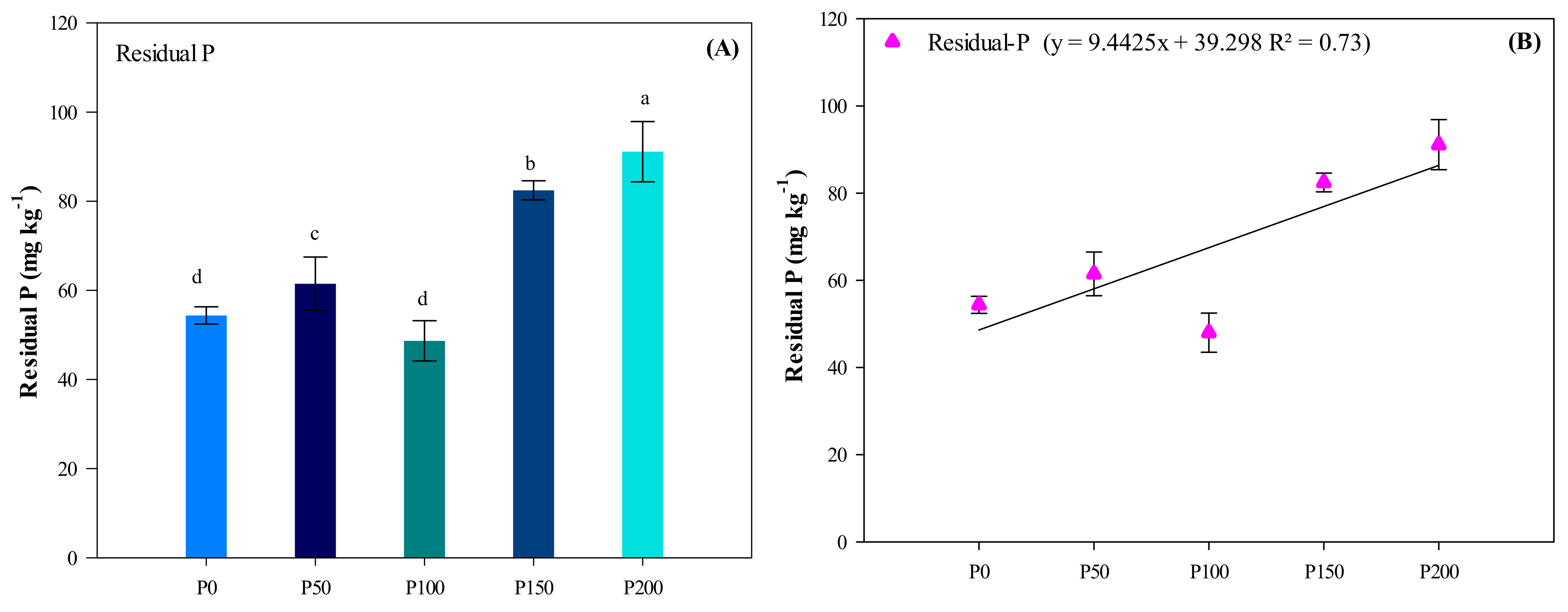
| Treatment | NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HClD-Pi | HClC-Pi | HClC-Po | Residual P | Total P | Grain Yield | P Uptake |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P0 | 13.1 e | 27 c | 24.5 e | 27 a,b | 165.5 d | 53.5 c | 59 b | 54.4 b,c | 424 e | 2964 c | 6.4 d |
| P50 | 24.3 d | 44 b | 40.3 b | 22 b | 189.2 c | 70.0 b | 59 b | 61.5 a,b,c | 510 d | 5156 b | 11.9 c |
| P100 | 40.5 c | 68 a | 29.5 d | 36 a | 216.3 b | 49.8 c | 72 b | 48.7 c | 551 c | 5845 a | 17.5 b |
| P150 | 48.7 b | 67 a | 33.7 c | 31 a,b | 219.0 b | 84.9 a | 79 b | 82.5 a,b | 646 b | 5582 a,b | 17.8 a,b |
| P200 | 61.3 a | 76 a | 47.0 a | 27 b | 245.9 b | 83.8 a | 124 a | 91.1 a | 756 a | 5851 a | 19.6 a |
| NaHCO3-Pi | NaHCO3-Po | NaOH-Pi | NaOH-Po | HClD-Pi | HClC-Pi | HClC-Po | Residual-P | Grain Yield | Total Biomass | Grain P Uptake | Total P Uptake | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaHCO3-Pi | 1 | |||||||||||
| NaHCO3-Po | 0.92 ** | 1 | ||||||||||
| NaOH-Pi | 0.63 ** | 0.51 * | 1 | |||||||||
| NaOH-Po | 0.19 | 0.33 | −0.38 | 1 | ||||||||
| HClD-Pi | 0.95 ** | 0.91 ** | 0.65 ** | 0.28 | 1 | |||||||
| HClC-Pi | 0.60 ** | 0.42 | 0.66 ** | −0.35 | 0.54 * | 1 | ||||||
| HClC-Po | 0.75 ** | 0.64 ** | 0.60 ** | 0.17 | 0.76 ** | 0.38 | 1 | |||||
| Residual-P | 0.72 ** | 0.52 * | 0.66 ** | −0.12 | 0.64 ** | 0.85 ** | 0.69 ** | 1 | ||||
| Grain yield | 0.79 ** | 0.87 ** | 0.59 ** | 0.18 | 0.80 ** | 0.44 | 0.42 | 0.40 | 1 | |||
| Total Biomass | 0.75 ** | 0.84 ** | 0.58 ** | 0.17 | 0.76 ** | 0.38 | 0.39 | 0.36 | 0.99 ** | 1 | ||
| Grain P uptake | 0.91 ** | 0.93 ** | 0.56 * | 0.26 | 0.91 ** | 0.49 * | 0.59 ** | 0.52 * | 0.93 ** | 0.91 ** | 1 | |
| Total P uptake | 0.91 ** | 0.93 ** | 0.56 ** | 0.25 | 0.91 ** | 0.49 * | 0.59 ** | 0.53 * | 0.92 ** | 0.90 ** | 0.99 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, M.; Tian, Y.; Ma, Q.; Ahmed, W.; Mehmood, S.; Hui, X.; Wang, Z. Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China. Agronomy 2020, 10, 1818. https://doi.org/10.3390/agronomy10111818
Mahmood M, Tian Y, Ma Q, Ahmed W, Mehmood S, Hui X, Wang Z. Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China. Agronomy. 2020; 10(11):1818. https://doi.org/10.3390/agronomy10111818
Chicago/Turabian StyleMahmood, Mohsin, Yi Tian, Qingxia Ma, Waqas Ahmed, Sajid Mehmood, Xiaoli Hui, and Zhaohui Wang. 2020. "Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China" Agronomy 10, no. 11: 1818. https://doi.org/10.3390/agronomy10111818
APA StyleMahmood, M., Tian, Y., Ma, Q., Ahmed, W., Mehmood, S., Hui, X., & Wang, Z. (2020). Changes in Phosphorus Fractions and Its Availability Status in Relation to Long Term P Fertilization in Loess Plateau of China. Agronomy, 10(11), 1818. https://doi.org/10.3390/agronomy10111818







