Leaf Nitrogen Traits in Response to Plant Density and Nitrogen Supply in Oilseed Rape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Treatments
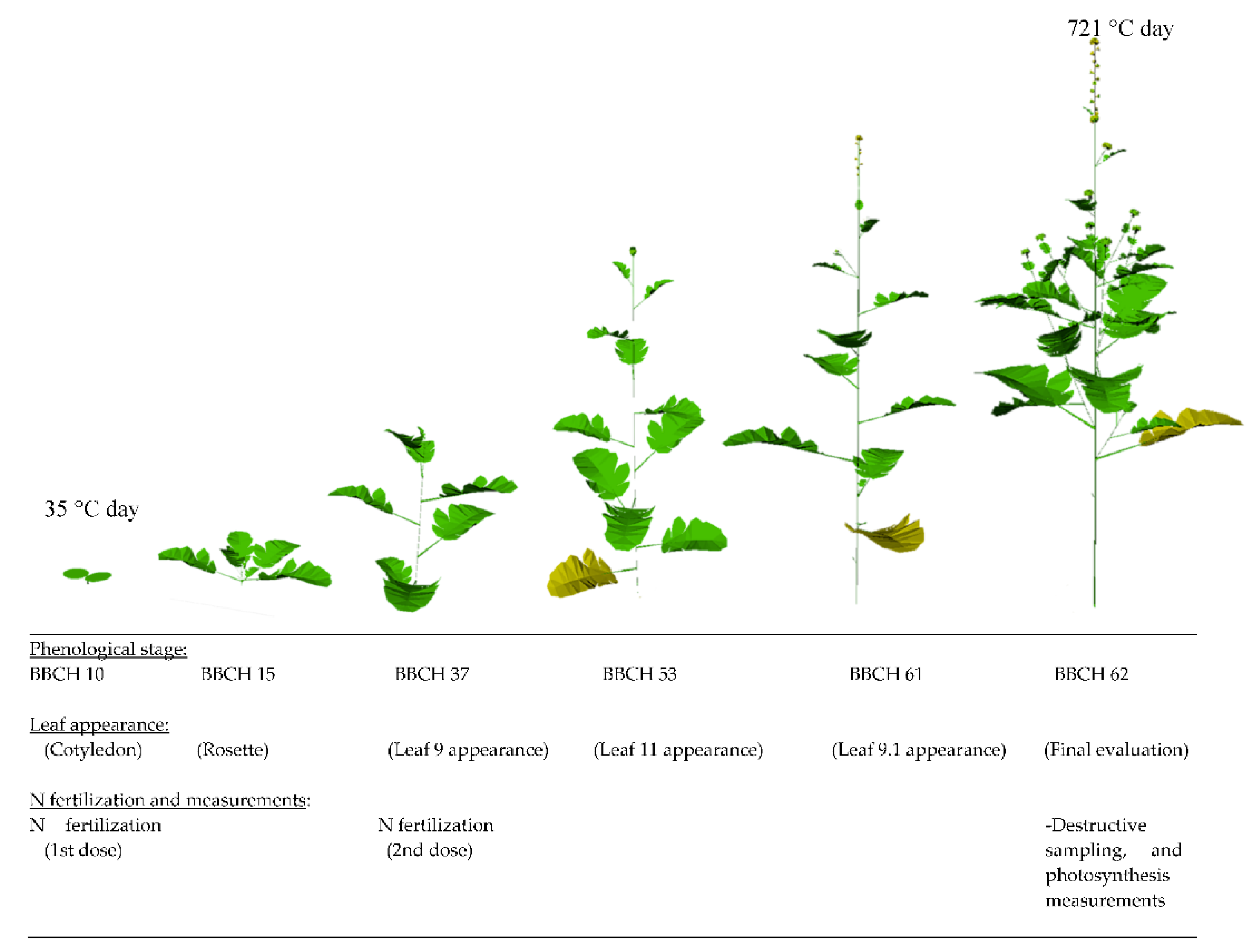
2.2. Temperature Conditions
2.3. Plant Measurements
2.4. Soil Sampling
2.5. Statistical Analysis
3. Results
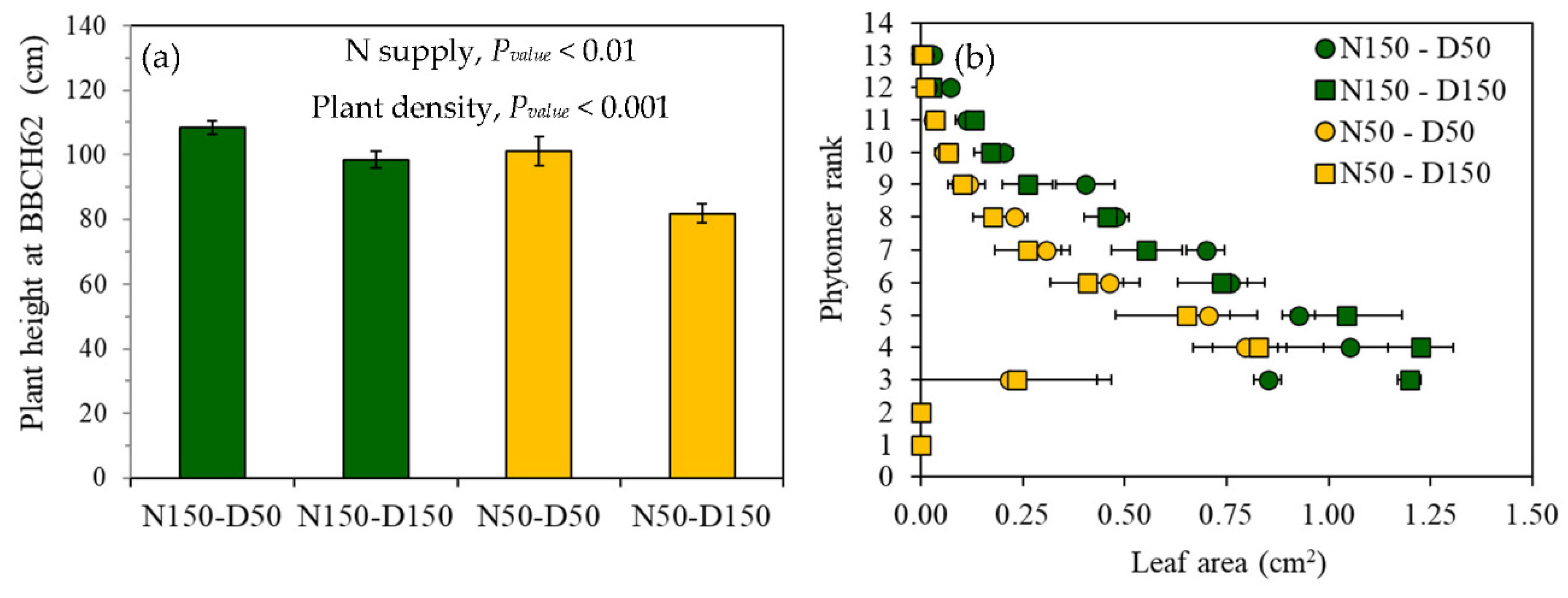
3.1. Plant and Leaf Characteristics
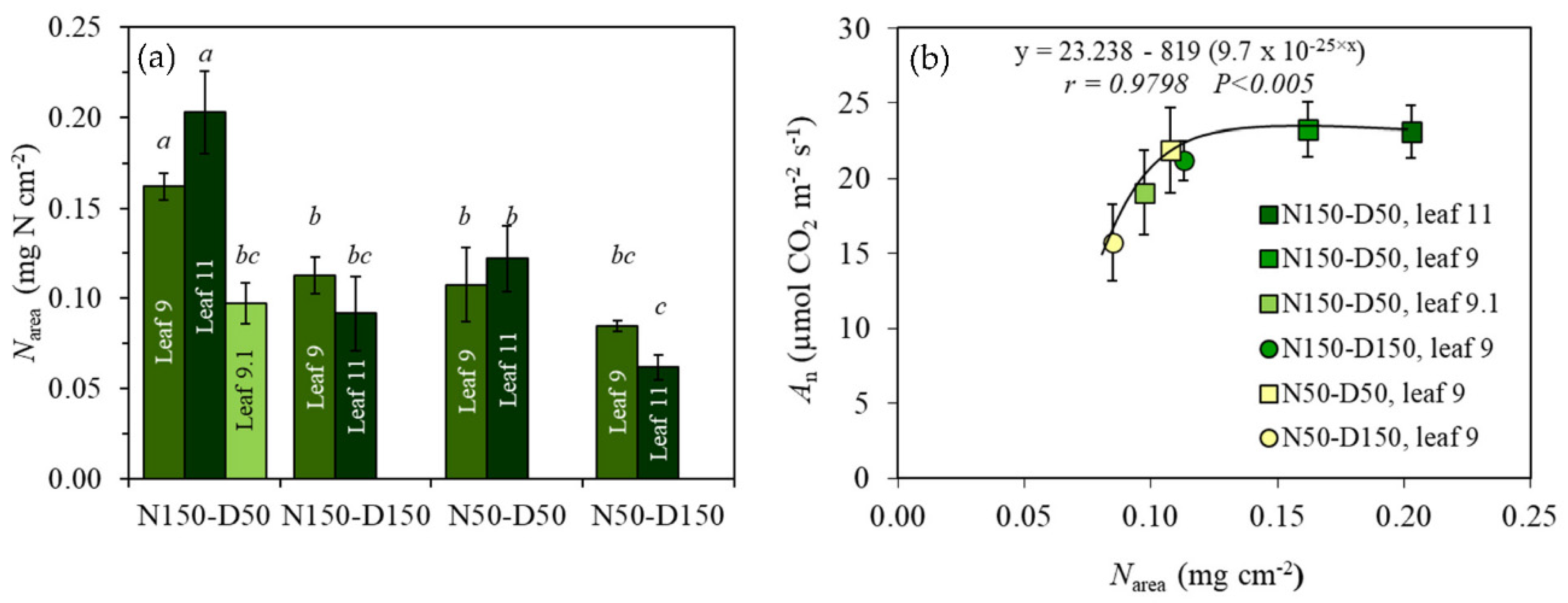
3.2. Leaf N Content (Narea) and Photosynthesis
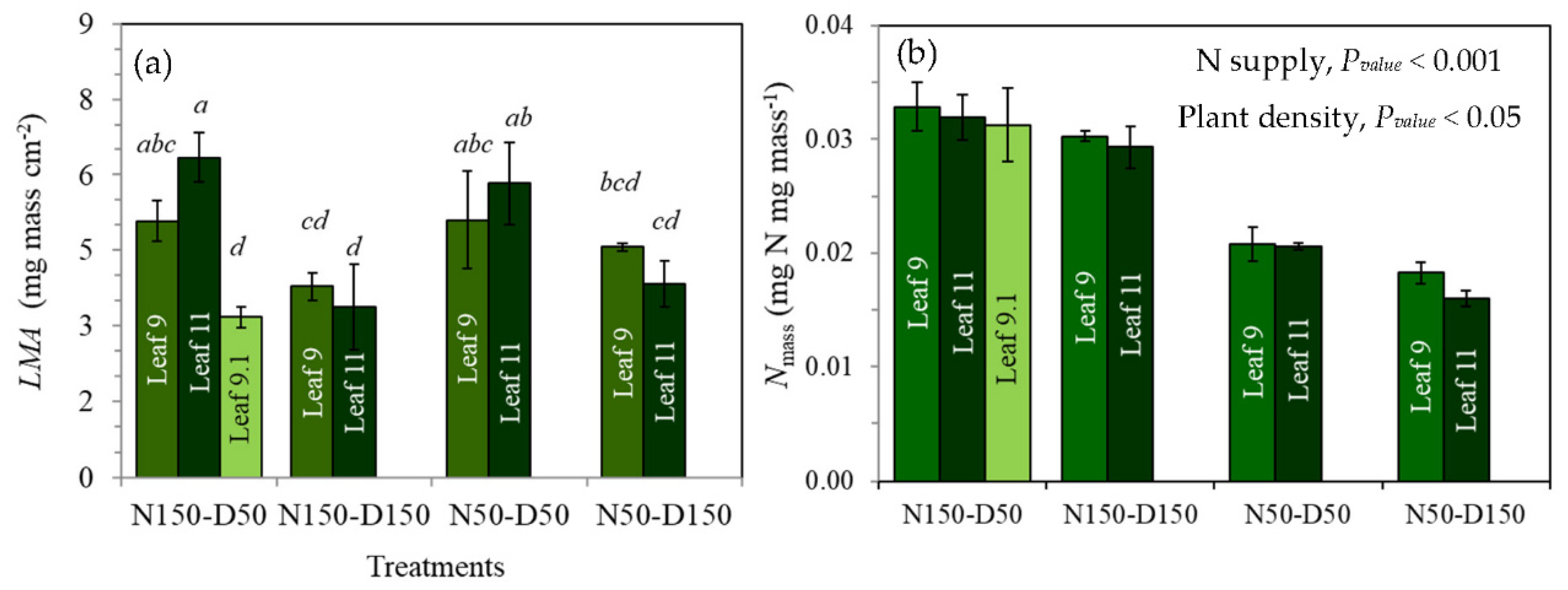
3.3. Physiological and Structural Determinants of Narea
4. Discussion
4.1. Effects at Plant Level
4.2. Effects at Leaf Level
4.3. Implications of the Present Results and Further Research
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momoh, E.J.; Song, W.J.; Li, H.Z.; Zhou, W.J. Seed yield and quality responses of winter oilseed rape (Brassica napus) to plant density and nitrogen fertilization. Indian J. Agric. Sci. 2004, 74, 420–424. [Google Scholar]
- Rondanini, D.P.; Gomez, N.V.; Agosti, M.B.; Miralles, D.J. Global trends of rapeseed grain yield stability and rapeseed-to-wheat yield ratio in the last four decades. Eur. J. Agron. 2012, 37, 56–65. [Google Scholar] [CrossRef]
- FAOSTAT. Crop production. Food and Agriculture Organization of the United Nations, Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 29 February 2020).
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crops Res. 2008, 107, 89–101. [Google Scholar] [CrossRef]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Vollrath, P.; Samans, B.; Frisch, M.; Wittkop, B.; Snowdon, R.J. Effect of breeding on nitrogen use efficiency-associated traits in oilseed rape. J. Exp. Bot. 2019, 70, 1969–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gastal, F.; Lemaire, G.; Durand, J.L.; Louarn, G. Quantifying crop responses to nitrogen and avenues to improve nitrogen-use efficiency. In Crop Physiology—Applications for Genetic Improvement and Agronomy, 2nd ed.; Sadras, V.O., Calderini, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 161–206. [Google Scholar]
- Roques, S.E.; Berry, P.M. The yield response of oilseed rape to plant population density. J. Agric. Sci. 2016, 154, 305–320. [Google Scholar] [CrossRef]
- Seepaul, R.; George, S.; Wright, D.L. Comparative response of Brassica carinata and B-napus vegetative growth, development and photosynthesis to nitrogen nutrition. Ind. Crop. Prod. 2016, 94, 872–883. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Bloomfield, K.J.; Farquhar, G.D.; Lloyd, J. Photosynthesis-nitrogen relationships in tropical forest tree species as affected by soil phosphorus availability: A controlled environment study. Funct. Plant Biol. 2014, 41, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Poorter, H. Inherent variation in growth-rate between higher-plants—A search for physiological causes and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar] [CrossRef]
- Gan, Y.; Stulen, I.; Van Keulen, H.; Kuiper, P. Physiological response of soybean genotypes to plant density. Field Crop. Res. 2002, 74, 231–241. [Google Scholar] [CrossRef]
- Gammelvind, L.H.; Schjoerring, J.K.; Mogensen, V.O.; Jensen, C.R.; Bock, J.G.H. Photosynthesis in leaves and siliques of winter oilseed rape (Brassica napus L.). Plant Soil 1996, 186, 227–236. [Google Scholar] [CrossRef]
- DeJong, T.M.; Day, K.R.; Johnson, R.S. Partitioning of leaf nitrogen with respect to within canopy light exposure and nitrogen availability in peach (Prunus persica). Trees 1989, 3, 89–95. [Google Scholar] [CrossRef]
- Amanullah, M.J.H.; Nawab, K.; Ali, A. Response of specific leaf area (SLA), leaf area index (LAI) and leaf area ratio (LAR) of maize (Zea mays L.) to plant density, rate and timing of nitrogen application. World Appl. Sci. J. 2007, 2, 235–243. [Google Scholar]
- Stenger, R.; Priesack, E.; Beese, F. Spatial variation of nitrate-N and related soil properties at the plot-scale. Geoderma 2002, 105, 259–275. [Google Scholar] [CrossRef]
- Werger, M.J.A.; Hirose, T. Leaf Nitrogen Distribution and Whole Canopy Photosynthetic Carbon Gain in Herbaceous Stands. Vegetatio 1991, 97, 11–20. [Google Scholar]
- Hikosaka, K.; Terashima, I.; Katoh, S. Effects of leaf age, nitrogen nutrition and photon flux-density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav) grown horizontally to avoid mutual shading of leaves. Oecologia 1994, 97, 451–457. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Miyazawa, K.; Hikosaka, K.; Nagashima, H.; Hirose, T. Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia 1998, 113, 314–324. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Schieving, F.; Werger, M.J.A. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C-3 and C-4 monocotyledonous and dicotyledonous species. Oecologia 1995, 101, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, J.C.; van Bodegom, P.M.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Connor, D.J.; Hall, A.J.; Sadras, V.O. Effect of Nitrogen-Content on the Photosynthetic Characteristics of Sunflower Leaves. Funct. Plant Biol. 1993, 20, 251–263. [Google Scholar] [CrossRef]
- Connor, D.J.; Sadras, V.O.; Hall, A.J. Canopy Nitrogen Distribution and the Photosynthetic Performance of Sunflower Crops during Grain Filling—A Quantitative-Analysis. Oecologia 1995, 101, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 2000, 30, 1–67. [Google Scholar]
- Al-Barzinjy, M.; Stolen, O.; Christiansen, J.L. Comparison of growth, pod distribution and canopy structure of old and new cultivars of oilseed rape (Brassica napus L.). Acta Agric. Scand. Sect. B Plant Soil Sci. 2003, 53, 138–146. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Schapendonk, A.H.C.M.; van Oijen, M.; Pot, C.S.; Rabbinge, R. Radiation and nitrogen use at the leaf and canopy level by wheat and oilseed rape during the critical period for grain number definition. Aust. J. Plant Physiol. 2000, 27, 899–910. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. The critical period for yield and quality determination in canola (Brassica napus L.). Field Crop. Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Schapendonk, A.H.C.M.; Slafer, G.A.; Rabbinge, R. Comparative response of wheat and oilseed rape to nitrogen supply: Absorption and utilisation efficiency of radiation and nitrogen during the reproductive stages determining yield. Plant Soil 2000, 220, 189–205. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Andrieu, B.; Struik, P.C. Cessation of tillering in spring wheat in relation to light interception and red: Far-red ratio. Ann. Bot. 2006, 97, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Retuerto, R.; Rochefort, L.; Woodward, F.I. The influence of plant density on the responses of Sinapis alba to CO2 and windspeed. Oecologia 1996, 108, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants. BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry, Ed.; Wissenschafts-Verlag: Berlin, Germany, 2001; 158p. [Google Scholar]
- Kirkegaard, J.A.; Sprague, S.J.; Lilley, J.M.; Mc Cormick, J.I.; Virgona, J.M.; Morrison, M.J. Physiological response of spring canola (Brassica napus) to defoliation in diverse environments. Field Crop. Res. 2012, 125, 61–68. [Google Scholar] [CrossRef]
- Bailey, L.H.; Bailey, E.Z. Hortus Third: A Concise Dictionary of Plants Cultivated in the United States and Canada; Macmillan: New York, NY, USA, 1976. [Google Scholar]
- Monsi, M.; Saeki, T. On the factor light in plant communities and its importance for matter production. Ann. Bot. 2005, 95, 549–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteith, J.L. Principles of environmental physics. Int. J. Environ. Stud. 1973, 5, 154–155. [Google Scholar]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Arkoun, M.; Sarda, X.; Jannin, L.; Laine, P.; Etienne, P.; Garcia-Mina, J.M.; Yvin, J.C.; Ourry, A. Hydroponics versus field lysimeter studies of urea, ammonium and nitrate uptake by oilseed rape (Brassica napus L.). J. Exp. Bot. 2012, 63, 5245–5258. [Google Scholar] [CrossRef]
- Laine, P.; Ourry, A.; Boucaud, J. Shoot control of nitrate uptake rates by roots of Brassica napus L. Effects of localized nitrate supply. Planta 1995, 196, 77–83. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, Z.H.; Stewart, B.A. Responses of crop plants to ammonium and nitrate N. Adv. Agron. 2013, 118, 205–397. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Payne, R.W.; Murray, D.A.; Harding, S.A.; Baird, D.B.; Soutar, D.M. An Introduction to GenStat for Windows, 14th ed.; VSN International: Hemel Hempstead, UK, 2011. [Google Scholar]
- Zhang, S.; Zhi, H.; Li, W.; Shan, J.; Tang, C.; Jia, G.; Tang, S.; Diao, X. SiYGL2 Is Involved in the Regulation of Leaf Senescence and Photosystem II Efficiency in Setaria italica (L.) P. Beauv. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Gan, S.; Amasino, R.M. Making Sense of Senescence (Molecular Genetic Regulation and Manipulation of Leaf Senescence). Plant Physiol. 1997, 113, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archontoulis, S.V.; Yin, X.; Vos, J.; Danalatos, N.G.; Struik, P.C. Leaf photosynthesis and respiration of three bioenergy crops in relation to temperature and leaf nitrogen: How conserved are biochemical model parameters among crop species? J. Exp. Bot. 2012, 63, 895–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapani, N.; Hall, A.J. Effects of leaf position and nitrogen supply on the expansion of leaves of field grown sunflower (Helianthus annuus L.). Plant Soil 1996, 184, 331–340. [Google Scholar] [CrossRef]
- Reddy, M.R.; Prasad, R. Effect of nitrogen doses and row direction on LAI, light transmission, plant height and dry-matter production of wheat cultivars grown in pure and mixed stands. Biol. Plant. 1979, 21, 85–91. [Google Scholar] [CrossRef]
- Yin, X.H.; Hayes, R.M.; Mc Clure, M.A.; Savoy, H.J. Assessment of plant biomass and nitrogen nutrition with plant height in early-to mid-season corn. J. Sci. Food Agric. 2012, 92, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Muharam, F.M.; Bronson, K.F.; Maas, S.J.; Ritchie, G.L. Inter-relationships of cotton plant height, canopy width, ground cover and plant nitrogen status indicators. Field Crop. Res. 2014, 169, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Kuai, J.; Sun, Y.Y.; Zhou, M.; Zhang, P.P.; Zuo, Q.S.; Wu, J.S.; Zhou, G.S. The effect of nitrogen application and planting density on the radiation use efficiency and the stem lignin metabolism in rapeseed (Brassica napus L.). Field Crop. Res. 2016, 199, 89–98. [Google Scholar] [CrossRef]
- Xue, J.; Gou, L.; Zhao, Y.S.; Yao, M.N.; Yao, H.S.; Tian, J.S.; Zhang, W.F. Effects of light intensity within the canopy on maize lodging. Field Crop. Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Li, Y.S.; Yu, C.B.; Zhu, S.; Xie, L.H.; Hu, X.J.; Liao, X.; Liao, X.S.; Che, Z. High planting density benefits to mechanized harvest and nitrogen application rates of oilseed rape (Brassica napus L.). Soil Sci. Plant Nutr. 2014, 60, 384–392. [Google Scholar] [CrossRef]
- Lafond, G.P. The Effects of Nitrogen, Row Spacing and Seeding Rate on the Yield of Flax under a Zero-Till Production System. Can. J. Plant Sci. 1993, 73, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Xiao, S.; Chen, S.Y.; Zhao, L.Q.; Wang, G. Density effects on plant height growth and inequality in sunflower populations. J. Integr. Plant Biol. 2006, 48, 513–519. [Google Scholar] [CrossRef]
- Thompson, W.A.; Kriedemann, P.E.; Craig, I.E. Photosynthetic response to light and nutrients in sun-tolerant and shade-tolerant rain-forest trees.1. Growth, leaf anatomy and nutrient content. Funct. Plant Biol. 1992, 19, 1–18. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, U.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Jullien, A.; Allirand, J.M.; Mathieu, A.; Andrieu, B.; Ney, B. Variations in leaf mass per area according to N nutrition, plant age, and leaf position reflect ontogenetic plasticity in winter oilseed rape (Brassica napus L.). Field Crop. Res. 2009, 114, 188–197. [Google Scholar] [CrossRef]
- Wright, I.J.; Groom, P.K.; Lamont, B.B.; Poot, P.; Prior, L.D.; Reich, P.B.; Schulze, E.D.; Veneklaas, E.J.; Westoby, M. Shorth communication: Leaf trait relationships in Australian plant species. Funct. Plant Biol. 2004, 31, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Dreccer, M.F.; Van Oijen, M.; Schapendonk, A.H.C.M.; Pot, C.S.; Rabbinge, R. Dynamics of vertical leaf nitrogen distribution in a vegetative wheat canopy. Impact on canopy photosynthesis. Ann. Bot. 2000, 86, 821–831. [Google Scholar] [CrossRef] [Green Version]
- Sorin, C.; Leport, L.; Cambert, M.; Bouchereau, A.; Mariette, F.; Musse, M. Nitrogen deficiency impacts on leaf cell and tissue structure with consequences for senescence associated processes in Brassica napus. Bot. Stud. 2016, 57, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dordas, C. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source-sink relations. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar] [CrossRef]
- de Jong, M.; George, G.; Ongaro, V.; Williamson, L.; Willetts, B.; Ljung, K.; McCulloch, H.; Leyser, O. Auxin and strigolactone signaling are required for modulation of Arabidopsis shoot branching by nitrogen supply. Plant Physiol. 2014, 166, 384–395. [Google Scholar] [CrossRef] [Green Version]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, J. The effects of nitrogen supply and stem density on leaf attributes and stem branching in potato (Solanum tuberosum L.). Potato Res. 1995, 38, 271–279. [Google Scholar] [CrossRef]
- Gombert, J.; Le Dily, F.; Lothier, J.; Etienne, P.; Rossato, L.; Allirand, J.M.; Jullien, A.; Savin, A.; Ourry, A. Effect of nitrogen fertilization on nitrogen dynamics in oilseed rape using N-15-labeling field experiment. J. Plant Nutr. Soil Sci. 2010, 173, 875–884. [Google Scholar] [CrossRef]
- Malagoli, P.; Laine, P.; Rossato, L.; Ourry, A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest. II. An N-15-labelling-based simulation model of N partitioning between vegetative and reproductive tissues. Ann. Bot. 2005, 95, 1187–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Symbol | Variable Name | Definition | Units |
|---|---|---|---|
| An | Rate of photosynthesis | Net CO2 uptake per leaf area per time | µmol CO2·m−2 s−1 |
| IPAR | PAR intercepted | PAR intercepted by the canopy | µmol·m−2·s−1 |
| k | Light extinction coefficient | Extinction coefficient for light in a stand | - |
| LAI | Leaf area index | Green leaf area/ground surface area | - |
| LMA | Leaf mass per area | Leaf dry matter/leaf area | mg mass·cm−2 |
| PAR | Photosynthetically active radiation | Spectral range of solar radiation from 400 to 700 nm | nm |
| PARinc | PAR incoming | PAR incoming above the canopy | µmol·m−2·s−1 |
| Narea | N content per green leaf area | LMA/Nmass | mg N·cm−2 |
| Nmass | N content per unit of mass | Amount of N/leaf dry weight | mg N·g−1 leaf |
| NO3− | Nitrate | Nitrate content | g·m−2 |
| Ntotal | Total mineral nitrogen | NO3− + NH4+ | g·m−2 |
| N uptake | Nitrogen uptake | N contained in the plant | g·plant−1 |
| TT | Thermal time | Accumulation of daily mean temperature above a base temperature | °C·day |
| Treatment | Leaf Position | Leaf Appearance (°C·Day) | Individual Leaf Area (cm2 per Leaf) | N content Per Leaf (mg N Per Leaf) | |||
|---|---|---|---|---|---|---|---|
| N150-D50 | Leaf 11 | 539.1 | d | 14.86 | cd | 2.868 | b |
| Leaf 9 | 473.1 | a | 60.74 | a | 9.916 | a | |
| Leaf 9.1 | 690.9 | e | 11.83 | de | 1.180 | cd | |
| 150-D150 | Leaf 11 | 535.2 | cd | 9.44 | def | 0.892 | cd |
| Leaf 9 | 470.4 | a | 19.26 | c | 2.184 | bc | |
| N50-D50 | Leaf 11 | 527.6 | bc | 6.27 | ef | 0.756 | d |
| Leaf 9 | 466.9 | a | 26.59 | b | 2.729 | b | |
| N50-D150 | Leaf 11 | 523.7 | b | 3.44 | f | 0.136 | d |
| Leaf 9 | 469.3 | a | 9.19 | def | 0.722 | d | |
| N supply (N) | *** | *** | *** | ||||
| Plant population density (D) | *** | *** | *** | ||||
| N × D × Leaf position | *** | NS | ** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labra, M.H.; Struik, P.C.; Calderini, D.F.; Evers, J.B. Leaf Nitrogen Traits in Response to Plant Density and Nitrogen Supply in Oilseed Rape. Agronomy 2020, 10, 1780. https://doi.org/10.3390/agronomy10111780
Labra MH, Struik PC, Calderini DF, Evers JB. Leaf Nitrogen Traits in Response to Plant Density and Nitrogen Supply in Oilseed Rape. Agronomy. 2020; 10(11):1780. https://doi.org/10.3390/agronomy10111780
Chicago/Turabian StyleLabra, Marcelo H., Paul C. Struik, Daniel F. Calderini, and Jochem B. Evers. 2020. "Leaf Nitrogen Traits in Response to Plant Density and Nitrogen Supply in Oilseed Rape" Agronomy 10, no. 11: 1780. https://doi.org/10.3390/agronomy10111780
APA StyleLabra, M. H., Struik, P. C., Calderini, D. F., & Evers, J. B. (2020). Leaf Nitrogen Traits in Response to Plant Density and Nitrogen Supply in Oilseed Rape. Agronomy, 10(11), 1780. https://doi.org/10.3390/agronomy10111780







