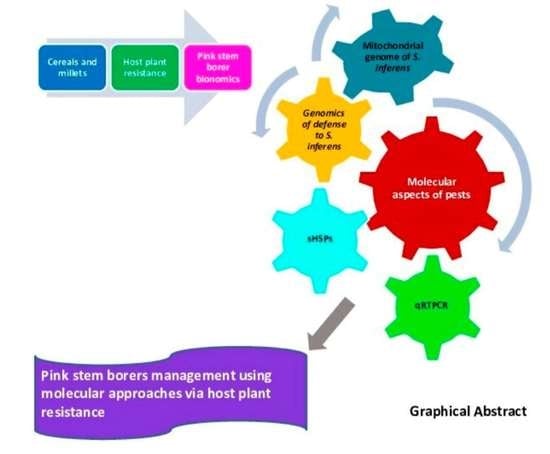
An Overview of the Bionomics, Host Plant Resistance and Molecular Perspectives of Sesamia inferens Walker in Cereals and Millets
Abstract
1. Introduction
1.1. Historical Perspectives
1.2. Pink Stem Borer—Host Range and Crop Losses
1.3. Taxonomy of Pink Stem Borer
1.4. Geographic Distribution
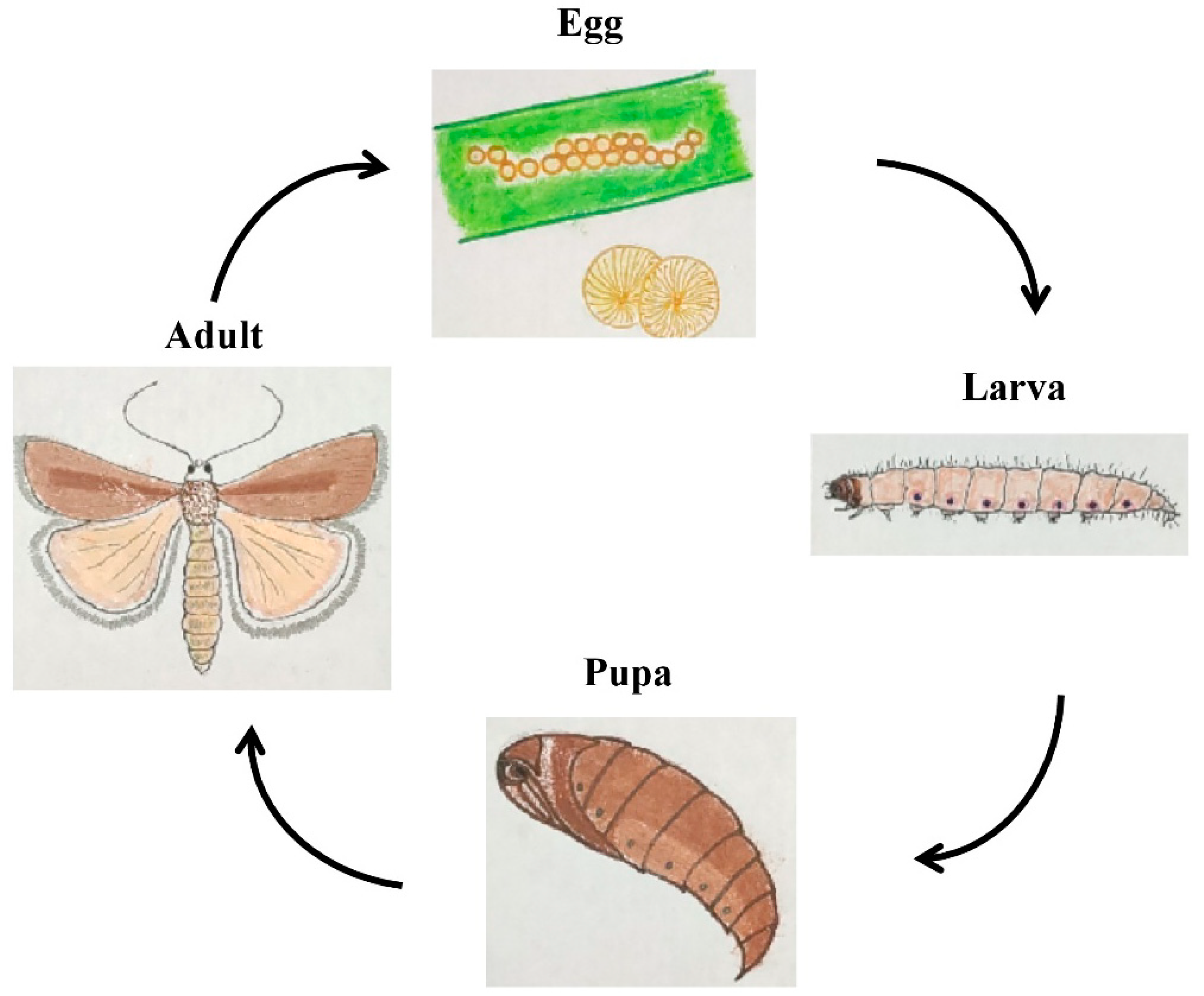
1.5. Bionomics and Life History on Different Host Plants
1.5.1. Egg
1.5.2. Larva
1.5.3. Pupa
1.5.4. Adult
1.6. Seasonal Abundance
1.7. Crop Damage
1.8. Genomics of Defense to S. inferens
2. Quantitative Trait Loci (QTLs)
2.1. Barnyard Millet Genome
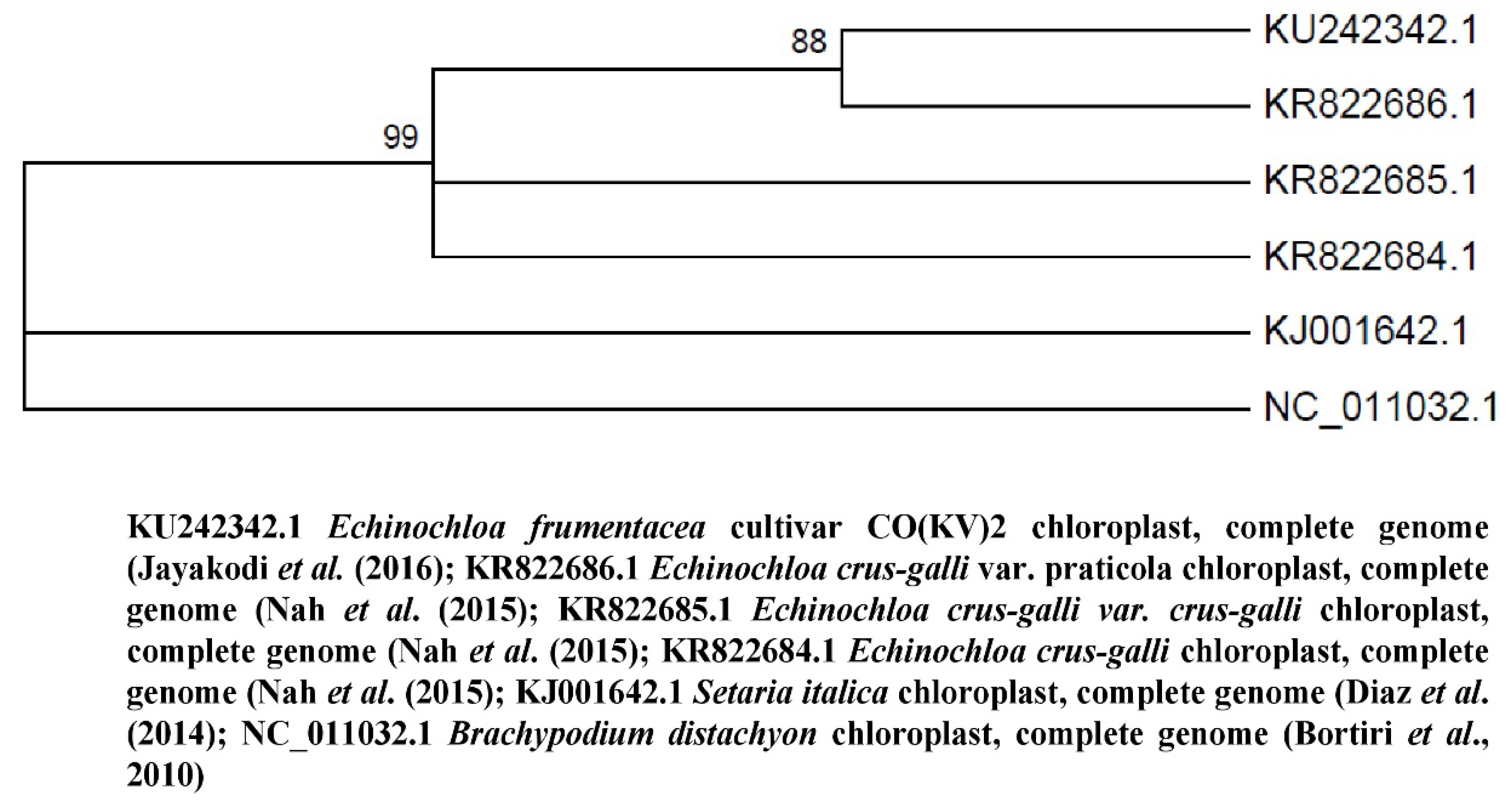
2.2. Phylogenetic Analysis of Echinochloa frumentacea
2.3. The Mitochondrial Genome of S. inferens
2.4. Temperature Shock Proteins in S. inferens
2.5. Quantitative Real-Time PCR Analysis in S. inferens
3. Status of Pink Stem Borer, S. inferens on Cereals and Millets
3.1. Finger Millet, Eleusine coracana
3.2. Maize, Zea mays
3.3. Barnyard Millet, Echinochloa frumentacea
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lucas, J. Advance in plant disease and pest management. J. Agric. Sci. 2011, 149, 91–114. [Google Scholar] [CrossRef]
- Gahukar, R.T. Problems and perspectives of pest management in the Shel: A case study of pearl millet. Trop. Pest Manag. 1988, 34, 35–48. [Google Scholar] [CrossRef]
- Nwanze, K.F. Some aspects of pest management and host plant resistance in pearl millet in the Sahel. Int. J. Trop. Insect Sci. 1985, 6, 461–465. [Google Scholar] [CrossRef]
- Renganathan, V.G.; Vanniarajan, C. Exploring the Barnyard Millet (Echinochloa frumentacea Roxb. Link) Segregating Population for Isolation of High Yielding, Iron and Zinc Content Genotype. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3611–3621. [Google Scholar] [CrossRef]
- Oishimaya, S.N. The Leading Millet Producing Countries in the World. World Atlas. April 2017. Available online: worldatlas.com/articles/the-leading-millet-producing-countries-in-the-world.html (accessed on 25 April 2017).
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Harris, K.M. Lepidopterous stem bores of cereals in Nigeria. Bull. Entomol. Res. 1962, 53, 139–171. [Google Scholar] [CrossRef]
- Ingram, W.R. The lepidopterous stemborers associated with Gramineae in Uganda. Bull. Entomol. Res. 1958, 49, 367–383. [Google Scholar] [CrossRef]
- Jepson, W.F. A Critical Review of the World Literature on the Lepidopterous Stalk Borers of Tropical Graminaceous Crops; Commonwealth Institute of Entomology: London, UK, 1954; pp. 1–127. [Google Scholar]
- Tams, W.H.T.; Boweden, J. A revision of African species of Sesamia Guenee and related genera (Agrotidae: Lepidoptera). Bull. Entomol. Res. 1953, 43, 645–648. [Google Scholar] [CrossRef]
- Kalaisekar, A.; Patil, J.V. Insect–Plant Relationships. In Insect Pests of Millets; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Judal, G.S. The Bionomics and Control Measures of the Pink Borer: Sesamia inferens Walker (Noctuidae: Lepidoptera) on Wheat: Triticum aestivum L. Master’s Thesis, Gujarat Agricultural University, Anand, India, 1978. [Google Scholar]
- Patel, G.D.; Patel, H.K. Kit Vidya”, Part-2; Government of Gujarat: Gujarat, India, 1970; p. 88.
- Seetharam, A.; Reley, K.W.; Harinarayana, G. Small Millets in Global Agriculture; Oxford & IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1986. [Google Scholar]
- Siddiqui, K.H.; Marwaha, K.K. The Vistas of Maize Entomology in India; Kalyani Publishers: Ludhiana, Punjab, India, 1993; p. 184. [Google Scholar]
- Muthusamy, S.; Shanker, C.; Mohan, M.; Padmavathi, S.K.; Katti, G. Emergence pattern, reproductive biology and courtship behaviour of rice pink stem borer, Sesamia inferens (Walker) (Noctuidae: Lepidoptera). Agrotechnology 2014, 2, 60. [Google Scholar]
- Xu, Y. Molecular Plant Breeding; CAB International: Wallingford, UK; Cambridge, MA, USA, 2010. [Google Scholar]
- Jiang, G.L. Plant Marker-Assisted Breeding and Conventional Breeding: Challenges and Perspectives. Adv. Crop Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Meihls, L.N.; Kaur, H.; Jander, G. Natural variation in maize defense against insect herbivores. Quant. Biol. 2012, 77, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.S. Management of insect and mite pests. In Twenty Years of Coordinated Wheat Research 1961–1986; Wheat Project Directorate, All India Coordinated Wheat Improvement Project; Tandon, J.P., Sethi, A.P., Eds.; ICAR: New Delhi, India, 1986; pp. 158–188. [Google Scholar]
- Fletcher, T.B. Report of the Imperial Entomologist. In Science Reports of Agriculture Research Institute; Department of Agriculture: Calcutta, India, 1920; pp. 68–94. [Google Scholar]
- Jhaveri, T.N. Juar Stem Borers (Chilo Simplox and Sesamia Inferens). In Report of Proceeding 4th Entomological Meeting Pusa; Tata Mc Graw-Hill Publishers: Calcutta, India, 1921; pp. 143–147. [Google Scholar]
- Khan, Z.R.; Litsinger, J.A.; Barrion, A.T.; Villanueva, F.F.D.; Fernandez, N.J.; Taylor, L.D. World Bibliography of Rice Stem Borers; International Rice Research Institute: Los Banos, Philippines, 1991; pp. 1–426. [Google Scholar]
- Rao, B.A. Techniques of Scoring for Resistance to Maize Stalk Borer (Sesamia Inferens). In Techniques of Scoring for Resistance to the Major Insect Pests of Maize; All India Co-ordinated Maize Improvement Project; Singh, J., Ed.; Indian Agricultural Research Institute: New Delhi, India, 1983; pp. 16–26. [Google Scholar]
- Deole, S.; Dubey, V.K.; Dipti, D.; Rashmi, G. Exploitation of neonate larval behaviour of Sesamia inferens on maize for its effective management. J. Pharmacogn. Phytochem. 2018, 7, 2685–2688. [Google Scholar]
- Ghai, S.; Ramamurthy, V.V.; Gupta, S.L. Lepidopterous insects associated with rice crop in India. Indian J. Entomol. 1979, 41, 65–90. [Google Scholar]
- Godhani, P.H. Bionomics, Varietal Susceptibility and Chemical Control of Wheat Stem Borer Sesamia Inferens Walker (Lepidoptera: Noctuidae). Ph.D. Thesis, Gujarat Agricutural University, Junagadh, India, 1987. [Google Scholar]
- Rao, V.P.; Nagaraja, H. Sesamia Species as Pests of Sugarcane. In Pests of Sugarcane; William, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1969; pp. 207–223. [Google Scholar]
- Kapur, A.P. Taxonomy of the rice stem borers. In The Major Insect Pests of the Rice; International Rice Research Institute: Los Baños, Philippines, 1967; pp. 3–43. [Google Scholar]
- Lefroy, H.M. Manual of Entomology; Agricole, Reprints Corporation: New Delhi, India, 1923; p. 541. [Google Scholar]
- Litsinger, J.A. Pests in Tropical Crops. In Diseases, Pests and Weeds in Tropical Crops; Kranz, J., Schmutterer, H., Koch, W., Eds.; Verlag Paul Parey: Berlin/Hamberg, Germany, 1977; pp. 453–498. [Google Scholar]
- Dale, D. Insect pests of rice plant their biology and ecology. In Biology and Management of Rice Insects; Heinrichs, E.A., Ed.; Wiley Eastern World Limilted: Delhi, India, 1994; pp. 364–486. [Google Scholar]
- Sekhar, J.C.; Sharma, V.K.; Reddy, R.K.; Chaudhary, M.L.K.; Singh, N.N. Pink stem borers (Sesamia spp.)—Major threat for tropical maize. In Stresses on Maize in Tropics; Zaidi, P.H., Singh, N.N., Eds.; Directorate of Maize Research: New Delhi, India, 2005; pp. 396–435. [Google Scholar]
- Pathak, M.D.; Khan, Z.R. Insect Pests of Rice; International Rice Research Institute: Manila, Philippines, 1994; pp. 5–17. [Google Scholar]
- Banerjee, S.N.; Pramanik, L.M. The lepidopterous stalk borers of rice and their life cycles in the tropics. In Major Insect Pests of the Rice Plant; International Rice Research Institute: Los Baños, Philippines, 1967; pp. 103–124. [Google Scholar]
- Singh, K.G. Recent progress in rice insect research in Malaysia. In Symposium on Rice Insects, Proceedings of a Symposium on Tropical Agriculture Researches, Tokyo, Japan, 19–24 July 1971; Tropical Agriculture Research Series 5; Japan International Research Center for Agricultural Sciences: Tokyo, Japan, 1971; pp. 109–121. [Google Scholar]
- Fletcher, T.B.; Ghosh, C. Borers in Sugarcane, rice etc. In Proceedings of the 3rd Entomon Meet, Pusa, India, 3–15 February 1920; pp. 354–417. [Google Scholar]
- Catling, H.D.; Alam, S. Rice Stem Borers. In Literature Review of Insect Pests and Diseases of Rice in Bangladesh; Bangladesh Rice Research Institute: Dacca, Bangladesh, 1977; pp. 5–29. [Google Scholar]
- Rajagopal, D.; Basavanna, C.G.P. Insect pests of maize in Karnataka. Mysore J. Agric. Sci. 1975, 9, 110–121. [Google Scholar]
- Garg, D.K. Host range and overwintering of rice pink stem borer (PSB) in a hilly region of India. Int. Rice Res. Notes 1988, 13, 2. [Google Scholar]
- Vreden, G.V.; Ahmadzabidi, A.L. Pests of Rice and Their Natural Enemies in Peninsular Malaysia; Cent Agric Publ Doc: Wageningen, The Netherlands, 1986; p. 230. [Google Scholar]
- Hampson, G.F. The Fauna of British India: Moths. In London World Bibliography of Rice Stem Borers; Oxfordshire Taylor and Francis: Milton Park, UK, 1984; Volume 284, pp. 1–426. [Google Scholar]
- Chatterji, S.M.; Young, W.R.; Sharma, G.C.; Sayi, I.V.; Chhal, B.S.; Khare, B.P.; Rathore, V.S.; Panwar, V.P.S.; Siddiqui, K.H. Estimation of loss in yield of maize due to insect pests with special reference to borers. Indian J. Entomol. 1969, 31, 109–115. [Google Scholar]
- Krishnamurti, B.; Usman, S. The Ragi Stem Borer, Sesamia Inferens Walker. In Bulletin of the Department of Agriculture; Abstract in CAB Abstracts, UK, AN: 19550500178; 1952; Volume 15, p. 70. Available online: https://www.cabdirect.org/cabdirect/abstract/19550500178 (accessed on 27 March 2016).
- Agarwal, R.A.; Siddiqui, Z.A. Sugarcane pests. Indian J. Entomolody 1964, 25, 155–159. [Google Scholar]
- Grist, D.H.; Lever, R.J.A.W. Pests of Rice; Green and Co, Ltd.: London/Harlow, UK, 1969; pp. 117–126. [Google Scholar]
- Aggarwal, R.; Singh, J.; Shukla, K.K. Biology of Pink Stem Borer, Sesamia Inferens Walker on Rice Crop. Indian J. Ecol. 2004, 31, 66–67. [Google Scholar]
- Das, I.K.; Padmaja, P.G. Biotic Stress Resistance in Millets; Academic Press: Cambridge, MA, USA, 2016; p. 246. [Google Scholar]
- Rajendra, A. Studies on Sesamia Inferens Wlk: The shoot-borer pest of sugarcane in Sri Lanka. J. Natn. Sci. Coun. Sri Lanka 1976, 4, 99–108. [Google Scholar]
- Aggarwal, R. Studies on the Biology of Pink Stem Borer, Sesamia inferens Walker on Rice Crop. Master’s Thesis, Punjab Agricultural University, Ludhiana, India, 2002. [Google Scholar]
- Joshi, G.; Ram, L.; Singh, R. Biology of pink borer, Sesamia inferens Walker on Taraori Basmati rice. Ann. Biol. 2009, 25, 41–45. [Google Scholar]
- Sekhar, J.C.; Kumar, P.; Rakshit, S.; Singh, K.P.; Dass, S. Differential preference for oviposition by Sesamia inferens Walker on maize genotypes. Ann. Plant Protec. Sci. 2009, 17, 46–49. [Google Scholar]
- Ateyim, S.T.S.; Obeng, -O.D.; Botchey, M.A.; Owusu, E.O. Some aspects of the biology and behaviour of Sesamia nonagrioides botanephaga (Lepidoptera: Noctuidae), a major stem borer pest of maize in Southern Ghana. W. Afr. J. Appl. Ecol. 2009, 8, 116–126. [Google Scholar] [CrossRef]
- Anuradha, M.; Reddy, M.L.; Sreelatha, D.; Sekhar, J.C. Seasonal Incidence of Stem Borers in Corn—Record of New Site and Stage of Oviposition of Sesamia Inferens. In Proceedings of the 12th Asian Maize Conference TS3–5, Food and Agricultural Organization of the United Nations, Bangkok, Thailand, 30 October–1 November 2014. [Google Scholar]
- Kaur, J.; Kumar, P.; Singh, J.; Suby, S.B.; Bajya, D.R. Egg laying pattern of Sesamia inferens on maize (Zea mays). Indian J. Agric. Sci. 2015, 85, 109–113. [Google Scholar]
- Atwal, A.S.; Chaudhary, J.P. Studies on the Insect Pests of Rice: Annual Report of 1966–67; Department of Zoology-Entomology, Punjab Agricultural University: Ludhiana, India, 1967; pp. 55–77. [Google Scholar]
- Rothschild, G.H.L. The biology and ecology of rice stem borers in Sarawak (Malaysian Borneo). J. Appl. Ecol. 1971, 8, 287–322. [Google Scholar] [CrossRef]
- Singh, B. Bionomics of Pink Stem Borer, Sesamia Inferens Walker (Lepidoptera: Noctuidae) in Paddy-Wheat Cropping System. Ph.D. Thesis, Punjab Agricultural University, Ludhiana, India, 2013. [Google Scholar]
- Nagarjuna, B.; Manjunatha, M.; Latha, M. Biology of maize stem borer, Sesamia inferens (Walker) Noctuidae: Lepidoptera. J. Ecofriendly Agric. 2015, 10, 90–91. [Google Scholar]
- Deole, S.; Dubey, V.K.; Mehta, N. First record of the pink stem borer Sesamia inferens Walker in maize crop at Raipur (Chhatisgarh) region. Insect Environ. 2013, 19, 164–165. [Google Scholar]
- Patel, R.K.; Verma, R. Sex dimorphism in pink stem borer, Sesamia inferens Walk (Lepidoptera: Noctuidae). Sci. Cult. 1980, 46, 195–196. [Google Scholar]
- Yanagihara, M. Results of studies on Sesamia inferens Walker, a serious pest of sugarcane in Taichu Perfecture, Formosa. J. Formosan. Sug. Plant 1934, 12, 53. [Google Scholar]
- Akhter, A.; Zia, S.; Haider, Z.; Makhdoom, A. Associating light trap catches of some major rice insect pests with prevailing environmental factors. Pak. J. Agric. Sci. 2015, 52, 716–722. [Google Scholar]
- Deole, S. Studies on Pink Stem Borer, Sesamia Inferens Walker of Maize, Zea Mays L. with Particular Reference to Neonate Larval Behavior and Its Management. Ph.D. Thesis, Indira Gandhi Krishi Vishwavidhyalaya, Raipur, India, 2016. [Google Scholar]
- Sidar, Y.; Deole, S.; Yadu, Y.K.; Ganguli, R.N. Seasonal incidence of major insect pests in maize crop (Zea mays L.) under chhattisgarh plains. Trends Biosci. 2015, 8, 4848–4854. [Google Scholar]
- Reddy, M.L. Bio-Ecology and Management of Sesamia inferens (Walker) on Maize. Ph.D. Thesis, Acharya NG Ranga Agricultural University, Rajendranagar, Hyderabad, India, 2001. [Google Scholar]
- Hashmi, A. Insect pest management. Pak. Agric. Res. Counc. 1994, 1, 14–17. [Google Scholar]
- Pavani, T. Studies on Management of Pink Borer Sesamia inferens Walker on Maize. Master’s Thesis, N.G. Ranga Agricultural University, Hyderabad, India, 2011. [Google Scholar]
- Friesen, D.K.; Palmer, A.F.E. Integrated Approaches to Higher Maize in Productivity in the New Millennium. In Proceedings of the 7th Eastern and Southern Africa Regional Maize Conference, Nairobi, Kenya, 11–15 February 2002; pp. 31–37. [Google Scholar]
- Mather, K. Biometrical Genetics, 1st ed.; Methuen: London, UK, 1949. [Google Scholar]
- Ordas, B.; Malvar, R.A.; Santiago, R.; Butron, A. QTL mapping for Mediterranean corn borer resistance in European flint germplasm using recombinant inbred lines. BMC Genome 2010, 11, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Butron, A.; Sandoya, G.; Revilla, P.; Malvar, R.A. Genetics of resistance to the pink stem borer (Sesamia nonagrioides) in maize (Zea mays). Ann. Appl. Biol. 2009, 154, 205. [Google Scholar] [CrossRef]
- Cartea, M.E.; Malvar, R.A.; Vales, M.I.; Butron, A.; Ordas, A. Inheritance of resistance to ear damage caused by Sesamia nonagrioides (Lepidoptera: Noctuidae) in maize. J. Econ. Entomol. 2001, 94, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Santosh, H.B.; Sekhar, J.C.; Rakshit, S.; Gadag, R.N.; Dass, S. Detection of epistatic interaction for susceptibility towards pink borer (Sesamia inferens Walker) in maize (Zea mays L.). Indian J. Genet. 2012, 72, 284–289. [Google Scholar]
- Sekhar, J.C.; Karjagi, C.G.; Kumar, B.; Rakshit, S.; Soujanya, L.; Kumar, P.; Singh, K.P.; Dhandapani, A.; Dass, S.; Kumar, R.S. Genetics of resistance to Sesamia inferens infestation and its correlation with yield in maize. Plant Breed. 2015, 134, 394–399. [Google Scholar] [CrossRef]
- Ordas, A.; Butron, P.; Soengas, A.; Ordas, R.; Malvar, A. Antibiosis of the pith of maize to Sesamia nonagrioides (Lepidoptera: Noctuidae). J. Econ. Entomol. 2002, 95, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Botstein, D. Mapping Mendelien factors underlying quantitative traits using RFLP linkage maps. Genetics 1989, 121, 185–199. [Google Scholar] [PubMed]
- Jayakodi, M.; Natesan, S.; Yang, T.J. The Complete Chloroplast Genome Sequence of Indian Barnyard Millet, Echinochloa frumentacea. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KU242342.1 (accessed on 27 March 2016).
- Ye, C.Y.; Lin, Z.; Li, G.; Wang, Y.Y.; Qiu, J.; Fu, F.; Zhang, H.; Chen, L.; Ye, S.; Song, W.; et al. Echinochloa chloroplast genomes: Insights into the evolution and taxonomic identification of two weedy species. PLoS ONE 2014, 9, e113657. [Google Scholar] [CrossRef]
- Nah, G.; Im, J.H.; Kim, J.W.; Kim, K.H.; Lim, J.S.; Choi, A.Y.; Choi, I.Y.; Yang, T.J.; Kim, D.-S. The Evolutionary Relationship of Three Echinochloa crus-galli Chloroplast Genomes. 2015; Unpublished. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KR822684.1 (accessed on 27 March 2016).
- Young, H.A.; Lanzatella, C.L.; Sarath, G.; Tobias, C.M. Chloroplast genome variation in upland and lowland switchgrass. PLoS ONE 2011, 6, e23980. [Google Scholar] [CrossRef]
- Maier, R.M.; Neckermann, K.; Igloi, G.L.; Kossel, H. Complete sequence of the maize chloroplast genome: Gene content hotspots of divergence and fine tuning of genetic information transcript editing. J. Mol. Biol. 2009, 251, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Diaz, V.M.; Keepers, K.G.; Tittes, S.B.; Kane, N.C. Complete genome Setaria italica (Foxtail millet). Available online: https://www.ncbi.nlm.nih.gov/nuccore/KJ001642.1 (accessed on 9 April 2014).
- Zhang, Z.; Ersoz, E.; Lai, C.Q.; Todhunter, R.J.; Tiwari, H.K.; Gore, M.A.; Bradbury, P.J.; Yu, J.; Arnett, D.K.; Ordovas, J.M.; et al. Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 2010, 42, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, N.; Peterson, G.; Stecher, M.; Kumar, N. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, P.H.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Salvato, P.; Simonato, M.; Battisti, A.; Negrisolo, E. The complete mitochondrial genome of the bag-shelter moth Ochrogaster lunifer (Lepidoptera, Notodontidae). BMC Genom. 2008, 9. [Google Scholar] [CrossRef]
- Liao, F.; Wang, L.; Wu, S.; Li, Y.P.; Zhao, L.; Huang, G.M.; Niu, C.J.; Liu, Y.Q.; Li, M.G. The complete mitochondrial genome of the fall webworm, Hyphantria cunea (Lepidoptera: Arctiidae). Int. J. Biol. Sci. 2016, 6, 172–186. [Google Scholar] [CrossRef]
- Yin, J.; Hong, G.Y.; Wang, A.M.; Cao, Y.Z.; Wei, Z.J. Mitochondrial genome of the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) and comparison with other Lepidopterans. Mitochondrial DNA 2010, 21, 160–169. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Zhou, G.L.; Fang, R.; Ye, J.; Yi, J.P. The complete sequence determination and analysis of Lymantria dispar (Lepidoptera: Lymantriidae) mitochondrial genome. Plant Quar. 2010, 4, 6–11. [Google Scholar]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1726–1780. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X.X. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to Holometabolous insects. BMC Genom. 2010, 11, 371. [Google Scholar] [CrossRef]
- Huan, N.C.; Zhou, Y.D. The Complete Mitochondrial Genome of the Pink Stem Borer, Sesamia inferens, in Comparison with Four Other Noctuid Moths. Int. J. Mol. Sci. 2012, 13, 10236–10256. [Google Scholar] [CrossRef]
- Meng, S.; Xiao, T.T.; Ming, X.L.; Wei, F.Y.; Zhou, D. Cold tolerance characteristics and overwintering strategy of Sesamia inferens (Lepidoptera: Noctuidae). Fla. Entomol. 2014, 97, 1544–1553. [Google Scholar]
- Meng, S.; Ming, X.L.; Xiao, T.T.; Zhou, Y.D. Exploring Valid Reference Genes for Quantitative Real-Time PCR Analysis in Sesamia inferens (Lepidoptera: Noctuidae). PLoS ONE 2015, 10, e0115979. [Google Scholar]
- Anthony Reetha, B.M.; Mohan, M. Development of SSRs and its application in genetic diversity study of Indian population of Sesamia inferens (Walker) (Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2018, 6, 26–32. [Google Scholar]
- Devi, P.B.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.G.; Priyadarisini, V.B. Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: A review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef]
- Kundu, G.G.; Kishore, P. Parasites of Sesamia inferens (Walker) and Atherigona mudiseta Rondani. Entomol. Newsl. 1971, 1, 13. [Google Scholar]
- Sasmal, A.; Mohapatra, A.K.B.; Pradhan, K.C. Screening of finger millet genotypes against pink stem borer (Sesamia inferens Walker, Noctuidae, Lepidoptera). J. Entomol. Zool. Stud. 2018, 6, 796–799. [Google Scholar]
- Singh, B. Incidence of the pink noctuid borer Sesamia inferens (Walker), on wheat under two tillage conditions and three sowing dates in north-western plains of India. J. Entomol. 2012, 9, 368–374. [Google Scholar] [CrossRef]
- Lingappa, S. Development of artificial diet for mass rearing of Sesamia inferens W (Lepidoptera, Noctuidae) and screening for resistance to finger millet germplasm. Mysore J. Agric. Sci. 1979, 8, 353. [Google Scholar]
- Murthi, T.K.; Harinarayana, G. Insect pests of Small millets and their management in India. In Small Millets in Global Agriculture; Seetharam, A.K., Reley, W., Harinarayana, G., Eds.; Oxford and IBH Publishing Co Pvt Ltd.: New Delhi, India, 1986; pp. 255–270. [Google Scholar]
- Kishore, P.; Jatwani, M.G. Screening of some improved genotypes of the finger millet (Eleusine coracana) for resistant of Sesamia inferens and Myllocerus maculosus. J. Entomol. Res. 1980, 4, 221–223. [Google Scholar]
- Dhamdhere, S.V. Reaction of finger millet varieties to stem borer and caterpillar insect pests. Millets News Lett. 1988, 7, 47. [Google Scholar]
- Sekhar, J.C.; Lakshmi, S.; Chikkappa, G.K.; Sunil, N.; Kaul, J.; Singh, K.P.; Kumar, P. Response of different maize accessions to pink stem borer Sesamia inferens Walker (Lepidoptera: Noctuidae). Electron. J. Plant Breed. 2016, 7, 132–136. [Google Scholar] [CrossRef]
- Lavakumar reddy, M.; Sekhar, J.C. Evaluation of maize germplasm to pink stem borer Sesamia inferens in maize. Indian J. Entomon 2002, 64, 402–404. [Google Scholar]
- Divekar, P.; Kumar, P.; Suby, S.B. Oviposition preference of pink stem borer, Sesamia inferens (Walker) in maize germplasm. J. Entomol. Zool. Stud. 2019, 7, 1115–1119. [Google Scholar]
- Laxmi, R.; Nautiyal, A.; Bisht, T.S.; Shambhoo, P.; Dinesh, N.; Kuldev, M.; Tewari, A. Screening of Barnyard Millet Germplasm against Shoot Fly and Stem Borer Damage under Field Conditions. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1221–1226. [Google Scholar] [CrossRef]

| Region | Reported Crop | Authors |
|---|---|---|
| Malaysia, Philippines, Taiwan, China, Japan, Bangladesh, Bhutan, Brunei, Hongkong, India, Indonesia, Korea, Nepal, Pakistan, Singapur, Srilanka, Thailand and Vietnam | Rice | [31,32,35] |
| Malaysia | Wheat | [36] |
| India | [9,26,37] | |
| Philippines | [29] | |
| Asia | Maize | [31] |
| Bangladesh | [38] | |
| India | [9,21,39,40,41] | |
| Malaysia | [29,41] | |
| India | Sorghum, Johnson grass, Sudan grass | [28] |
| India | Millets | [37] |
| Asia | Sugarcane | [31] |
| India | [9,29,39,42,43] | |
| Asia | Guinea grass | [31] |
| India | [37,39] | |
| Asia | Graminaceous and Cyperus weeds | [31] |
| S.No | Name of the Inbred Lines | Source Germplasm | Reaction to Pink Stem Borer |
|---|---|---|---|
| 1 | DMRE1 | PT963128 | Resistant |
| 2 | DMRE2 | Antigua Gr.1 | Resistant |
| 3 | CML287 | Population 24 | Susceptible |
| 4 | CML451 | Pool 25 | Susceptible |
| Crop Description | Accession Number | Genome Size | Country | Reference |
|---|---|---|---|---|
| Echinochloa frumentacea cultivar CO(KV)2 chloroplast, complete genome | KU242342.1 | 139593 bp | Tamil Nadu, India | [78] |
| Echinochloa crus-galli var. crus-galli chloroplast, complete genome | KJ000047.1 | 139800 bp | China | [79] |
| Echinochloa crus-galli var. praticola chloroplast, complete genome | KR822686.1 | 139846 bp | China | [80] |
| Echinochloa crus-galli chloroplast, complete genome | KR822684.1 | 139857 bp | ||
| Echinochloa crus-galli var. crus-galli chloroplast, complete genome | KR822685.1 | 139860 bp | ||
| Panicum virgatum chloroplast, complete genome | NC_015990.1 | 139619 bp | USA | [81] |
| Zea mays chloroplast, complete genome | NC_001666.2 | 140384 bp | Germany | [82] |
| Setaria italica chloroplast, complete genome | KJ001642.1 | 138833 bp | USA | [83] |
| Crop | Category | Reported by | |
|---|---|---|---|
| Tolerant | Resistant | ||
| Finger millet, Eleusine coracana | - | PRM 9002, KOPN 933, OEB 28, RAU 8 and Champabati | [103] |
| IE 932, IE 982 and IE 1037 | - | [105] | |
| KM 1 RAU 1, RAU 3, INDAF 7, INDAF 8, HR 154, HR 374, HR 1523, PES, 110, PES 400, WR 9 and VL 110 | - | [106] | |
| - | PES 9, PES 144, PES 224, KM 1, KM 14, HR 228, JNR 1008 and T 36-B. | [107] | |
| - | VL 109, VR 530, PR 202, HR 374 | [108] | |
| Crop | Category | Reported by | |
|---|---|---|---|
| Susceptible | Resistant | ||
| Maize, Zea mays | - | The entries namely, 221267-A, full season maturity line 221267-D, PAC-9703, BIO-9637, PAC-9735, medium stage maturity line KH-5354, BIO-9691, 127868, JKMH4095MMH-1765 and R-51 (Early maturity) | [110] |
| IC331939, IC340368, IC369174, IC369184, IC406420, IC549985, IC549989, IC549990, IC569669, IC547811 | IC258225, IC319533, IC321053, IC321110, IC321111, IC321119, IC326886, IC331795, IC 338827 and IC 350198 | [109] | |
| DC-2, HKI-193-2 and E-62 | WP-21, E-63 and HKI-193-1 | [111] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeevanandham, N.; Ramiah, N.; Chockalingam, V.; Jegadeesan, R. An Overview of the Bionomics, Host Plant Resistance and Molecular Perspectives of Sesamia inferens Walker in Cereals and Millets. Agronomy 2020, 10, 1705. https://doi.org/10.3390/agronomy10111705
Jeevanandham N, Ramiah N, Chockalingam V, Jegadeesan R. An Overview of the Bionomics, Host Plant Resistance and Molecular Perspectives of Sesamia inferens Walker in Cereals and Millets. Agronomy. 2020; 10(11):1705. https://doi.org/10.3390/agronomy10111705
Chicago/Turabian StyleJeevanandham, Niranjanadevi, Nalini Ramiah, Vanniarajan Chockalingam, and Ramalingam Jegadeesan. 2020. "An Overview of the Bionomics, Host Plant Resistance and Molecular Perspectives of Sesamia inferens Walker in Cereals and Millets" Agronomy 10, no. 11: 1705. https://doi.org/10.3390/agronomy10111705
APA StyleJeevanandham, N., Ramiah, N., Chockalingam, V., & Jegadeesan, R. (2020). An Overview of the Bionomics, Host Plant Resistance and Molecular Perspectives of Sesamia inferens Walker in Cereals and Millets. Agronomy, 10(11), 1705. https://doi.org/10.3390/agronomy10111705