Morphology, Phenology, Yield, and Quality of Durum Wheat Cultivated within Organic Olive Orchards of the Mediterranean Area
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Microclimatic Characteristics of Treatments
2.4. Wheat Phenology
2.5. Leaf Characteristics of Wheat
2.6. Plant Phenotyping at Maturity
2.7. Grain Quality Analysis
2.8. Statistical Analysis
3. Results
3.1. Microclimatic Dynamics within Treatments
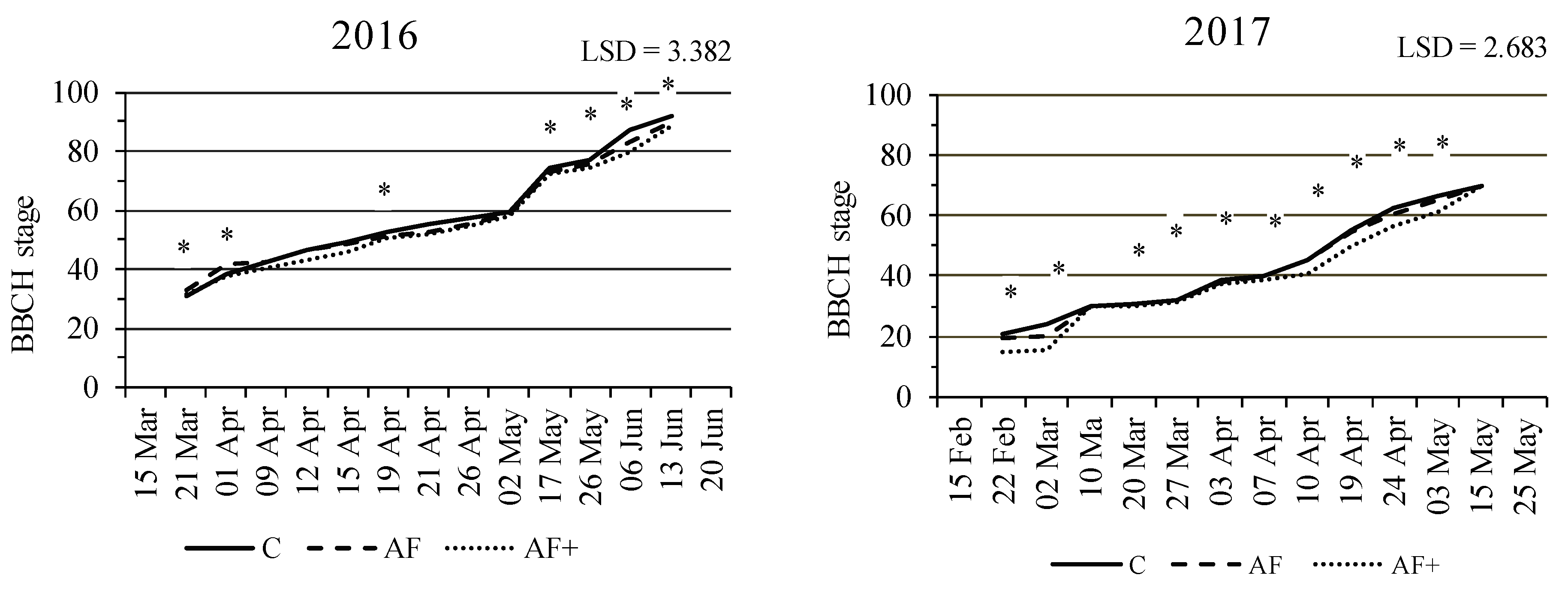
3.2. Durum Wheat Phenology
3.3. Leaf Greenness
3.4. Plant Morphology
3.5. Yield and Yield Components
3.6. Quality Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moriondo, M.; Bindi, M. Impact of climate change on the phenology of typical Mediterranean crops. Ital. J. Agrometeorol. 2007, 3, 5–12. [Google Scholar]
- Ferrise, R.; Moriondo, M.; Bindi, M. Probabilistic assessments of climate change impacts on durum wheat in the Mediterranean region. Nat. Hazards Earth Syst. Sci. 2011, 11, 1293–1302. [Google Scholar] [CrossRef]
- Gouache, D.; Le Bris, X.; Bogard, M.; Deudon, O.; Pagé, C.; Gate, P. Evaluating agronomic adaptation options to increasing heat stress under climate change during wheat grain filling in France. Eur. J. Agron. 2012, 39, 62–70. [Google Scholar] [CrossRef]
- Xiao, D.; Bai, H.; Liu, D.L. Impact of future climate change on wheat production: A simulated case for china’s wheat system. Sustainability 2018, 10, 1277. [Google Scholar] [CrossRef]
- Mosquera-Losada, M.R.; Santiago-Freijanes, J.; Rois-Díaz, M.; Moreno, G.; Herder, M.D.; Aldrey-Vázquez, J.; Ferreiro-Domínguez, N.; Pantera, A.; Pisanelli, A.; Rigueiro-Rodríguez, A. Agroforestry in Europe: A land management policy tool to combat climate change. Land Use Policy 2018, 78, 603–613. [Google Scholar] [CrossRef]
- Taguas, E.V.; Peña, A.; Ayuso, J.L.; Pérez, R.; Yuan, Y.; Giráldez, J.V. Rainfall variability and hydrological and erosive response of an olive tree microcatchment under no-tillage with a spontaneous grass cover in Spain. Earth Surf. Process. Landforms 2010, 35, 750–760. [Google Scholar] [CrossRef]
- Kavvadias, V.; Koubouris, G. Sustainable soil management practices in olive groves. In Soil Fertility Management for Sustainable Development; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 167–188. [Google Scholar]
- Duarte, F.; Jones, N.; Fleskens, L. Traditional olive orchards on sloping land: Sustainability or abandonment? J. Environ. Manag. 2008, 89, 86–98. [Google Scholar] [CrossRef]
- Swieter, A.; Langhof, M.; Lamerre, J.; Greef, J.M. Long-term yields of oilseed rape and winter wheat in a short rotation alley cropping agroforestry system. Agrofor. Syst. 2019, 93, 1853–1864. [Google Scholar] [CrossRef]
- Pardon, P.; Reubens, B.; Mertens, J.; Verheyen, K.; De Frenne, P.; De Smet, G.; Van Waes, C.; Reheul, D. Effects of temperate agroforestry on yield and quality of different arable intercrops. Agric. Syst. 2018, 166, 135–151. [Google Scholar] [CrossRef]
- Garatuza-Payan, J.; Argentel-Martinez, L.; Yépez, E.A.; Arredondo, T. Initial response of phenology and yield components of wheat (Triticum durum L., CIRNO C2008) under experimental warming field conditions in the Yaqui Valley. PeerJ 2018, 6, e5064. [Google Scholar] [CrossRef]
- Mäkinen, H.; Kaseva, J.; Trnka, M.; Balek, J.; Kersebaum, K.; Nendel, C.; Gobin, A.; Olesen, J.; Bindi, M.; Ferrise, R.; et al. Sensitivity of European wheat to extreme weather. Field Crops Res. 2018, 222, 209–217. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Phenological development-yield relationships in durum wheat cultivars under late-season high-temperature stress in a semiarid environment. ISRN Agron. 2012, 2012, 456856. [Google Scholar] [CrossRef]
- Carboni, G. Evaluation of Conservation Tillage and Rotation with Legumes as Adaptation and Mitigation Strategies of Climate Change on Durum Wheat in Sardinia. Ph.D. Thesis, Università degli Studi di Sassari, Sassari, Italy, 2011. [Google Scholar]
- Dufour, L.; Metay, A.; Talbot, G.; Dupraz, C. Assessing light competition for cereal production in temperate agroforestry systems using experimentation and crop modelling. J. Agron. Crop. Sci. 2012, 199, 217–227. [Google Scholar] [CrossRef]
- Artru, S.; Garré, S.; Dupraz, C.; Hiel, M.-P.; Blitz-Frayret, C.; Lassois, L. Impact of spatio-temporal shade dynamics on wheat growth and yield, perspectives for temperate agroforestry. Eur. J. Agron. 2017, 82, 60–70. [Google Scholar] [CrossRef]
- Li, F.; Meng, P.; Fu, D.; Wang, B. Light distribution, photosynthetic rate and yield in a Paulownia-wheat intercropping system in China. Agrofor. Syst. 2008, 74, 163–172. [Google Scholar] [CrossRef]
- Wang, B.J.; Zhang, W.; Ahanbieke, P.; Gan, Y.W.; Xu, W.L.; Li, L.H.; Christie, P. Interspecific interactions alter root length density, root diameter and specific root length in jujube/wheat agroforestry systems. Agrofor. Syst. 2014, 88, 835–850. [Google Scholar] [CrossRef]
- Retkute, R.; Smith-Unna, S.E.; Smith, R.W.; Burgess, A.J.; Jensen, O.; Johnson, G.N.; Preston, S.P.; Murchie, E.H. Exploiting heterogeneous environments: Does photosynthetic acclimation optimize carbon gain in fluctuating light? J. Exp. Bot. 2015, 66, 2437–2447. [Google Scholar] [CrossRef]
- Ehret, M.; Gras, R.; Wachendorf, M. The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems. Agrofor. Syst. 2015, 89, 557–570. [Google Scholar] [CrossRef]
- Barro, R.S.; Varella, A.C.; Lemaire, G.; De Medeiros, R.B.; De Saibro, J.C.; Nabinger, C.; Bangel, F.V.; Carassai, I.J. Forage yield and nitrogen nutrition dynamics of warm-season native forage genotypes under two shading levels and in full sunlight. Rev. Bras. Zootec. 2012, 41, 1589–1597. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Sharma, S.C.; Beri, V.; Yadav, S.; Gupta, N. Yield and carbon sequestration potential of wheat (Triticum aestivum)-poplar (Populus deltoides) based agri-silvicultural system. Indian J. Agric. Sci. 2010, 80, 129–135. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Hoel, B.O. Effect of irradiance on chlorophyll estimation with the minolta SPAD-502 leaf chlorophyll meter. Ann. Bot. 1998, 82, 389–392. [Google Scholar] [CrossRef]
- Chang, S.X.; Robison, D.J. Nondestructive and rapid estimation of hardwood foliar nitrogen status using the SPAD-502 chlorophyll meter. For. Ecol. Manag. 2003, 181, 331–338. [Google Scholar] [CrossRef]
- Dachkevitch, T.; Autran, J.C. Prediction of baking quality of bread wheats in breeding programs by size-exclusion high-performance liquid chromatography. Cereal Chem. 1989, 66, 448–456. [Google Scholar]
- Morel, M.-H.; Dehlon, P.; Autran, J.C.; Leygue, J.P.; Bar-L’Helgouac’h, C. Effects of temperature, sonication time, and power settings on size distribution and extractability of total wheat flour proteins as determined by size-exclusion high-performance liquid chromatography. Cereal Chem. J. 2000, 77, 685–691. [Google Scholar] [CrossRef]
- Samson, M.-F.; Morel, M.-H. Heat denaturation of durum wheat semolina β-amylase effects of chemical factors and pasta processing conditions. J. Food Sci. 1995, 60, 1313–1320. [Google Scholar] [CrossRef]
- Morel, M.-H.; Bar-L’Helgouac’H, C. Reliable Estimates of Gliadin, Total and Unextractable Glutenin Polymers and Total Protein Content, from Single Se-Hplc Analysis of Total Wheat Flour Protein Extract; Royal Society of Chemistry (RSC): London, UK, 2007; pp. 140–143. [Google Scholar]
- Panozzo, A.; Huan, H.Y.; Bernazeau, B.; Meunier, F.; Turc, O.; Duponnois, R.; Prin, Y.; Desclaux, D. Impact of olive trees on the microclimatic and edaphic environment of the understorey durum wheat in a Mediterranean alley cropping system. Arch. Agron. Soil. Sci. (under review).
- Bisht, N.; Sah, V.K.; Satyawali, K.; Tewari, S.; Kandpal, G. Assessment of soil quality and wheat yield under open and poplar based farming system in Tarai region of Uttarakhand. Indian J. Agric. Res. 2018, 52. [Google Scholar] [CrossRef]
- Sida, T.S.; Baudron, F.; Kim, H.; Giller, K.E. Climate-smart agroforestry: Faidherbia albida trees buffer wheat against climatic extremes in the Central Rift Valley of Ethiopia. Agric. For. Meteorol. 2018, 248, 339–347. [Google Scholar] [CrossRef]
- Francis, G.; Oliver, J.; Sujatha, M. High yielding and trait specific genotypes and genetic associations among yield and yield contributing traits in Jatropha curcas L. Agrofor. Syst. 2017, 92, 1417–1436. [Google Scholar] [CrossRef]
- Yang, T.; Duan, Z.P.; Zhu, Y.; Gan, Y.W.; Wang, B.J.; Hao, X.D.; Xu, W.L.; Zhang, W.; Li, L.H. Effects of distance from a tree line on photosynthetic characteristics and yield of wheat in a jujube tree/wheat agroforestry system. Agrofor. Syst. 2018, 93, 1545–1555. [Google Scholar] [CrossRef]
- Kanzler, M.; Böhm, C.; Mirck, J.; Schmitt, D.; Veste, M. Microclimate effects on evaporation and winter wheat (Triticum aestivum L.) yield within a temperate agroforestry system. Agrofor. Syst. 2019, 93, 1821–1841. [Google Scholar] [CrossRef]
- Inurreta-Aguirre, H.D.; Lauri, P.-E.; Dupraz, C.; Gosme, M. Yield components and phenology of durum wheat in a Mediterranean alley-cropping system. Agrofor. Syst. 2018, 92, 961–974. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Jeuffroy, M.-H. Effects of nitrogen and radiation on dry matter and nitrogen accumulation in the spike of winter wheat. Field Crops Res. 2004, 87, 221–233. [Google Scholar] [CrossRef]
- Campuzano, G.E.; Miralles, D.J.; Slafer, G.A. Yield determination in triticale as affected by radiation in different development phases. Eur. J. Agron. 2008, 28, 597–605. [Google Scholar] [CrossRef]
- Renaud, V.; Innes, J.L.; Dobbertin, M.; Rebetez, M. Comparison between open-site and below-canopy climatic conditions in Switzerland for different types of forests over 10 years (1998−2007). Theor. Appl. Clim. 2010, 105, 119–127. [Google Scholar] [CrossRef]
- Gosme, M.; Dufour, L.; Inurreta-Aguirre, H.; Dupraz, C. Microclimate effect of agroforestry on diurnal temperature cycle. In Proceedings of the 3rd European Agroforestry Conference—Montpellier, Montpellier, France, 23–25 May 2016; pp. 183–186. [Google Scholar]
- García, G.A.; Dreccer, M.F.; Miralles, D.J.; Serrago, R.A. High night temperatures during grain number determination reduce wheat and barley grain yield: A field study. Glob. Chang. Biol. 2015, 21, 4153–4164. [Google Scholar] [CrossRef]
- García, G.A.; Miralles, D.J.; Serrago, R.A.; Alzueta, I.; Huth, N.I.; Dreccer, M.F. Warm nights in the Argentine Pampas: Modelling its impact on wheat and barley shows yield reductions. Agric. Syst. 2018, 162, 259–268. [Google Scholar] [CrossRef]
- Hossain, A.; Da Silva, J.A.T. Phenology, growth and yield of three wheat (Triticum aestivum L.) varieties as affected by high temperature stress. Not. Sci. Biol. 2012, 4, 97–109. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of shading on morphology, physiology and grain yield of winter wheat. Eur. J. Agron. 2010, 33, 267–275. [Google Scholar] [CrossRef]
- Gill, R.I.S.; Singh, B.; Kaur, N. Productivity and nutrient uptake of newly released wheat varieties at different sowing times under poplar plantation in north-western India. Agrofor. Syst. 2009, 76, 579–590. [Google Scholar] [CrossRef]
- Arenas-Corraliza, M.G.; López-Díaz, M.L.; Moreno, G. Winter cereal production in a Mediterranean silvoarable walnut system in the face of climate change. Agric. Ecosyst. Environ. 2018, 264, 111–118. [Google Scholar] [CrossRef]
- Lakshmanakumar, P.; Bana, O.P.S.; Guru, S.K. Physiological basis of yield variability in wheat (Triticum aestivum L.) under varying degree of shades. Indian J. Plant. Physiol. 2013, 18, 164–168. [Google Scholar] [CrossRef]
- Gu, L.; Baldocchi, D.; Verma, S.B.; Black, T.A.; Vesala, T.; Falge, E.; Dowty, P.R. Advantages of diffuse radiation for terrestrial ecosystem productivity. J. Geophys. Res. Space Phys. 2002, 107, 2. [Google Scholar] [CrossRef]
- Mu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Jing, Q.; Cao, W. Long-term low radiation decreases leaf photosynthesis, photochemical efficiency and grain yield in winter wheat. J. Agron. Crop. Sci. 2010, 196, 38–47. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, Y.; He, M.; Zhang, Y.; Lu, S.; Li, Q.; Shi, S. Allocation of photosynthates and grain growth of two wheat cultivars with different potential grain growth in response to pre- and post-anthesis shading. J. Agron. Crop. Sci. 2003, 189, 280–285. [Google Scholar] [CrossRef]
- Panozzo, A.; Bernazeau, B.; Desclaux, D. Durum wheat in organic olive orchard: Good deal for the farmers? Agrofor. Syst. 2019, 94, 707–717. [Google Scholar] [CrossRef]

| Plant Height (cm) | Spike Length (cm) | Last Internode Length (cm) | Leaf MTA (°) | Leaf Growth Habit (1–9 Visual Scale) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 2015 | 2016 | 2017 | Average | 2015 | 2016 | 2017 | Average | 2015 | 2016 | 2017 | Average | Average 2017 | Average 2017 |
| C | 81.84 (±2.57) a | 73.78 (±2.34) a | 75.17 (±2.26) a | 75.95 (±1.41) a | 7.18 (±0.19) a | 5.91 (±0.17) a | 6.02 (±0.19) a | 6.21 (±0.12) a | 14.22 (±0.63) a | 11.37 (±0.59) a | 18.17 (±0.76) a | 14.66 (±0.49) a | 64.18 (±0.32) b | 2.79 (±0.11) a |
| AF | 71.67 (±2.27) b | 63.03 (1.47) b | 72.72 (±2.75) a | 68.66 (±1.38) b (−10%) | 6.10 (±0.18) b | 4.46 (±0.13) b | 4.61 (±0.14) b | 4.86 (±0.10) b (−22%) | 14.37 (±0.69) a | 12.44 (±0.45) a | 18.67 (±0.66) a | 15.31 (±0.43) a (+4%) | 62.19 (±0.38) c (−3%) | 2.96 (±0.12) a (+6%) |
| AF+ | 70.89 (±2.58) b | 62.10 (±1.56) b | 59.98 (±1.74) b | 62.25 (±1.11) c (−18%) | 5.28 (±0.19) c | 4.31 (±0.13) b | 3.53 (±0.10) c | 4.09 (±0.09) c (−34%) | 16.09 (±0.83) a | 10.78 (±0.44) a | 16.62 (±0.62) a | 14.00 (±0.44) a (−5%) | 66.27 (±0.49) a (+4%) | 1.56 (±0.09) b (−44%) |
| Treatment | Grain Yield (t/ha) | HI | Biomass/Plant (g DM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | Average (%var/C) | 2016 | 2017 | Average (%var/C) | 2016 | 2017 | Average (%var/C) | |
| C | 0.97 (±0.12) a | 1.58 (±0.10) a | 2.29 (±0.09) a | 1.76 (±0.07) a | 0.28 (±0.01) b | 0.34 (±0.01) a | 0.31 (±0.01) a | 112.40 (±4.75) a | 105.74 (±3.96) a | 109.07 (±3.09) a |
| AF | 1.21 (±0.07) a | 0.91 (±0.05) b | 0.99 (±0.08) b | 1.00 (±0.04) b (−43%) | 0.32 (±0.01) a | 0.34 (±0.01) a | 0.33 (±0.01) a (+6%) | 49.42 (±2.94) b | 66.59 (±4.11) b | 58.00 (±2.66) b (−47%) |
| AF+ | 0.40 (±0.04) b | 0.48 (±0.03) c | 0.10 (±0.01) c | 0.30 (±0.02) c (−83%) | 0.25 (±0.01) b | 0.20 (±0.01) b | 0.23 (±0.01) b (−27%) | 46.98 (±2.96) b | 21.02 (±1.90) c | 34.00 (±2.18) c (−69%) |
| Plants/m² | No. Spikes/Plant | No. Grains/Spike | TGW (g/1000 Kernels) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 2015 | 2016 | 2017 | Average (%var/C) | 2015 | 2016 | 2017 | Average (%var/C) | 2015 | 2016 | 2017 | Average (%var/C) | 2015 | 2016 | 2017 | Average (%var/C) |
| C | 81.25 (±10.88) b | 249.80 (±13.94) b | 241.09 (±5.92) b | 213.67 b (±8.72) | 3.22 (±0.19) a | 1.49 (±0.11) a | 2.86 (±0.16) a | 2.37 (±0.11) a | 41.32 (±4.89) a | 22.02 (1.17) a | 20.81 (±0.76) a | 25.18 (±1.31) a | 44.67 (±1.22) a | 34.90 (±1.11) c | 39.59 (±0.58) a | 38.64 (±0.65) b |
| AF | 108.45 (±10.12) a | 283.31 (±13.52) b | 310.98 (±6.22) a | 260.44 (±9.27) a (+22%) | 2.33 (±0.11) b | 1.05 (±0.04) b | 1.82 (±0.08) b | 1.61 (±0.06) b (−32%) | 25.58 (±1.20) b | 12.76 (±0.95) b | 14.28 (±0.92) b | 15.91 (±0.73) b (−37%) | 45.36 (±1.18) a | 45.26 (±1.10) a | 40.80 (±0.56) a | 43.42 (±0.57) a (+12%) |
| AF+ | 129.56 (±10.45) a | 327.06 (±13.40) b | 138.60 (±8.91) c | 220.15 (±11.40) b (+3%) | 1.71 (±0.11) c | 0.94 (±0.03) b | 1.17 (±0.08) c | 1.14 (±0.04) c (−52%) | 18.91 (±1.96) b | 10.24 (±0.79) c | 6.59 (±0.58) c | 9.70 (±0.61) c (−62%) | 40.02 (±1.22) b | 39.32 (±1.25) b | 36.90 (±1.19) b | 38.33 (±0.77) b (−0.8%) |
| Treatment | Grain Protein % DM (% var/C) | F1 | F2 | F3 | F4 | F5 | Gliadins/Glutenins Ratio (%var/C) | Fi | %UPP | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | Average (%var/C) | |||||||||
| C | 10.17 (±0.12) c | 7.51 (±0.09) a | 20.80 (±0.16) b | 9.96 (±0.08) b | 33.33 (±0.26) b | 20.15 (±0.16) a | 0.92 (±0.01) b | 8.26 (±0.26) a | 18.94 (±0.72) a | 25.92 (±0.72) a | 22.39 (±0.62) a |
| AF | 11.72 (±0.12) b (+15%) | 7.32 (±0.10) a | 21.72 (±0.16) a | 9.74 (±0.07) b | 35.03 (±0.24) a | 18.80 (±0.13) b | 0.97 (±0.01) a (+6%) | 7.39 (±0.22) b (−11%) | 18.62 (±0.68) a | 21.63 (±0.73) b | 20.14 (±0.52) b (−10%) |
| AF+ | 13.91 (±0.18) a (+37%) | 7.04 (±0.21) a | 20.57 (±0.24) b | 10.73 (±0.35) a | 35.46 (±0.37) a | 18.69 (±0.20) b | 1.02 (±0.02) a (+11%) | 7.51 (±0.26) ab (−9%) | 18.30 (±0.83) a | 23.97 (±0.82) ab | 21.19 (±0.65) ab (−5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panozzo, A.; Huang, H.; Bernazeau, B.; Vamerali, T.; Samson, M.F.; Desclaux, D. Morphology, Phenology, Yield, and Quality of Durum Wheat Cultivated within Organic Olive Orchards of the Mediterranean Area. Agronomy 2020, 10, 1789. https://doi.org/10.3390/agronomy10111789
Panozzo A, Huang H, Bernazeau B, Vamerali T, Samson MF, Desclaux D. Morphology, Phenology, Yield, and Quality of Durum Wheat Cultivated within Organic Olive Orchards of the Mediterranean Area. Agronomy. 2020; 10(11):1789. https://doi.org/10.3390/agronomy10111789
Chicago/Turabian StylePanozzo, Anna, Hsinya Huang, Bruno Bernazeau, Teofilo Vamerali, Marie Françoise Samson, and Dominique Desclaux. 2020. "Morphology, Phenology, Yield, and Quality of Durum Wheat Cultivated within Organic Olive Orchards of the Mediterranean Area" Agronomy 10, no. 11: 1789. https://doi.org/10.3390/agronomy10111789
APA StylePanozzo, A., Huang, H., Bernazeau, B., Vamerali, T., Samson, M. F., & Desclaux, D. (2020). Morphology, Phenology, Yield, and Quality of Durum Wheat Cultivated within Organic Olive Orchards of the Mediterranean Area. Agronomy, 10(11), 1789. https://doi.org/10.3390/agronomy10111789






