Evaluation of Factors Affecting Direct Organogenesis in a Somatic Tissue Culture of Sinningia speciosa (Lodd.) Hiern
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Induction of In Vitro Organogenesis from Leaf Explants
2.3. Root Formation and Plantlet Acclimatization
2.4. Statistical Analysis
3. Results and Discussion
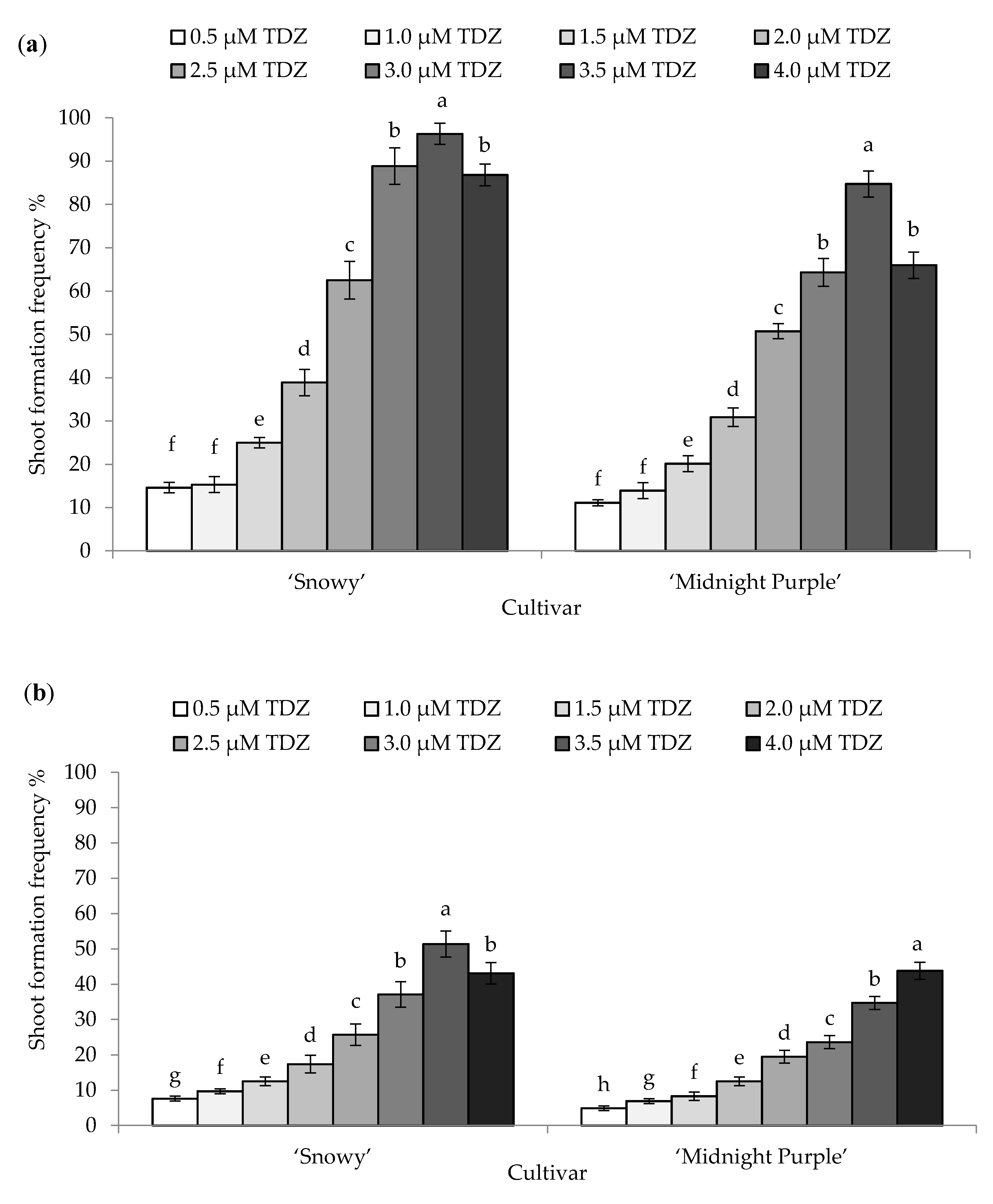
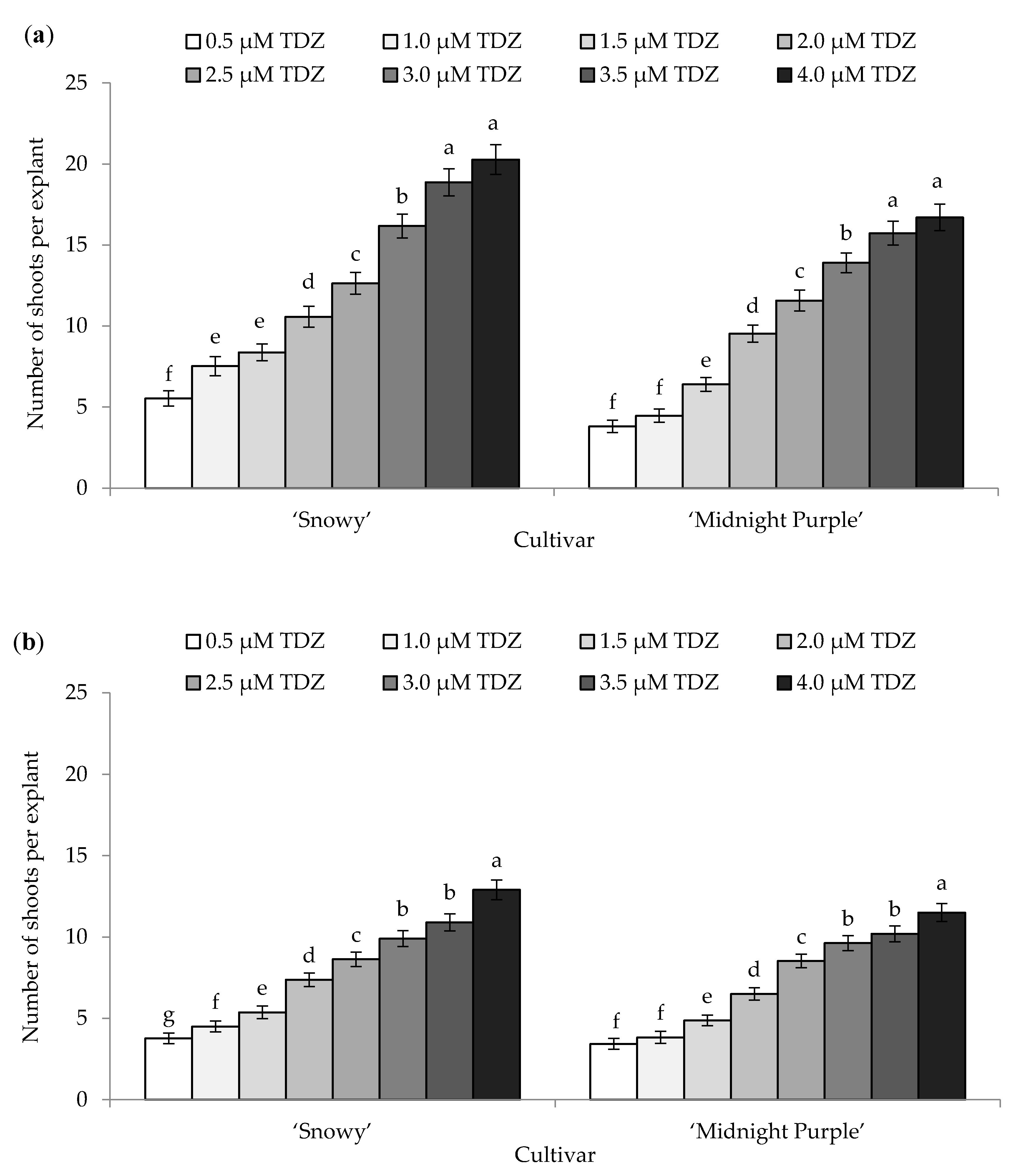
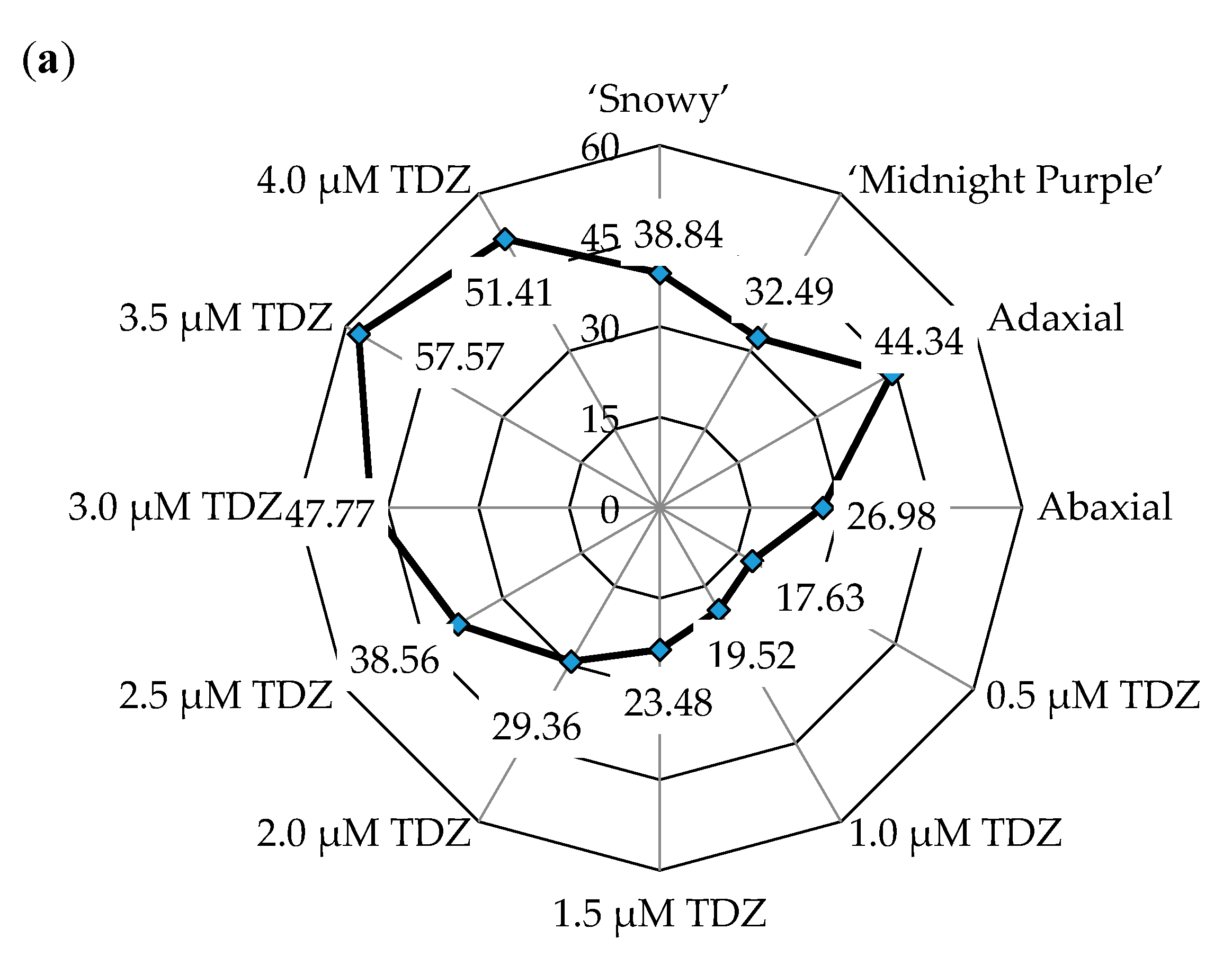
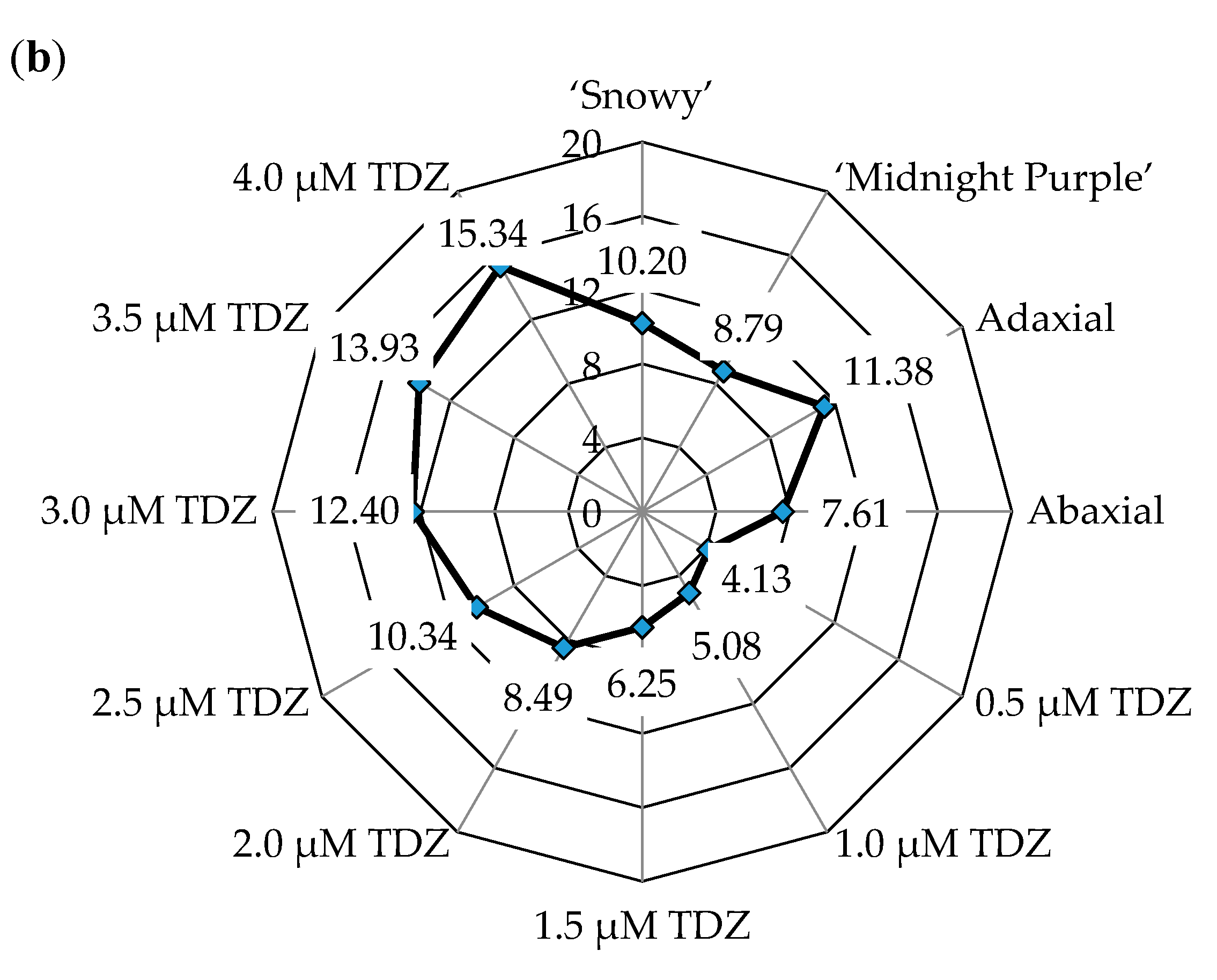
3.1. Adventitious Shoot Formation from Leaf Explants
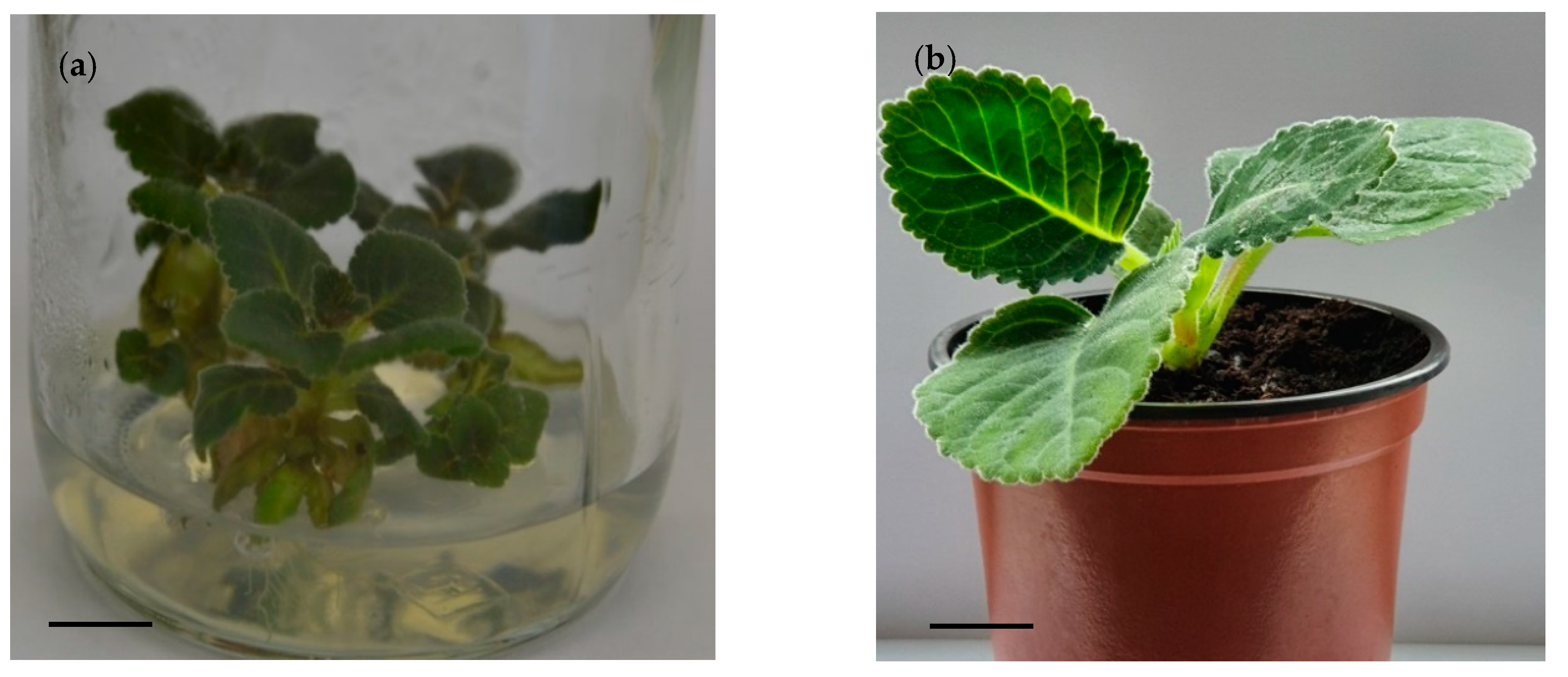
3.2. Root Formation and Plantlets Acclimatization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scaramuzzi, F.; Apollonio, G.; D’Emerico, S. Adventitious shoot regeneration from Sinningia speciosa leaf discs in vitro and stability of ploidy level in subcultures. Vitr. Cell Dev. Biol.-Plant 1999, 35, 217–221. [Google Scholar] [CrossRef]
- Nhut, D.T.; Phu, T.H.; Huyen, P.X.; Thuy, D.T.T. Effects of in vitro leaf explants and leaf size on direct shoot regeneration of gloxinia. Propag. Ornam. Plants 2007, 7, 16–22. [Google Scholar]
- Takagi, H.; Sugawara, S.; Saito, T.; Tasaki, H.; Yuanxue, L.; Kaiyun, G.; Han, D.S.; Godo, T.; Nakano, M. Plant regeneration via direct and indirect adventitious shoot formation and chromosome-doubled somaclonal variation in Titanotrichum oldhamii (Hemsl.) Solereder. Plant Biotechnol. Rep. 2011, 5, 187–195. [Google Scholar] [CrossRef]
- Chautems, A.; Baracho, G.S.; Filho, J.S. A new species of Sinningia (Gesneriaceae) from northeastern Brazil. Brittonia 2000, 52, 49–53. [Google Scholar] [CrossRef]
- Zaitlin, D.; Pierce, A.J. Nuclear DNA content in Sinningia (Gesneriaceae); intraspecific genome size variation and genome characterization in S. speciosa. Genome 2010, 53, 1066–1082. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant. A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef] [PubMed]
- Mithila, J.; Hall, J.C.; Victor, J.M.R.; Saxena, P.K. Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl.). Plant Cell Rep. 2003, 21, 408–414. [Google Scholar] [CrossRef]
- Cui, J.; Chen, J.J.; Henny, R.J. Regeneration of Aeschynanthus radicans via direct somatic embryogenesis and analysis of regenerants with flow cytometry. Vitr. Cell Dev. Biol. Plant 2009, 45, 34–43. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Murch, S.J.; Sullivan, J.A.; Saxena, P.K. Development of an efficient protocol for high frequency in vitro regeneration of a horticultural plant Primulina tamiana (B.L. Burtt) Mich. Möller & A. Webber. Can. J. Plant Sci. 2014, 94, 1281–1287. [Google Scholar]
- Pang, J.L.; Wang, L.L.; Hu, J.Q.; Xiang, T.H.; Liang, H.M. Synergistic promotion of gibberellin and cytokinin on direct regeneration of floral buds from in vitro cultures of sepal segments in Sinningia speciosa Hiern. Vitr. Cell Dev. Biol. Plant 2006, 42, 450–454. [Google Scholar] [CrossRef]
- Pang, J.; Wang, L.; Xiang, T.; Zhong, D.; Yu, H. High-frequency floral bud regeneration from petal segment cultures of Sinningia speciosa Hiern. Chin. J. Cell Biol. 2012, 34, 279–285. [Google Scholar]
- Macas-Palacios, G.C.; Pucha-Pauta, E.E.; Delgado-Paredes, G.E.; Rojas-Idrogo, C.; Minchala-Patino, J. In vitro plant regeneration in gloxinia (Sinningia speciosa (Lodd.) Hiern.). J. Biol. 2015, 3, 6–14. [Google Scholar]
- Xu, Q.L.; Hu, Z.; Li, C.Y.; Wang, X.Y.; Wang, C.Y. Tissue culture of Sinningia speciosa and analysis of the in vitro-generated tricussate whorled phyllotaxis (twp) variant. Vitr. Cell Dev. Biol. Plant 2009, 45, 583–590. [Google Scholar] [CrossRef]
- Park, E.H.; Bae, H.; Park, W.T.; Kim, Y.B.; Chae, S.C.; Park, S.U. Improved shoot organogenesis of gloxinia (Sinningia speciosa) using silver nitrate and putrescine treatment. Plant Omics 2012, 5, 6–9. [Google Scholar]
- Sharma, S.K.; Sharma, M. Improved protocol for in vitro propagation of gloxinia (Sinningia sp.). J. Cell Tissue Res. 2013, 13, 3545–3548. [Google Scholar]
- Zhou, G.Y.; Zhou, W.H.; Cheng, L. Preliminary study on dormancy of gloxinia in vitro. Acta Agric. Shanghai 2000, 16, 69–72. [Google Scholar]
- Lu, C. The use of thidiazuron in tissue culture. Vitr. Cell Dev. Biol. Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Singh, N.D.; Sahoo, L.; Sarin, N.B.; Jaiwal, P.K. The effect of TDZ on organogenesis and somatic embryogenesis in pigeonpea (Cajanus cajan (L.) Millsp). Plant Sc. 2003, 164, 341–347. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Wang, Y.H.; Raman, V.; Khan, I.A. TDZ-induced high frequency plant regeneration through direct shoot organogenesis in Stevia rebaudiana Bertoni: An important medicinal plant and a natural sweetener. Am. J. Plant Sci. 2013, 4, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Vujovic, T.; Ruzic, D.; Cerovic, R.; Momirovic, G.S. Adventitious regeneration in blackberry (Rubus fruticosus L.) and assessment of genetic stability in regenerants. Plant Growth Regul. 2010, 61, 265–275. [Google Scholar] [CrossRef]
- Cappelletti, R.; Sabbadini, S.; Mezzetti, B. The use of TDZ for the efficient in vitro regeneration and organogenesis of strawberry and blueberry cultivars. Sci. Hortic. 2016, 207, 117–124. [Google Scholar] [CrossRef]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Raudonius, S. Application of statistics in plant and crop research: Important issues. Zemdirb. Agric. 2017, 104, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Compton, M.E. Statistical methods suitable for the analysis of plant tissue culture data. Plant Cell Tissue Organ Cult. 1994, 37, 217–242. [Google Scholar]
- Datta, S.K.; Chakrabarty, D.; Saxena, M.; Mandal, A.K.A.; Biswas, A.K. Direct shoot generation from florets of chrysanthemum cultivars. J. Genet. Plant Breed. 2001, 61, 373–376. [Google Scholar]
- Chen, X.; Kane, M.E.; Chen, J. Effect of genotype, explant source, and plant growth regulators on indirect shoot organogenesis in Dieffenbachia cultivars. Vitr. Cell Dev. Biol. Plant 2008, 44, 282–288. [Google Scholar]
- Yusnita, Y.; Pungkastiani, W.; Hapsoro, D. In vitro organogenesis of two Sansevieria cultivars on different concentrations of benzyladenine (BA). Agrivita 2011, 33, 147–153. [Google Scholar]
- Burbulis, N.; Blinstrubienė, A.; Jonytienė, V. In vitro regeneration from leaf explants of Petunia hybrida L. Propag. Ornam. Plants 2015, 15, 47–52. [Google Scholar]
- Murthy, B.N.S.; Murch, S.J.; Saxena, P.K. Thidiazuron: A potent regulator of in vitro plant morphogenesis. Vitr. Cell Dev. Biol. Plant 1998, 34, 267–275. [Google Scholar] [CrossRef]
- Murch, S.J.; Saxena, P.K. Molecular fate of thidiazuron and its effects on auxin transport in hypocotyl tissues of Pelargonium hortorum Bailey. Plant Growth Regul. 2001, 35, 269–275. [Google Scholar] [CrossRef]
- Jones, M.P.A.; Cao, J.; O’Brien, R.; Murch, S.J.; Saxena, P.K. The mode of action of thidiazuron: Auxins, indoleamines, and ion channels in the regeneration of Echinacea purpurea L. Plant Cell Rep. 2007, 26, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Mundhara, R.; Rashid, A. TDZ-induced triple-response and shoot formation on intact seedlings of Linum, putative role of ethylene in regeneration. Plant Sci. 2006, 170, 185–190. [Google Scholar] [CrossRef]
- Taha, R.M.; Daud, N.; Hasbullah, N.A.; Awal, A. Somatic embryogenesis and production of artificial seeds in Saintapaulia ionantha Wendl. Acta Hortic. 2009, 829, 331–336. [Google Scholar] [CrossRef]
- Ma, G.H.; Lu, J.; da Silva, J.A.T.; Zhang, X.; Zhao, J. Shoot organogenesis and somatic embryogenesis from leaf and shoot explants of Ochna integerrima (Lour.). Plant Cell Tissue Organ Cult. 2011, 104, 157–162. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. Thidiazuron (TDZ) induced plant regeneration from cotyledonary petiole explants of elite genotypes of Jatropha curcas: A candidate biodiesel plant. Ind. Crop. Prod. 2012, 39, 62–68. [Google Scholar] [CrossRef]

| Effect | F-Values of Shoot Formation Frequency (%) | p-Values for Shoot Formation Frequency | F-Values of Number of Shoots per Explant | p-Values for Number of Shoots per Explant |
|---|---|---|---|---|
| Cultivar (C) | 95.21 ** | <0.001 | 20.13 ** | <0.001 |
| TDZ concentration (T) | 710.67 ** | <0.001 | 141.96 ** | <0.001 |
| Position of explant on culture medium (P) | 279.06 ** | <0.001 | 87.89 ** | <0.001 |
| C × T | 8.08 ** | 0.006 | 6.62 * | 0.012 |
| C × P | 4.95 ** | <0.001 | 0.48 ns | 0.842 |
| T × P | 30.95 ** | <0.001 | 5.6 ** | <0.001 |
| C × T × P | 2.45 ** | 0.027 | 0.19ns | 0.986 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blinstrubienė, A.; Burbulis, N.; Jonytienė, V.; Masienė, R. Evaluation of Factors Affecting Direct Organogenesis in a Somatic Tissue Culture of Sinningia speciosa (Lodd.) Hiern. Agronomy 2020, 10, 1783. https://doi.org/10.3390/agronomy10111783
Blinstrubienė A, Burbulis N, Jonytienė V, Masienė R. Evaluation of Factors Affecting Direct Organogenesis in a Somatic Tissue Culture of Sinningia speciosa (Lodd.) Hiern. Agronomy. 2020; 10(11):1783. https://doi.org/10.3390/agronomy10111783
Chicago/Turabian StyleBlinstrubienė, Aušra, Natalija Burbulis, Vaida Jonytienė, and Ramunė Masienė. 2020. "Evaluation of Factors Affecting Direct Organogenesis in a Somatic Tissue Culture of Sinningia speciosa (Lodd.) Hiern" Agronomy 10, no. 11: 1783. https://doi.org/10.3390/agronomy10111783
APA StyleBlinstrubienė, A., Burbulis, N., Jonytienė, V., & Masienė, R. (2020). Evaluation of Factors Affecting Direct Organogenesis in a Somatic Tissue Culture of Sinningia speciosa (Lodd.) Hiern. Agronomy, 10(11), 1783. https://doi.org/10.3390/agronomy10111783





