1. Introduction
Water is the most limiting natural resource for sustainable agricultural development in arid and semi-arid areas of Mediterranean and more specifically under climate change scenarios [
1]. In this regard, several works have reported the impact of climate change on agriculture [
2,
3], concluding that crop water demand will increase substantially due to higher evapotranspiration rates, temporal variability of rainfall or heat weaves events [
4].
Within these scenarios, implementing drought-tolerant crops in irrigated zones and the application of deficit irrigation (DI) strategies are being considered, especially in the last few years [
5,
6]. Developing water-saving strategies in Mediterranean woody crops involves many considerations relative to environmental constraints, sustainable yields, and product marketability. However, these must be deeply studied, establishing the most profitable strategies to maximize the fruit production, minimizing the irrigation water consumption and maintaining (or even improving) the fruit quality.
Almond (
Prunus dulcis Mill.) is the largest tree nut crop, and the world surface dedicated to its cultivation during 2018 amounted to 2,071,884 ha, with Spain being the country with the largest area devoted to this crop, with 657,768 ha, followed by the USA, with 441,107 ha [
7]. However, in production terms, relevant differences are found, with 1,872,500 and 339,033 t for the USA and Spain, respectively. Moreover, for the period 2010–2018 in the USA (3500–5608 kg ha
−1) and Spain (279–515 kg ha
−1), the production per area were highly different. In 2018, the Mediterranean basin (Spain, Italy, Greece, Syria, Tunisia, Argelia and Morocco) produced roughly 29% of the world almond production, with most plantations under rainfed conditions and located in marginal areas. In Spain, the almond cultivation is mainly concentrated in Andalusia (S Spain) (31% of total area), and since approximately 85% of almond crops are rainfed, this provokes important fluctuations in productivity [
8].
Today, the increase in almond prices from 2014 to 2019, when it reached an average price of 5€ per kg [
9], has resulted in an increase of surface devoted to almond cultivation with different techniques [
10], explicitly in irrigated areas that were traditionally occupied by other crops (cereals, cotton and sunflower, among others) [
11]. This increase in cultivated area under irrigation from 2014 to 2018 amounted to 25% by using new cultivars [
12], which allows to obtain higher yields (>1500 kg ha
−1) than those from traditional rainfed plantations (150–500 kg ha
−1) [
13].
By taking into consideration the advantage of the positive adaptation of almond to drought scenarios [
14,
15], and its sharp phenology, many authors have reported the positive responses and opportunities of DI for almond cultivation, obtaining competitive yields under moderate-to-severe water stress situations [
16,
17,
18,
19].
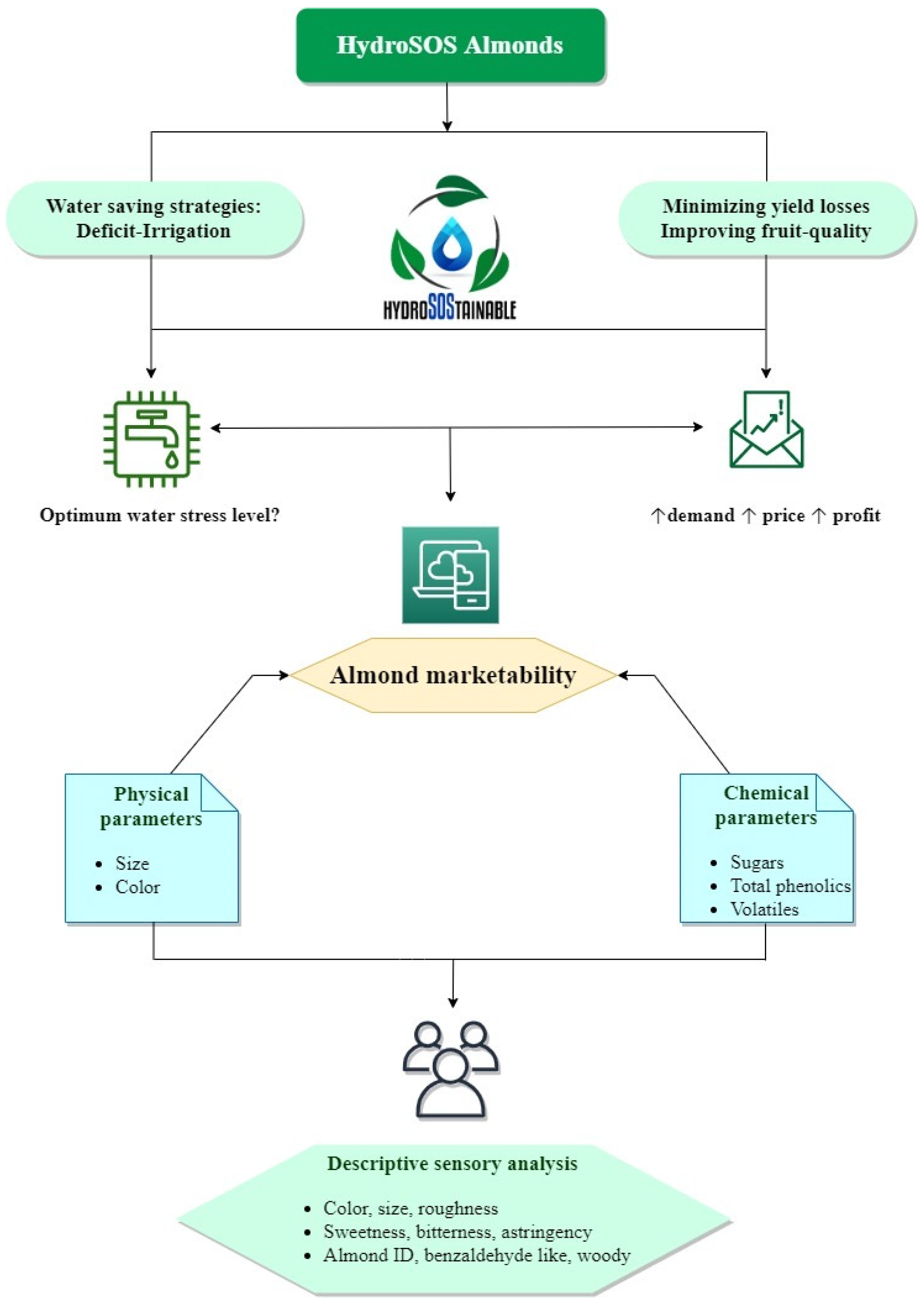
Recently, a novelty research line, focused on food production under hydro-sustainable strategies (hydroSOStainable products), has been successfully developed [
20,
21,
22]. This also showed advantages of different Mediterranean crops, with significant improvements in the fruit quality, sensory profile and consumer acceptance in crops such as pistachios [
23], olives [
24] and almonds [
25]. In this context, there is in an interest in those characteristics or parameters of raw almonds related to their marketability that could be affected by DI strategies. In other words, the main limitation of DI implementation is ultimately the yield reduction (in comparison to the potential rate when almond is grown under full-irrigated conditions), affecting the plantation viability and its competitiveness. In this regard, Lipan et al. [
25,
26] reported relevant results, concluding that some fruit-quality parameters could be improved (or at least not affected) when DI strategies are imposed. If that is the case, these kinds of strategies would encourage the product marketability and the consumer acceptance, allowing a recovering in terms of final price, minimizing the losses when these are analyzed in monetary terms. In addition, many aspects are still not clear, such as if these effects would be similar for the new high-yielding cultivars or the dependence in relation to the irrigation strategy imposed or crop physiological status during the water stress period.
On the other hand, consumer appreciation, and hence, almond marketability, can be determined by a wide number of variables that could be classified into physical and chemical parameters, all of them determining the sensory appreciation and the almond appeal. Raw almonds are mainly composed by fats (44–61%), proteins (16–23%) and dietary fiber (11–14%) and high concentrations of vitamin E [
27,
28]. Despite these being the main compounds of raw almonds, their influence in taste receptors is negligible. In this line, other compounds, more related to flavor properties and sensory and chemical characteristics, can be found. According to Civille et al. [
29] the main taste properties of raw almonds are mainly defined by astringency and sweetness degree, and to a lesser extent, the tactile dimensions (almond texture) [
30]; this is the highest variability in almond flavor related to odor-active volatiles compounds. That is, volatile compounds are responsible of characteristic flavor properties of raw and processed almonds and contribute to their high consumer acceptance [
25,
31]. In particular, benzaldehyde is one of the main volatiles in bitter almonds, but its presence in sweet almonds is very variable (highly cultivar dependent), and, overall, it is found in very low concentrations [
32]. Despite these low concentrations, its presence is responsible of the typical almond flavor and derivates such as marzipan [
33].
Considering that the hydroSOStainable almond production could be a key factor for sustainable development in a semiarid Mediterranean environment; the objective of this study was to evaluate the effects of sustained deficit irrigation strategies and almond cultivars on the main physico-chemical parameters involved in nut sensory profile and improvements in marketability.
2. Material and Methods
2.1. Plant Material, Growing Conditions and Experimental Design
The trial was conducted during 2019 in a commercial orchard of almonds (
Prunus dulcis Mill.,
cvs Guara, Marta and Lauranne), grafted onto GN15 rootstock, and located in the Guadalquivir river basin (37°30′27.4′′ N; 5°55′48.7′′ O) (Seville, SW Spain) (
Figure 1). The plantation contained seven-year-old almond trees, 8 × 6 m spaced and drip irrigated by using two pipelines with emitters of 2.3 L h
−1. The soil is a silty loam typical Fluvisol [
34], more than 2 m deep, fertile and with an organic matter content of 15.0 g kg
−1. Roots were located predominately in the first 50 cm of soil, corresponding to the intended wetting depth, although these exceed more than one meter in depth. The climatology in the study area is attenuated meso-Mediterranean, with an annual reference evapotranspiration rate (ET
0) of 1400 mm and an annual rainfall of 540 mm, mainly distributed from October to April. More details about the experimental site can be found in Gutiérrez-Gordillo et al. [
35].
Three irrigation treatments were designed: (i) a full-irrigated treatment (FI), which received 100% of irrigation requirements (IR) during the irrigation period, and two sustained-deficit irrigation treatments, which received 75% (SDI
75) and 65% (SDI
65) of IR. Irrigation was applied from April to October, the IR being estimated according to the methodology proposed by Allen et al. [
36], obtaining the values of ET
0 by using a weather station installed in the same experimental orchard (Davis Advance Pro2, Davis Instruments, Valencia, Spain). The local crop coefficients used during the experimental period ranged from 0.4 to 1.2, according to the results obtained by García-Tejero et al. [
37].
2.2. Field Measurements
Physiological response to different irrigation doses was evaluated throughout measurements of leaf water potential (
Ψleaf) in shaded leaves, these readings being taken between 12:00 and13:30 GTM, and on a weekly basis. Measurements of
Ψleaf were developed by using a pressure chamber (Soil Moisture Equipment Corp., Sta. Barbara, CA, USA), monitoring eight trees per irrigation treatment (two leaves per tree), located in the north side of the tree and being totally mature, fresh and shaded, at 1.5 m of height. These readings were used to quantify the water stress supported by the crop for each week and the whole kernel-filling period by means of the stress integral (
ΨInt), following the methodology proposed by Myers [
38] (Equation (1)). This index allows to quantify the effect of water stress provided by the water restriction beyond its temporal distribution, integrating the global stress supported by the crop in comparison to the punctual measurements:
where
ΨInt is the stress integral in terms of
Ψleaf values,
is the average leaf water potential for any interval (in our case, for each week),
is the maximum value of
Ψleaf weekly registered, during the experimental period and n is the days numbers within each interval, in our case
n = 7.
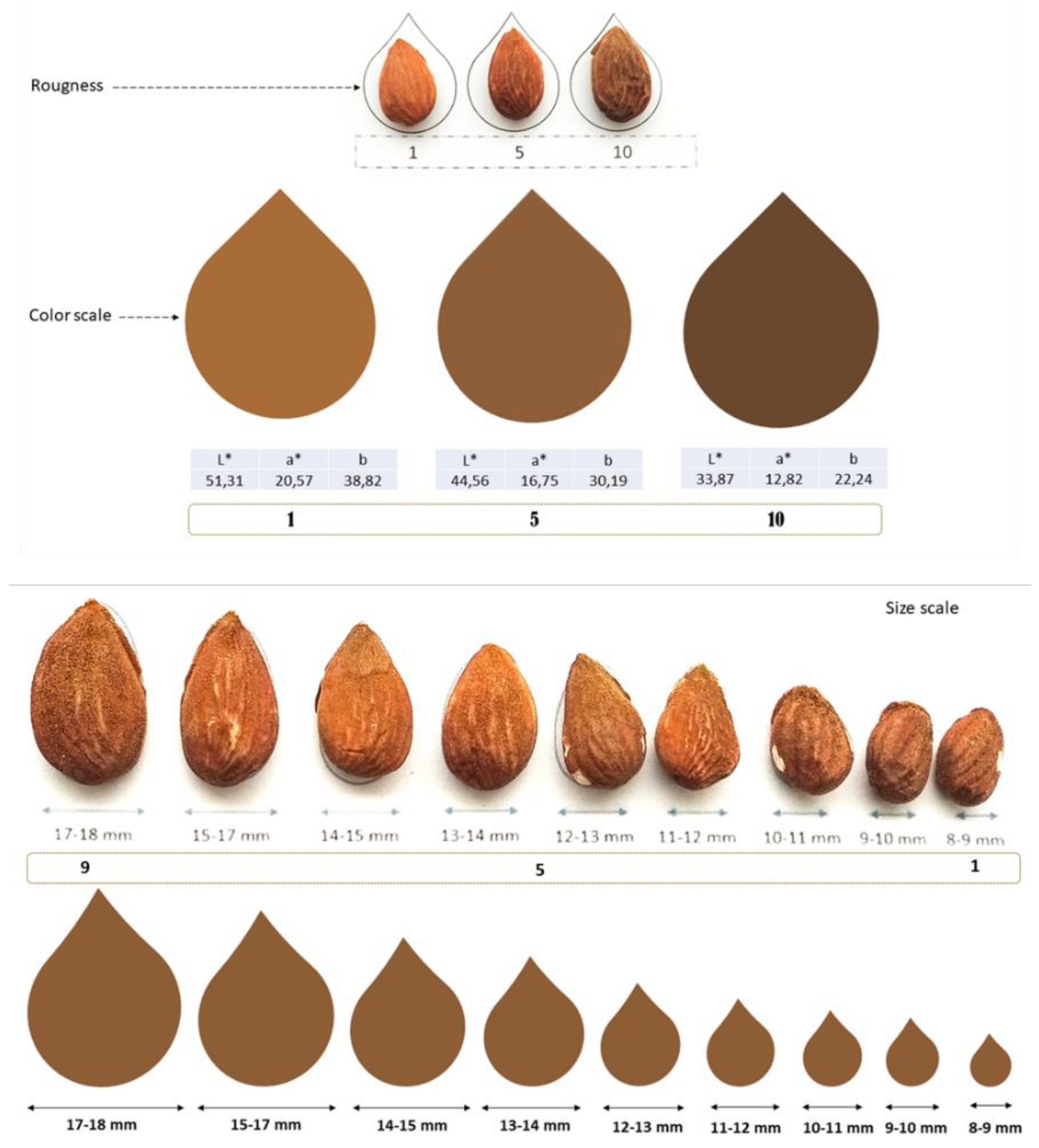
At the end of each season, monitored trees (eight per cultivar and irrigation strategy) were harvested. This process was carried out by using a mechanic vibrator to throw the almond on the ground (previously covered with a plastic mesh). Collected almonds were processed with a mechanic peeling to remove the hull. Finally, once cleaned, almonds were left to air dry and weighed once reached a humidity content around 6%. Around 3 kg of in-shell almonds were sent to Miguel Hernández University for quality and sensory analysis, where the main morphological, physical and chemical parameters were analyzed (
Figure 2).
2.3. Morphological and Physical Parameters
The ratio between the mass of in-shell almonds and kernel was calculated from ~1 kg of nuts per cultivar and irrigation treatment. Additionally, 225 almonds (25 samples × 3 varieties × 3 treatments) were randomly selected and analyzed by measuring the weight and size (length, width and thickness) of almonds (both in-shell and kernel) using a digital caliper (Mitutoyo 500-197-20, Kawasaki, Japan) and a scale (Mettler Toledo model AG204, Barcelona, Spain), respectively.
A Minolta Colorimeter CR-300 (Minolta, Osaka, Japan) was used to perform the color measurements in 75 kernels per each variety. This colorimeter uses a D65 illuminant and a 10° observer as references. The color was provided as CIEL*a*b* coordinates defining the color in a three-dimensional space and it was expressed in three numerical values, which includes L* for the lightness (L* = 0 black; L* = 100 white), a* for the green-red (a* = red; −a* = green) and b* for the blue-yellow components (b* = yellow; −b* = blue).
2.4. Chemical Composition
2.4.1. Total Sugars
Sugars were determined using a high-performance liquid chromatography (HPLC) equipment. The extraction consisted of 1 g of grinded almond in a Moulinex grinder (AR110830) for 10 s, homogenized with 5 mL of phosphate buffer with an homogenizer (Ultra Turrax T18 Basic) over 2 min at 11.3 rpm, while the tube was maintained in an ice bath and after it was centrifuged for 20 min at 15,000 rpm and 4 °C (Sigma 3–18 K; Sigma Laborzentrifugen, Osterode and Harz, Germany) followed by filtration and injection in the HPLC equipment. Sugar content was determined by using a Supelcogel TM C-610H column (30 cm × 7.8 mm) with a pre-column (Supelguard 5 cm × 4.6 mm; Supelco, Bellefonte, PA, USA) and it was detected by a refractive index detector (RID). Organic acid absorbance was measured at 210 nm in the same HPLC condition using a diode-array detector (DAD). Analyses were triplicated and results were expressed as g kg−1 dry weight.
2.4.2. Volatile Compounds
For the extraction of the volatile compounds, headspace solid phase microextraction (HS-SPME) was used. Ground almond (1 g) was added to a hermetic vial with polypropylene cap and PTFE (polytetrafluoroethylene)/silicone septa, together with 500 µL salty water (12.5% NaCl) and 2.5 μL of 2-acethylthiazole (1000 mg L−1) internal standard, needed for the semi-quantification of the volatile compounds. To simulate the mouth temperature, the vial was heated in a laboratory hot plate up to 50 °C. When the temperature was reached and was stable, a 50/30 μm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) fiber was introduced in the headspace of the vial for 35 min. A gas chromatograph Shimadzu GC-17A (Shimadzu Corporation, Kyoto, Japan) coupled with mass spectrometer (MS) detector Shimadzu QP-5050A were used for isolation and identification of the volatile compounds. The Gas Chromatography—Mass Spectrometry (GC-MS) was equipped with a SLB-5ms Fused Silica Capillary Column of 30 m × 0.25 mm × 0.25 µm film thickness, 5% diphenyl and 95% dimethyl siloxane (Supelco Analytical). Helium was used as gas carrier at a flow rate of 0.9 mL min−1 in a split ratio of 1:5. The oven program was: (a) initial temperature 50 °C, (b) rate of 4.0 °C min−1 to 130 °C, (c) rate of 10 °C min−1 from 130 °C to 180 °C, (d) rate of 20 °C from 180 °C to 280 °C. The injector and the detector were held at 250 °C. The identification of the volatile compounds was performed using three methods: (a) retention indices, (b) GC-MS retention times of authentic chemicals and (c) mass spectra compounds were extracted using HS-SPME.
Simultaneously, the quantification of the volatile compounds was done on a gas chromatograph, Shimadzu 2010, with a flame ionization detector (FID). The column and chromatographic conditions were those previously reported for the GC-MS analysis. The injector temperature was 200 °C and nitrogen was used as carrier gas (1 mL min−1). The quantification was obtained from electronic integration measurements using flame ionization detection (FID). 2-Acethylthiazole (2.5 μL of 1000 mg L−1) was used as internal standard.
2.5. Descriptive Sensory Analysis
The descriptive sensory analysis was held by a trained panel with a 10 highly qualified panelists from the Food Quality and Safety Group (Miguel Hernández University of Elche, Orihuela, Alicante, Spain). The descriptive sensory analysis was performed to estimate if the significant differences among treatments were found. Although the panelists were highly trained, having more than 600 h of experience with different types of food products, three orientation sessions were done prior to almond tasting, where the panelists were trained with reference products for each attribute according to the lexicon previously described by Lipan et al. [
25]. The samples were served in odor-free 30 mL covered plastic cup and randomly coded with three digits. To clean the palate between samples, water and unsalted crackers were served. The descriptive test was performed in a special tasting room with individual booths (controlled temperature of 21 ± 1 °C and combined natural/artificial light), and to collect panelists’ evaluations, ballot charts were used. The samples were presented based on a randomized block design to avoid biases. Numerical scale from 0 to 10 was used by the panelists to quantify the intensity of the almond attributes, where 0 represents no intensity and 10 extremely strong with a 0.5 increment (
Figure 2).
2.6. Statistical Analysis
The stress integral of Ψleaf and yield were analyzed by Sigma Plot statistical software (version 12.5, Systat Software, Inc., San Jose, CA, USA). Initially, a descriptive analysis for each treatment and cultivar was done, applying a Levene’s test to check the variance homogeneity of the whole of data. Once completed, a one-way analysis of variance (ANOVA) was performed to determine whether there were statistical differences (p < 0.05) between irrigation treatments and within each cultivar, applying a Tukey’s test to find the differences among them.
Relating to the quality and sensorial parameters, a two-way analysis of variance (ANOVA) was performed, with the cultivar and irrigation being the two factors. Moreover, a Tukey’s multiple range test was carried out to establish the means that were significantly different from each other. XLSTAT Premium 2016 (Addinsoft, New York, NY, USA) was used to perform statistically significant differences, with a significant level p < 0.05.