Abstract
Viral plant diseases represent a serious problem in agricultural production, causing large shortages in the production of food crops. Eco-friendly approaches are used in controlling viral plant infections, such as biocontrol agents. In the current study, Streptomyces cellulosae isolate Actino 48 is tested as a biocontrol agent for the management of tobacco mosaic virus (TMV) and inducing tomato plant systemic resistance under greenhouse conditions. Foliar application of a cell pellet suspension of Actino 48 (2 × 107 cfu. mL−1) is performed at 48 h before inoculation with TMV. Peroxidase activity, chitinase activity, protein content, and the total phenolic compounds are measured in tomato leaves at 21 dpi. On the other hand, the TMV accumulation level and the transcriptional changes of five tomato defense-related genes (PAL, PR-1, CHS, PR-3, and PR-2) are studied. Treatment with Actino 48 before TMV inoculation (48 h) induced tomato plants to increase their levels of peroxidase and chitinase enzymes. Furthermore, a significant increase in the concentration of total phenolic compounds was observed in Actino 48 and TMV-treated tomato plants compared to TMV-treated tomato plants alone. Treatment with Actino 48 reduced the TMV accumulation level (53.8%) compared to treatment with the virus alone. Actino 48 induced plant growth, where the fresh and dry weights of tomato plants increased. Additionally, significant increases of the PAL, PR-1, CHS, and PR-3 transcripts were observed. On the other hand, a higher induction of PR-2 was only observed in TMV-treated tomato plants. In conclusion, S. cellulosae isolate Actino 48 can be used as a biocontrol agent for the reduction of symptoms and severity of TMV.
1. Introduction
Tobacco mosaic virus (TMV, genus Tobamovirus) comprises positive-sense single-stranded RNA and infects over 885 plant species in 65 families, especially tobacco and tomato plants, as well as other members of the family Solanaceae [1,2]. Infection with TMV causes mosaic symptoms on leaves and the yellowing of plant tissue. The virus causes severe economic losses worldwide [3]. The management of TMV is very difficult because it is easily dispersed wherever it is transmitted mechanically, and symptoms show at 7 to 14 days past infection (dpi) once a susceptible plant is infected [4].
The management of viral plant diseases using beneficial microbes has received much interest, because it is a safe and friendly approach for controlling viruses [5]. Most of the biocontrol agents used for controlling viral plant diseases are bacteria, for example, Bacillus spp. [6] and Pseudomonas fluorescens [7], besides some fungi, such as Trichoderma spp. [8]. Streptomyces spp. is characterized as a large group of actiobacteria that contains more than 780 species and 30 subspecies [9]. Additionally, Streptomyces spp. has a great role in inhibiting the interactions between plant and pathogens, as well as in the biocontrol of plant fungal and bacterial diseases [10]; however, even now, the utilization of Streptomyces spp. for the biocontrol of viral plant diseases is limited and its prospective mechanisms against viral diseases are not clear [11].
Induced resistance (IR) can be divided into two major mechanisms. The first is systemic acquired resistance (SAR), which is induced by plant pathogens or chemical compounds, and its regulation is based on salicylic acid (SA). This resistance pathway mainly features pathogenesis-related proteins and other disease resistance proteins. A number of the pathogenesis-related proteins (PRs) play important roles as anti-pathogenic agents [12]. The induction of plants to synthesize PRs is accomplished by infection with viruses, bacteria, fungi, or viroids [13,14,15]. Additionally, Neetu et al. [16] documented that the level of various PR proteins, such as isozymes of peroxidase and chitinase, are increased by biotic inducers. Defense enzyme activities can be induced by Streptomyces spp., which represents an important group of plant-associated microorganisms [17]. Defense-related proteins have been shown to be induced in tomato plants treated with P. fluorescens that have been infected with the viral tomato spotted wilt virus (TSWV) pathogen [18]. The second major mechanism is induced systemic resistance (ISR), which is induced by plant microbes that promote growth, and its regulation is based on jasmonic acid and ethylene, which enables faster defense responses and greater defensive capabilities in plants when resisting disease [19,20,21]. Alazem and Lin [22] confirmed that plant resistance for viral infection may be related to both mechanisms.
Among the secondary metabolites, polyphenolic compounds play many crucial roles in plant growth, development, and resistance against various biotic and abiotic stresses [23]. Beside it being the first enzyme in the phenylpropanoid pathway, PAL is involved in the biosynthesis of salicylic acid [24]. Upon pathogen infection, the activation of SA is usually correlated with the accumulation of PR-1 as a SA marker gene [25]. Peroxidase, which is considered an imperative pathogen-related protein or defense protein, is implicated in various physiological responses in plants to biotic stresses, as well as for studying pollutant degradation and management [26,27]. Galal [28] reported that treatment with certain Streptomyces strains induced systemic acquired resistance (SAR) for virus infections, while P. aeruginosa was effective at enhancing the resistance of tobacco plants to TMV [29]. In addition, an antiviral agent from S. noursei var xichangensisn has been shown to induce systemic resistance to TMV [30]. Li et al. [11] documented that S. pactum Act12 induced systemic resistance in tomato plants against tomato yellow leaf curl virus (TYLCV), where the levels of salicylic and jasmonic acids increased in tomato plants. Induction of resistance was accomplished in tomato plants against tomato mottle virus by the application of PGPR isolates, B. amyloliquefaciens 937b, and B. pumilus SE-34 [31].
Different bioactive compounds extracted from various strains of Streptomyces have been effective at reducing the local lesions of TMV on Datura metel plant leaves [4].Additionally, a bioactive compound characterized as ε-poly-L-lysine, produced by S. ahygroscopicus, has exhibited a significant protective activity and curative activity against TMV [32].The reduction percentage of mosaic symptoms caused by zucchini yellow mosaic virus (ZYMV) has been shown to change to 95% and 100% with the foliar treatment of cucumbers with S. albovinaceus and S. sparsogenes, respectively [33]. Also, T. harzianum has reduced symptoms of TMV on tomato plants by inducing systemic resistance [34]. Li et al. [35] reported that Enterobacte rasburiae BQ9 induced resistance to TYLCV under greenhouse conditions and reduced disease severity, reaching 42%, even at 45 dpi.
In the current study, we investigate the controlling activity of S. cellulosae isolate Actino 48 against TMV when it is applied as a foliar treatment 48 h before inoculation with TMV. We also examine its efficiency for inducing tomato plant growth and systemic resistance under greenhouse conditions. The peroxidase activity, chitinase activity, protein content, and total phenolic compounds are evaluated. In addition, we study the TMV accumulation level (TMV-coat protein gene), and the transcriptional levels of tomatodefense genes, such as phenylalanine ammonia-lyase (PAL), pathogen-related protein 1 (PR-1), chalcone synthase (CHS), pathogen-related protein 3 (PR-3), and pathogen-related protein 2 (PR-2).
2. Materials and Methods
2.1. Plant Materials and Source of Viral Isolate
Virus-free seeds of the GS 12 cultivar of the tomato (Solanum lycopersicum L.) plant were obtained from the Ministry of Agriculture, Agriculture Research Center, Egypt. The source of the tobacco mosaic virus (accession No., MG264131) was previously isolated from infected tomato plants [36] and continuously maintained on tobacco plants under greenhouse conditions.
2.2. Actinobacterial Isolate
Actinobacterial isolate Actino 48, registered in GenBank as S. cellulosae with the accession number of MT573878 (https://www.ncbi.nlm.nih.gov/nuccore/MT573878.1/ 8 June 2020), was provided by Dr. Gaber A. Abo-Zaid of the City of Scientific Research and Technological Applications (SRTA-City).
2.3. Cultivation of Actinobacteria
S. cellulosae isolate Actino 48 was streaked on a starch nitrate agar medium (SNA) containing the following (as g L−1): starch, 20; KNO3, 1; MgSO4·7H2O, 0.5; NaCL, 2; FeSO4·7H2O, 0.01. Colonies of S. cellulosae were inoculated into an ISP-2 medium containing the following (as g L−1): yeast extract, 4; malt extract, 10; dextrose, 4; pH of 7 [37]. The culture was cultivated at 30 °C and shaken at 200 rpm for 7 days until reaching 2 × 107 cfu. mL−1. The culture was centrifuged at 5590× g for 20 min and a cell pellet was collected, washed, and centrifuged as with the previous conditions. After that, the cell pellet of Actino 48 was suspended in sterile distilled water.
2.4. Greenhouse Experimental Design and Antiviral Activity Assay
The antiviral activity of the cell pellet suspension of S. cellulosae isolate Actino 48 (2 × 107 cfu. mL−1) was firstly checked with Datura stramonium plants, as a local lesion host for TMV, calculated according to the inhibition percentage towards the number of local lesions developed on the leaves. After surface sterilization of the tomato seeds, the cultivation process occurred in plastic pots (20 cm in diameter) supplied with sterilized soil (clay and sand in a 1:1 ratio) ininsect-proof greenhouse conditions. After the 28 days of seed growth, the tomato seedlings were transplanted into new pots, and after one week, the two upper true leaves of each tomato plant were mechanically inoculated with 1 mL of semi-purified TMV using carborundum, as described previously [38,39]. We carried out the experiment with four treatments, each treatment comprised of three replicates and each pot featuring three tomato plants (Table 1). The first treatment featured mocktomato plants (control sample), in which plants were mechanically inoculated with a viral inoculation buffer, besides foliar spraying of sterile distilled water on the plants. The second treatment included tomato plants inoculated with TMV, besides the foliar spraying of sterile distilled water (infected). The third treatment featured tomato plants treated by foliar spraying of the cell pellet suspension of Actino 48 (2 × 107 cfu. mL−1). The fourth treatment contained the tomato plants that underwent foliar spraying with the pellet suspension of bacteria 48 h before mechanical inoculation with TMV. All plants were kept inan insect-proof greenhouse under conditions of 28 °C/16 °C (day/night) and 70% relative humidity. The plants were observed daily for the recording of symptom development. For all treatments, three biological replicates of tomato leaves from three different plants were collected at 21 dpi and subjected for the determination of enzyme activity, protein content, total phenolic compounds, and RNA extraction. The fresh and dry weights of the shoots and root systems were recorded for all treatments.

Table 1.
Greenhouse experiment scheme.
2.5. Determination of Enzymes Activity and Protein Content
2.5.1. Sample Extraction
One gram of macerated leaf tissue, using liquid nitrogen, was homogenized with 4 mL of a 0.1 M phosphate buffer solution at a pH of 7. Filtration of the extracts was accomplished by a nylon cloth. After that, the extracts were centrifuged at 10,000× g for 20 min at 4 °C [40]. The supernatants were stored at −80 °C and used to estimate the activity of peroxidase, chitinase, and for determination of protein content.
2.5.2. Peroxidase Assay (POD)
The determination of the peroxidase (POD) enzyme activity was performed according to Angelinai et al. [41] by adding 80 μL of the crude extract to 500 μL of a 0.1 M phosphate buffer at pH 7, 500 μL of 5 mM of guaiacol, and 60 μL of 2 mM of hydrogen peroxide (H2O2). The total solution was incubated at 30 °C for 10 min (tetraguaiacol will thus be formed). After that, the absorbance was measured at 480 nm, where ε = 26,600 M−1 cm−1.
2.5.3. Chitinase Assay
The determination of the chitinase enzyme activity was performed according to Reissig et al. [42]. One hundred μL of the crude extract was incubated with 400 μL of colloidal chitin (1%) suspended in a citrate phosphate buffer (0.1 M at pH 6.5) at 30 °C for 2 h in shaking conditions. For stopping the reaction, 1 mL of the DNS (3,5-Dinitrosalicylic acid) reagent was added and kept in a boiling water bath for 5 min to improve the color. The tubes were cooled, centrifuged at 5000× g for 10 min, and the absorbance was measured at 575 nm. One unit of chitinase was defined as the amount of enzyme which releases 1 μmol of N-acetylglucosamine per min under the reaction condition.
2.5.4. Protein Content Determination
The Bradford method was used for the determination of protein concentrations with bovine serum albumin as the standard [43].
2.6. Determination of Total Phenolic Compounds (TCP)
The extraction from dried leaves was carried out using 80% methanol (v/v) and distilled water. Each sample of 0.5 g was weighed into conical flask that was covered with aluminum foil, and a 25 mL 80% methanol solution was added. The mixtures were then incubated and shaken at the temperature of 30 °C and at 150 rpm for 24 h. The samples were then centrifuged at 3200× g for 20 minto obtain a clean solution.
The Folin–Ciocalteau (FC) assay, described by Singleton and Rossi [44], was used for the estimation of total phenolic compounds (TPC) of samples with faint changes. Four hundred μL of the extracted samples was added into test tubes, followed by adding 2 mL of the FC reagent, then the mixtures were vortexed. After that, the mixtures were kept for 5 minat room temperature. A volume of 1.6 mL 7.5% Na2CO3 was added into the mixture and vortexed again. The mixtures were allowed to stand for 1h in the dark at room temperature (20 ± 5 °C). The absorbance was measured at 765 nm and the calibration curve was prepared using gallic acid. The results were presented as mg gallic acid equivalents (GAE)/0.5 g of the dried samples.
2.7. Quantitative Real-Time PCR Analysis of TMV and Defense-Related Genes
2.7.1. Plant Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from tomato leaves (0.1 g, fresh weight) using the RNeasyPlant Mini Kit according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). The concentration and purity of the extracted RNA was determined using the SPECTRO star Nano instrument (BMG Labtech, Ortenberg, Germany), while the integrity was assessed by agarose gel electrophoresis. One microgram of DNase-treated RNA was used to synthesize cDNA in a reverse transcription reaction, as described previously [45]. The RT-PCR reaction mixture was stored at −20 °C until used.
2.7.2. qRT-PCR Assay and Data Analysis
The TMVcoat protein (TMV-CP) accumulation level and transcriptional levels of five tomato defense genes (PAL, PR-1, CHS, PR-3, and PR-2, Table 2) were assayed and evaluated through the qRT-PCR technique, as performed previously [46,47]. The housekeeping gene, β-actin (Table 2), was used as a reference gene for the normalization of the expression levels of different genes [48]. By using the SYBR Green PCR Master Mix (Thermo, Applied Biosystems, Foster, CA, USA), each biological treatment was performed with three technical replicates and run on a Rotor-Gene 6000 instrument (QIAGEN, Germantown, AL, USA). The qRT-PCR was composed of 20 µL containing 1 µL of 10 pmol µL−1 of each primer, 10 µL of 2 × SYBR Green PCR Master Mix, 1 µL of the cDNA template, and 7 µL of nuclease-free water. The relative transcriptional level was precisely determined according to Livak and Schmittgen [49].

Table 2.
Nucleotide sequences of qRT-PCR primers used in this study.
2.8. Statistical Analysis
All data and the relative expression levels were analyzed by a one-way ANOVA using the CoStat software package, while the significant differences were calculated according to Tukey’s honest significant differences method (H.S.D.) ata p ≤ 0.05 level of probability. Standard deviation (±SD) is shown as a column bar here. Compared to the control, relative expression levels higher than 1 were displayed as an increase in gene expression (upregulation), while values lower than 1 denoted a decrease in the expression levels (downregulation).
3. Results
3.1. Effect of Actino 48 on TMV Symptoms Development
Under greenhouse conditions, the protective antiviral activity of S. cellulosae isolate Actino 48 against TMV was evaluated with tomato plants. The results show that the foliar spraying of a cell pellet suspension of Actino 48 (48 h before viral inoculation) significantly reduced disease symptoms, increased plant growth, and decreased TMV accumulation levels of treated tomato tissues when compared to non-treated plants (Figure 1). The symptoms of TMV, including the mosaic pattern and chlorosis symptoms, were clearly observed for the infected tomato plants at 14 dpi whenever a delay in symptom development for three days in the Actino 48 + virus-treated plants was observed (Figure 1). No symptoms were observed for either the mock (control) or Actino 48-treated plants (Figure 1).

Figure 1.
Disease symptoms on tomato leaves infected with tobacco mosaic virus (TMV) at 21 days post-inoculation. (A) Mock-treated plants. (B) Plants inoculated with TMV only. (C) Plants treated with Streptomyces cellulosae isolate Actino 48 only. (D) Plants treated with Actino 48 before inoculation with TMV (48 h).
3.2. Determination of Enzymes Activity and Protein Content
3.2.1. Peroxidase (POD) Activity
Treatment that included tomato plants inoculated with TMV was more significant in terms of increasing the activity of peroxidase enzyme than other treatments followed by the treatment of tomato plants with a cell pellet suspension of Actino 48 and inoculated with TMV. The peroxidase activities reached 100.7 and 79.3 U L−1 min−1, respectively. Treatment by a cell pellet suspension of Actino 48 achieved the lowest activity of peroxidase, reaching 35 U L−1 min−1 (Figure 2A).
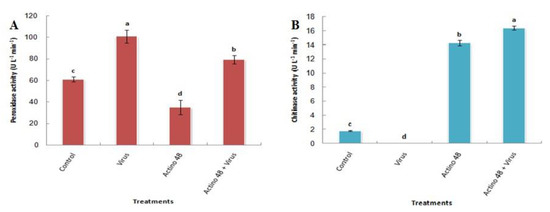
Figure 2.
Effect of Streptomyces cellulosae isolate Actino 48 on the induction of (A) peroxidase (POD) activity and (B) chitinase activity in tomato leaves at 21 days post-inoculation. Columns with the same letter denote an insignificant difference.
3.2.2. Chitinase Activity
In this study, cell pellet suspension of Actino 48 in the presence of TMV showed significant increases in chitinase activity, followed by cell pellet suspension alone when compared to the control and TMV treatments. Chitinase activity was recorded as 16.4 U L−1 min−1 in tomato plants treated with the cell pellet suspension and inoculated with TMV, while it reached 14.3 U L−1 min−1 in tomato plants treated with the cell pellet suspension alone. No response was observed for chitinase activity in tomato plants inoculated with TMV (Figure 2B).
3.2.3. Protein Content
Protein content significantly increased with the treatment with TMV compared to the control and other treatments, reaching a maximum value of 558.7 mg mL−1. Tomato plants that were treated with the cell pellet suspension of Actino 48 and inoculated with TMV recorded a protein content of 537.7 mg mL−1, with no significant differences between this group and the control treatment, which observed a protein content of 532.6 mg mL−1 (Figure 3A).
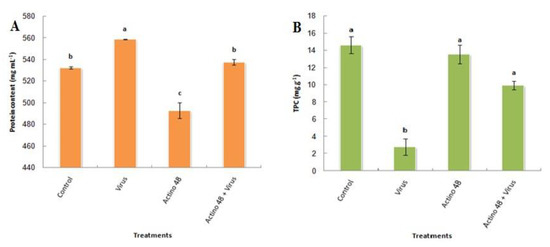
Figure 3.
Effect of Streptomyces cellulosae isolate Actino 48 on the (A) protein content and (B) total phenolic compounds in tomato leaves at 21 days post-inoculation. Columns with the same letter denote insignificant differences.
3.3. Determination of Total Phenolic Compounds
TMV infection in tomato plants significantly decreased the total phenolic compounds concentration to reach the lowest value of 2.8 mg g−1. On the other hand, the total phenolic compounds recorded significantly increasing with the control treatment, cell pellet suspension of Actino 48, and in tomato plants treated with the cell pellet suspension that were inoculated with TMV, reaching 14.6, 13.6, and 9.9 mg g−1, respectively, with no significant differences between each group (Figure 3B).
3.4. Effect of Actino 48 on TMV Accumulation Level and Growth Parameters
Compared to the control, the highest accumulation level of TMV-CP with a relative expression level of 133.4-fold was reported in the virus-treated plants (Figure 4). On the other hand, a significant decrease in the viral accumulation level was observed, with a relative transcriptional level of 61.7-fold in the Actino 48 + virus-treated plants (Figure 4).
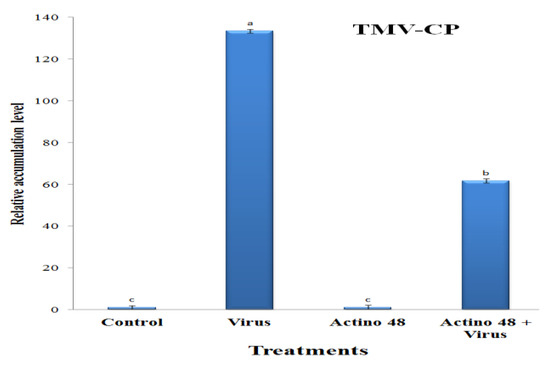
Figure 4.
The relative expression level of the TMV-CP (coat protein) gene in TMV-infected tomato plants at 21 days post-inoculation. Columns with the same letter denote insignificant differences.
Treatment with a cell pellet suspension of Actino 48 before TMV-inoculation was sufficient in terms of increasing the fresh and dry weights of the shoot and root systems of tomato plants. Actino 48 significantly increased the fresh weights of the shoot and root systems of tomato plants inoculated with TMV, recorded as 33.7 and 4.5 g, i.e., increase percentages of 28.3% and 19.7%, respectively. On the other hand, the tomato plants treated with TMV had lower fresh weights for their shoot systems (24.2 g) and root systems (3.6 g) compared to the control plants (Table 3). Moreover, treatment with Actino 48 before TMV inoculation revealed significant increases in the dry weights of shoot systems (3.4 g) and root systems (0.5 g) of tomato plants, with increase percentages of 28% and 35.6%, respectively. In contrast, significant decreases were observed in the dry weights of the shoot and root systems of tomato plants treated with TMV alone (Table 4).

Table 3.
Effect of treatment with a cell pellet suspension of Streptomyces cellulosae Actino 48 on the fresh weights of shoot and root systems in tomato plants inoculated with tobacco mosaic virus.

Table 4.
Effect of treatment with cell pellet suspension of Streptomyces cellulosae Actino 48 on dry weight of shoot and root systems in tomato plants inoculated with tobacco mosaic virus.
3.5. Effect of Actino 48 on Defense-Related Gene Expression in Tomato
Significant increases in the relative expression levels of PAL, PR-1, CHS, PR-2, and PR-3 were observed in the treated plants when compared with the non-treatment plants (p ≤ 0.05) at 21 dpi. Compared to the controls, a significant upregulation of PAL, with relative expression levels representing 1.6- and 1.5-fold changes, were observed in the Actino 48 and Actino 48 + virus-treated plants, respectively. On the other hand, virus-treated plants showed a downregulation of PAL, with a relative expression level representing a 0.7-fold change lower than the control (Figure 5). Like the PAL transcript, PR-1 was significantly upregulated, with relative transcription levels representing 2.1- and 11.2-fold changes higher than the control in the Actino 48 and Actino 48 + virus-treated tomato plants, respectively. On the other hand, the virus-treated plants showed a reduction of PR-1 with relative expression levels representing a 0.8-fold change, where no significant changes were reported when compared with the control (Figure 5).
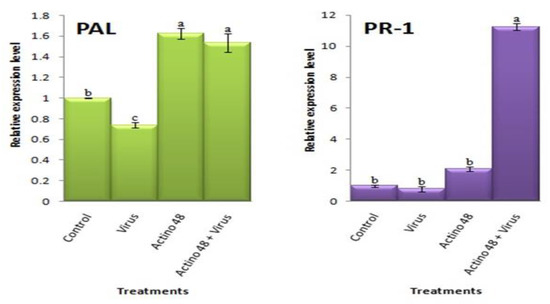
Figure 5.
The relative expression level of the phenylalanine ammonia-lyase (PAL) and pathogen-related protein 1 (PR-1) genes in TMV-infected tomato plants at 21 days post-inoculation. Columns with the same letter do not differ significantly.
Regarding the CHS transcript profiles, the induction of CHS was reported in all treatments when compared to the control plants. Both treatments of the virus and Actino 48 were triggered CHS transcriptions with relative expression levels representing 1.4- and 1.5-fold changes, respectively, higher than control. The highest CHS transcript level was observed in Actino 48 + virus-treated plants, with a relative expression level representing a 2.3-fold change over the control (Figure 6). For the PR-3 transcripts levels, only the treatment of Actino 48 + virus exhibited an induction of PR-3 with a relative expression level representing a 1.2-fold change higher than the control. Both treatments by the virus and Actino 48 showed CHS transcriptions with relative expression levels representing 0.95- and 0.96-fold changes, respectively (Figure 6). On the other hand, the induction of PR-2 with a relative expression level representing a 7-fold change higher than the control was only reported in the virus-treated tomato plants. Although the Actino 48 + virus-treated plants showed a slight induction of PR-2, with relative expression levels representing a 1.3-fold change, no significant changes were reported when compared with the controls (Figure 6).

Figure 6.
The relative expression levels of the chalcone synthase (CHS), protein 3 (PR-3), and pathogen-related protein 2 (PR-2) genes in TMV-infected tomato plants at 21 days post-inoculation. Columns with the same letter do not differ significantly.
4. Discussion
Plant diseases, especially viral plant infections, are responsible for severe crop losses in crop production worldwide, resulting in critical problems for food security [50]. The application of Actino bacteria as biocontrol agents for the management of viral plant diseases has been limited until now. In the current study, the antiviral activity of S. cellulosae isolate Actino 48 against TMV on tomato plants has been evaluated. Moreover, the peroxidase activity, chitinase activity, protein content and total phenolic compounds have been determined in leaves at 21 dpi. On the other hand, the effects of Actino 48 on the accumulation levels of TMV-CP and expression of five defense-related genes (PAL, PR-1, CHS, PR-3, and PR-2) at 21 dpi have been evaluated.
In the current study, peroxidase activity increased significantly in tomato plants infected with TMV followed by treatment with Actino 48 and TMV. S. pactum Act12 has enhanced peroxidase activity in tomato plants against tomato yellow leaf curl virus (TYLCV) [11]. Cucumber plants treated with Streptomyces as a biocontrol agent have shown an increasing peroxidase activity [51]. Also, the application of Streptomyces spp. has induced peroxidase activity as a defense enzyme in response to CMV [52]. Das and Roychoudhury [53] reported that peroxidase is supported by plant defense systems and is responsible for the reduction of the harmful effects of stresses via the scavenging of reactive oxygen species (ROS).
In our study, Actino 48 induced chitinase activity to reach its maximum value in tomato plants treated with Actino 48 and TMV compared to the control plants. These data are in accordance with the data obtained by Shafie et al. [52]. Shoman et al. [54] documented that Streptomyces spp. induces plant resistance against tobacco necrosis virus (TNV), with an increasing level of chitinase enzymes as one of the PR proteins. In addition, Kandan et al. [18] reported that the treatment with P. fluorescens increased chitinase activity in tomato plants in response to tomato spotted wilt virus (TSWV).
In the current study, protein content significantly increased in tomato plants infected with TMV followed by the treatment with Actino 48 and TMV, and the untreated tomato plants (control plants) showed no significant differences between each other. The obtained results may be related to the presence of the coat protein of TMV. Our results are similar to the results obtained by Barakat et al. [55], who reported an increasing protein content in watermelon plants infected with watermelon mosaic virus-2 (WMV-2) compared to other treatments.
Our study has revealed significant increases in the total phenolic compounds in untreated tomato plants (control), tomato plants treated with Actino 48 alone, and tomato plants treated with Actino 48 and TMV, with no significant differences between each other. On the other hand, treatment with TMV decreased the total phenolic compounds. Phenolic compounds play an important role in plant resistance to viruses, and they are implicated in phytoalexin accumulation, the biosynthesis of lignin, and the formation of structural barriers [56].
Under greenhouse experiments, the foliar application of Actino 48 (48 h before viral inoculation) significantly increased the plant growth parameters, reduced disease symptoms, and decreased viral accumulation levels compared to infected tomato plants without any treatment. Treatment with Actino 48 induced the growth of tomato plants treated with TMV compared to virus-treated tomato plants alone. The fresh and dry weights of the shoot and root systems increased significantly in tomato plants treated with Actino 48 and TMV but showed a significant reduction in the fresh and dry weights of the shoot and root systems of tomato plants infected with TMV. The increase in plant growth may be related to the ability of Actino 48 to induce the synthesis of various phytohormones and the ability of Actino 48 to produce different hormones. For example, the synthesis of indole acetic acid (IAA) in plants was stimulated by Streptomyces sp. in greenhouse experiments [57,58]. Li et al. [35] reported that S. pactum Act12 promoted tomato plant growth, where plant biomass and fruit yield were increased, while Streptomyces spp. isolate GS-93-23 was more efficient in terms of enhancing the forage weight of alfalfa [59]. Additionally, the synthesis of plant hormones, such as cytokinins, IAA, and gibberellins, has been induced by P. fluorescens strains, leading to increased plant growth [60]. On the other hand, Zamoum et al. [61] reported that the S. rochei strain PTL2 enhanced the dry weight of tomato plants, where it had the ability to produce phytohormones of IAA and gibberellin (GA3). A significant decrease in the TMV accumulation levels in the Actino 48 + virus-treated tomato plants of 53.8% compared to the virus-treated tomato plants confirmed the efficiency of the biocontrol activity of Actino 48 against TMV infection. These results suggest that Actino 48 may produce secondary metabolites which can play a major role in SAR. Li et al. [11] reported that the pre-inoculation of tomato plants with Act12 reduced TYLCV by 37.9%. Thus, Actino 48 may activate induced systemic resistance (ISR) in tomato plants against TMV infection.
In the present study, non-treated tomato plants challenged with TMV showed decreasing PAL and PR-1 levels, with relative expression levels representing 0.7- and 0.8-fold changes, respectively, which is lower than control samples. Interestingly, both treatments, i.e., Actino 48-treated and Actino 48 + virus-treated plants, exhibited an induction and overexpression of PAL and PR-1. The Actino 48 + virus-treated plants showed the highest expression level of PR-1, with a relative transcriptional level representing a 11.2-fold change, while the Actino 48-treated plants exhibited the highest expression level of PAL, with a relative expression level representing a 1.6-fold change higher than the control plants. Consequently, we suggest that Actino 48 may work as an elicitor molecule that induces the immune defense system, which may result SAR activation. In this context, tomato plants pre-treated with Act12 exhibited an induction of PR-1 and enhanced POD and PAL activities in tomato leaves, resulting in the development of SAR against TYLCV [11].
Compared to the mock plants at 21 dpi, the transcripts of chalcone synthase (CHS) were induced after challenging the tomato plants with TMV and/or Actino 48. The greatest transcriptional levels of CHS were reported in the Actino 48 + virus-treated plants, with a relative expression level representing a 2.3-fold higher change than the control plants. Consequently, the application of Actino 48 showed the greatest induction of CHS, which is strictly required for the production of naringeninchalcones. CHS, the first enzyme in the flavonoid pathway, is responsible for the conversion of p-coumaroyl CoA to naringeninchalcones and is strictly required in various plant tissues for flavonoid production [62,63]. Naringeninchalconesare considered as the primary precursors and main intermediates for the biosynthesis of many flavonoids via the action other enzymes [64,65].
In the present study, only the Actino 48 + virus treatment showed a slight induction of PR-3, encodeschitinase activity [65], with a relative expression level representing a 1.2-fold higher change than the control plants. We assumed that PR-3 expression may not be specific to TMV infection in tomato plants [36]. In addition, the upregulation or downregulation of PR-3 may depend on the viral isolate, type of plant, and plant genotype [66,67,68].
It has been reported that TMV increases the activity of PR-2 to facilitate its movement through cells [36,69]. In the current study, the virus treatment only exhibited the induction of PR-2, with a relative transcriptional level representing a 7-fold increase over the controls. These results are similar to previous studies that have reported a clear induction of PR-2 during viral infections in Arabidopsis [70], tobacco [71,72,73], onion [74], potato [75], and tomato [36] plants. Moreover, a deficiency of tobacco PR-2 decreases susceptibility to viral infection [75], while overexpression results in expediting the spread of PVY (potato virus Y) infection between cells [76,77]. Interestingly, although both treatments of Actino 48 and Actino 48 + virus exhibited slight increases in PR-2, there were no significant changes reported with the controls. Thus, the application of Actino 48 may have diminished PR-2, resulting in inhibiting long distance viral infection between cells.
Overall, the present study has shown that S. cellulosae isolate Actino 48 enhances tomato plant growth, decreases the TMV accumulation level, induces systemic resistance, and increases the production of some defense enzymes. Consequently, Actino 48 could be introduced as a biocontrol agent against TMV infection. However, further examinations are needed for analyzing potential field and commercial applications.
5. Conclusions
S. cellulosae isolate Actino 48 is sufficient in terms of inducing plant systemic resistance and controlling TMV. Actino 48 induced TMV-inoculated tomato plants to increase the level of peroxidase, chitinase, and total phenolic compounds. Actino 48 was effective in reducing the symptoms and severity of TMV. Treatment with Actino 48 reduced the TMV accumulation level (53.8%) compared to treatment with the virus alone. In addition, Actino 48 increased the fresh and dry weights of the shoot and root systems of tomato plants here. Tomato plants treated with Actino 48 and TMV showed significant increases in upregulation of the relative expression levels of the PAL, PR-1, CHS, and PR-3 genes.
Author Contributions
Planning and designing the research, A.A. and G.A.A.-Z.; methodology, A.A. and G.A.A.-Z.; software, A.A. and G.A.A.-Z.; writing—original draft preparation, G.A.A.-Z. and A.A.; writing—review and editing, G.A.A.-Z., A.A. and S.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ge, Y.; Liu, K.; Zhang, J.; Mu, S.; Hao, X. The limonoids and their antitobacco mosaic virus (TMV) activities from Munronia unifoliolata Oliv. J. Agric. Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Sanan-Mishra, N.A. Comparative analysis of the suppressor activity of tobacco mosaic virus proteins in the tomato plant. Jordan J. Biol. Sci. 2018, 11, 469–473. [Google Scholar]
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Ara, I.; Bukhari, N.A.; Aref, N.M.; Shinwari, M.M.A.; Bakir, M.A. Antiviral activities of streptomycetes against tobacco mosaic virus (TMV) in Datura plant: Evaluation of different organic compounds in their metabolites. Afr. J. Biotechnol. 2012, 11, 2130–2138. [Google Scholar]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 inhibits Fusarium oxysporum growth and induces systemic resistance to cucumber mosaic virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, P.S.; Agrawal, L.; Raj, R.; Srivastava, A.; Gupta, S.; Mishra, S.K.; Yadav, S.; Singh, P.C.; Raj, S.K.; et al. Paenibacillus lentimorbus inoculation enhances tobacco growth and extenuates the virulence of cucumber mosaic virus. PLoS ONE 2016, 11, e0149980. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, F.; Yang, J.; Qian, Y.; Dong, X.; Zhan, H. Control of tobacco mosaic virus by Pseudomonas fluorescens CZ powder in greenhouses and the field. Crop Prot. 2014, 56, 87–90. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Ozaki, K.; Hyakumachi, M. Induction of systemic resistance against cucumber mosaic virus in Arabidopsis thaliana by Trichoderma asperellum SKT-1. Plant Pathol. J. 2013, 29, 193. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.-H.; Chan, K.-G.; Khan, T.M.; Bukhari, S.I.; Saokaew, S.; Duangjai, A.; Pusparajah, P.; Lee, L.-H.; Goh, B.-H. Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front. Pharmacol. 2017, 8, 276. [Google Scholar] [CrossRef]
- Schrey, S.D.; Tarkka, M.T. Friends and foes: Streptomycetes as modulators of plant disease and symbiosis. Antonie Van Leeuwenhoek 2008, 94, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Li, Y.; Sun, Y.; Xue, Q.; Lai, H. Streptomyces pactum Act12 controls tomato yellow leaf curl virus disease and alters rhizosphere microbial communities. Biol. Fertil. Soils 2019, 55, 149–169. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Pierpoint, W.S.; Boller, T.H.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Conejero, V.; Picazo, I.; Segado, P. Citrus exocortis viroid (CEV): Protein alterations in different hosts following viroid infection. Virology 1979, 97, 454–456. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Ahl, P.; Cornu, A.; Scalla, R.; Cassini, R. First report of host b-protein appearance in response to a fungal infection in tobacco. Physiol. Plant Pathol. 1980, 16, 337–342. [Google Scholar] [CrossRef]
- Metraux, J.P.; Boller, T.H. Local and systemic induction of chitinase in cucumber plants in response to viral, bacterial and fungal infections. Physiol. Mol. Plant Pathol. 1986, 28, 161–169. [Google Scholar] [CrossRef]
- Singh, N.; Pandey, P.; Dubey, R.C.; Maheshwari, D.K. Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (Sarg.) by rhizosphere competent Bacillus subtilis BN1. World J. Microbiol. Biotechnol. 2008, 24, 1669. [Google Scholar] [CrossRef]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Große, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef]
- Kandan, A.; Ramiah, M.; Vasanthi, V.J.; Radjacommare, R.; Nandakumar, R.; Ramanathan, A.; Samiyappan, R. Use of Pseudomonas fluorescens-based formulations for management of tomato spotted wilt virus (TSWV) and enhanced yield in tomato. Biocontrol Sci. Technol. 2005, 15, 553–569. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F. Priming: Getting ready for battle. Mol. PlantMicrobe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; Lin, N. Roles of plant hormones in the regulation of host–virus interactions. Mol. Plant Pathol. 2015, 16, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.; Verardo, V. Phenolic compounds in the potato and its byproducts: An overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.M.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic acid biosynthesis and metabolism. Arab. Book Am. Soc. Plant Biol. 2011, 9, e0156. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Safaie, N.; Sadeghi, A.; Shamsbakhsh, M. Streptomyces strains induce resistance to Fusarium oxysporum f. sp. lycopersici race 3 in tomato through different molecular mechanisms. Front. Microbiol. 2019, 10, 1505. [Google Scholar] [CrossRef]
- Abd El-Rahim, W.M.; Moawad, H.; Azeiz, A.Z.A.; Sadowsky, M.J. Optimization of conditions for decolorization of azo-based textile dyes by multiple fungal species. J. Biotechnol. 2017, 260, 11–17. [Google Scholar] [CrossRef]
- Galal, A.M. Induction of systemic acquired resistance in cucumber plant against cucumber mosaic cucumovirus by local Streptomyces strains. Plant Pathol. J. 2006, 5, 343–349. [Google Scholar]
- De Meyer, G.; Audenaert, K.; Höfte, M. Pseudomonas aeruginosa 7NSK2-induced systemic resistance in tobacco depends on in planta salicylic acid accumulation but is not associated with PR1a expression. Eur. J. Plant Pathol. 1999, 105, 513–517. [Google Scholar] [CrossRef]
- Han, Y.; Luo, Y.; Qin, S.; Xi, L.; Wan, B.; Du, L. Induction of systemic resistance against tobacco mosaic virus by ningnanmycin in tobacco. Pestic. Biochem. Physiol. 2014, 111, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.F.; Zehnder, G.W.; Schuster, D.J.; Sikora, E.J.; Polston, J.E.; Kloepper, J.W. Plant growth-promoting rhizobacterial mediated protection in tomato against tomato mottle virus. Plant Dis. 2000, 84, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Xia, Z.; Zhao, X.; Wu, Y.; An, M. Purification and structural analysis of the effective anti-TMV compound ε-poly-L-lysine produced by Streptomyces ahygroscopicus. Molecules 2019, 24, 1156. [Google Scholar] [CrossRef]
- Askora, A.A. Antiphytoviral Studies from Certain Actinomycetal Isolates. Ph.D. Thesis, Zagazig University, Zagazig, Egypt, 2005. [Google Scholar]
- Kolase, S.V.; Sawant, D.M. Isolation and efficacy of antiviral principles from Trichoderma spp. against tobacco mosaic virus (TMV) on tomato. J. Maharashtra Agric. Univ. 2007, 32, 108–110. [Google Scholar]
- Li, H.; Ding, X.; Wang, C.; Ke, H.; Wu, Z.; WANG, Y.; Liu, H.; Guo, J. Control of tomato yellow leaf curl virus disease by Enterobacter asburiae BQ9 as a result of priming plant resistance in tomatoes. Turk. J. Biol. 2016, 40, 150–159. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to tobacco mosaic virus. J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Shirling, E.B.T.; Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Sanan-Mishra, N. Differential expression profiles of tomato miRNAs induced by tobacco mosaic virus. J. Agric. Sci. Technol. 2019, 21, 475–485. [Google Scholar]
- Hafez, E.E.; El-Morsi, A.A.; El-Shahaby, O.A.; Abdelkhalek, A.A. Occurrence of iris yellow spot virus from onion crops in Egypt. Virus Dis. 2014, 25, 455–459. [Google Scholar] [CrossRef]
- De Souza, M.B.; Stamford, N.P.; Silva, E.V.; Berger, L.R.R.; E Silva, S.C.E.R.; Costa, A.F.; Ferraz, A.P.F. Defense response by inter-active bio-protector and chitosan to Sclerotium rolfsii Wilt disease on cowpea, Brazilian oxisol. Afr. J. Agric. Res. 2018, 13, 1053–1062. [Google Scholar]
- Angelini, R.; Manes, F.; Federico, R. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 1990, 182, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Reissig, J.L.; Strominger, J.L.; Leloir, L.F. A modified colorimetric method for the estimation of N-acetylamino sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Abdelkhalek, A.; Elmorsi, A.; Alshehaby, O.; Sanan-Mishra, N.; Hafez, E. Identification of genes differentially expressed in onion infected with Iris yellow spot virus. Phytopathol. Mediterr. 2018, 57, 334–340. [Google Scholar]
- Behiry, S.I.; Ashmawy, N.A.; Abdelkhalek, A.A.; Younes, H.A.; Khaled, A.E.; Hafez, E.E. Compatible-and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J. Plant Dis. Prot. 2018, 125, 197–204. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Hafez, E. Iris yellow spot virus–induced chloroplast malformation results in male sterility. J. Biosci. 2019, 44, 142. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Dessoky, E.S.; Hafez, E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with tobacco mosaic virus. Biosci. Res. 2018, 15. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Salla, T.D.; Astarita, L.V.; Santarém, E.R. Defense responses in plants of Eucalyptus elicited by Streptomyces and challenged with Botrytis cinerea. Planta 2016, 243, 1055–1070. [Google Scholar] [CrossRef]
- Shafie, R.M.; Hamed, A.H.; El-Sharkawy, H.H.A. Inducing systemic resistance against cucumber mosaic cucumovirus using Streptomyces spp. Egypt J. Phytopathol. 2016, 44, 127–142. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Shoman, S.A.; Abd-Allah, N.A.; El-Baz, A.F. Induction of resistance to tobacco necrosis virus in bean plants by certain microbial isolates. Egypt. J. Biol. 2003, 5, 8–10. [Google Scholar]
- Barakat, O.S.; Goda, H.A.; Mahmoud, S.M.; Emara, K.S. Induction of systemic acquired resistance in watermelon against watermelon mosaic virus-2. Arab. J. Biotech. 2012, 15, 1–22. [Google Scholar]
- Sudhakar, N.; Nagendra-Prasad, D.; Mohan, N.; Murugesan, K. Induction of systemic resistance in Lycopersicon esculentum cv. PKM1 (tomato) against cucumber mosaic virus by using ozone. J. Virol. Methods 2007, 139, 71–77. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; VALADON, L.R.G.; ABD-EL-RAHEEM, E.-S. Biosynthesis and metabolism of indole-3-acetic acid in Streptomyces mutabilis and in Streptomyces atroolivaceus. Microbios Lett. 1987, 36, 85–95. [Google Scholar]
- El-Shanshoury, A.B.D.E.-R.R. Biosynthesis of indole-3-acetic acid in Streptomyces atroolivaceus and its changes during spore germination and mycelial growth. Microbios 1991, 67, 159–164. [Google Scholar]
- Xiao, K.; Kinkel, L.L.; Samac, D.A. Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol. Control 2002, 23, 285–295. [Google Scholar] [CrossRef]
- Dubeikovsky, A.N.; Mordukhova, E.A.; Kochetkov, V.T.; Polikarpova, F.Y.; Boronin, A.M. Growth promotion of blackcurrant softwood cuttings by recombinant strain Pseudomonas fluorescens BSP53a synthesizing an increased amount of indole-3-acetic acid. Soil Biol. Biochem. 1993, 25, 1277–1281. [Google Scholar] [CrossRef]
- Zamoum, M.; Goudjal, Y.; Sabaou, N.; Mathieu, F.; Zitouni, A. Development of formulations based on Streptomyces rochei strain PTL2 spores for biocontrol of Rhizoctonia solani damping-off of tomato seedlings. Biocontrol Sci. Technol. 2017, 27, 723–738. [Google Scholar] [CrossRef]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.-F.; Larondelle, Y.; Evers, D.; et al. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Kang, J.-H.; McRoberts, J.; Shi, F.; Moreno, J.E.; Jones, A.D.; Howe, G.A. The flavonoid biosynthetic enzyme chalconeisomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014, 164, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The stereochemistry of flavonoids. In The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Van Loon, L.C. Occurrence and properties of plant pathogenesis-related proteins. In Pathogenesis-Related Proteins in Plants; Datta, S.K., Muthukrishnan, S., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 1–19. [Google Scholar]
- Mandadi, K.K.; Scholthof, K.-B.G. Characterization of a viral synergism in the monocot Brachypodium distachyon reveals distinctly altered host molecular processes associated with disease. Plant Physiol. 2012, 160, 1432–1452. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, K.K.; Pyle, J.D.; Scholthof, K.-B.G. Comparative analysis of antiviral responses in Brachypodium distachyon and Setariaviridis reveals conserved and unique outcomes among C3 and C4 plant defenses. Mol. Plant Microbe Interact. 2014, 27, 1277–1290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andronic, L.; Port, A.; Duca, M. Expression of some genes in barley under viral infection. Buletinul Academiei de Ştiinţe a Moldovei. Ştiinţelevieţii 2015, 326, 59–65. [Google Scholar]
- Iglesias, V.A.; Meins, F., Jr. Movement of plant viruses is delayed in a β-1,3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Linthorst, H.J.; Melchers, L.S.; Mayer, A.; Van Roekel, J.S.; Cornelissen, B.J.; Bol, J.F. Analysis of gene families encoding acidic and basic beta-1,3-glucanases of tobacco. Proc. Natl. Acad. Sci. USA 1990, 87, 8756–8760. [Google Scholar] [CrossRef]
- Rezzonico, E.; Flury, N.; Meins, F.; Beffa, R. Transcriptional down-regulation by abscisic acid of pathogenesis-related β-1,3-glucanase genes in tobacco cell cultures. Plant Physiol. 1998, 117, 585–592. [Google Scholar] [CrossRef]
- Šindelářová, M.; Šindelář, L. Isolation of pathogenesis-related proteins from TMV-infected tobacco and their influence on infectivity of TMV. Plant Prot. Sci. 2005, 41, 52–57. [Google Scholar] [CrossRef]
- ElMorsi, A.; Abdelkhalek, A.; Alshehaby, O.; Hafez, E.E. Pathogenesis-related genes as tools for discovering the response of onion defence system against Iris yellow spot virus infection. Botany 2015, 93, 735–744. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef] [PubMed]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F., Jr.; Iglesias, V.A. Local expression of enzymatically active class I β-1,3-glucanase enhances symptoms of TMV infection in tobacco. Plant J. 2001, 28, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Dobnik, D.; Baebler, Š.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K.; et al. β-1,3-glucanase class III promotes spread of PVY NTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).