Effects of Fruit and Vegetable Wastes and Biodegradable Municipal Wastes Co-Mixed Composts on Nitrogen Dynamics in an Oxisol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Co-Composting
2.2. Nitrogen Mineralization Study: Soil Incubation
2.3. Statistical Analyses
3. Results and Discussion
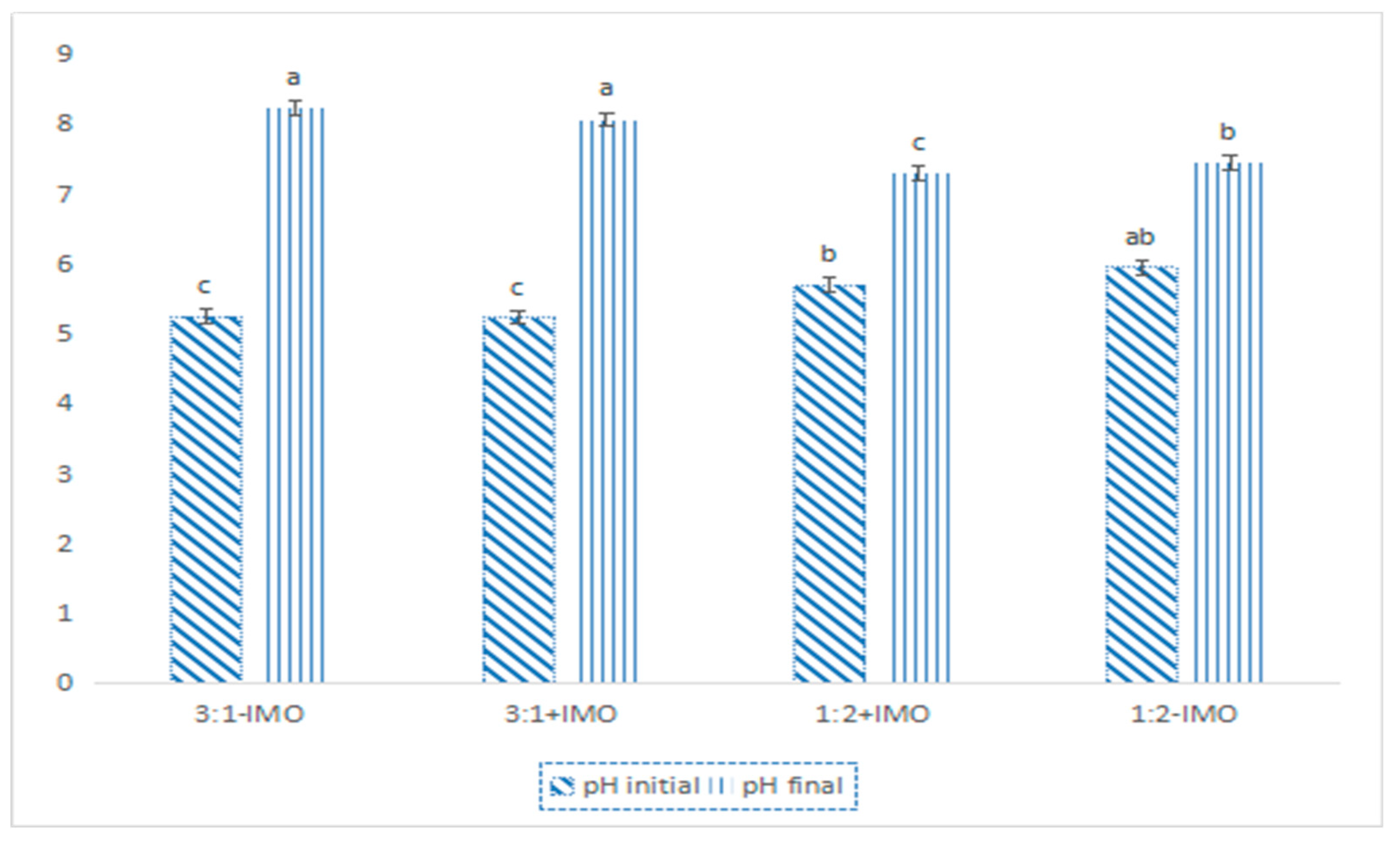
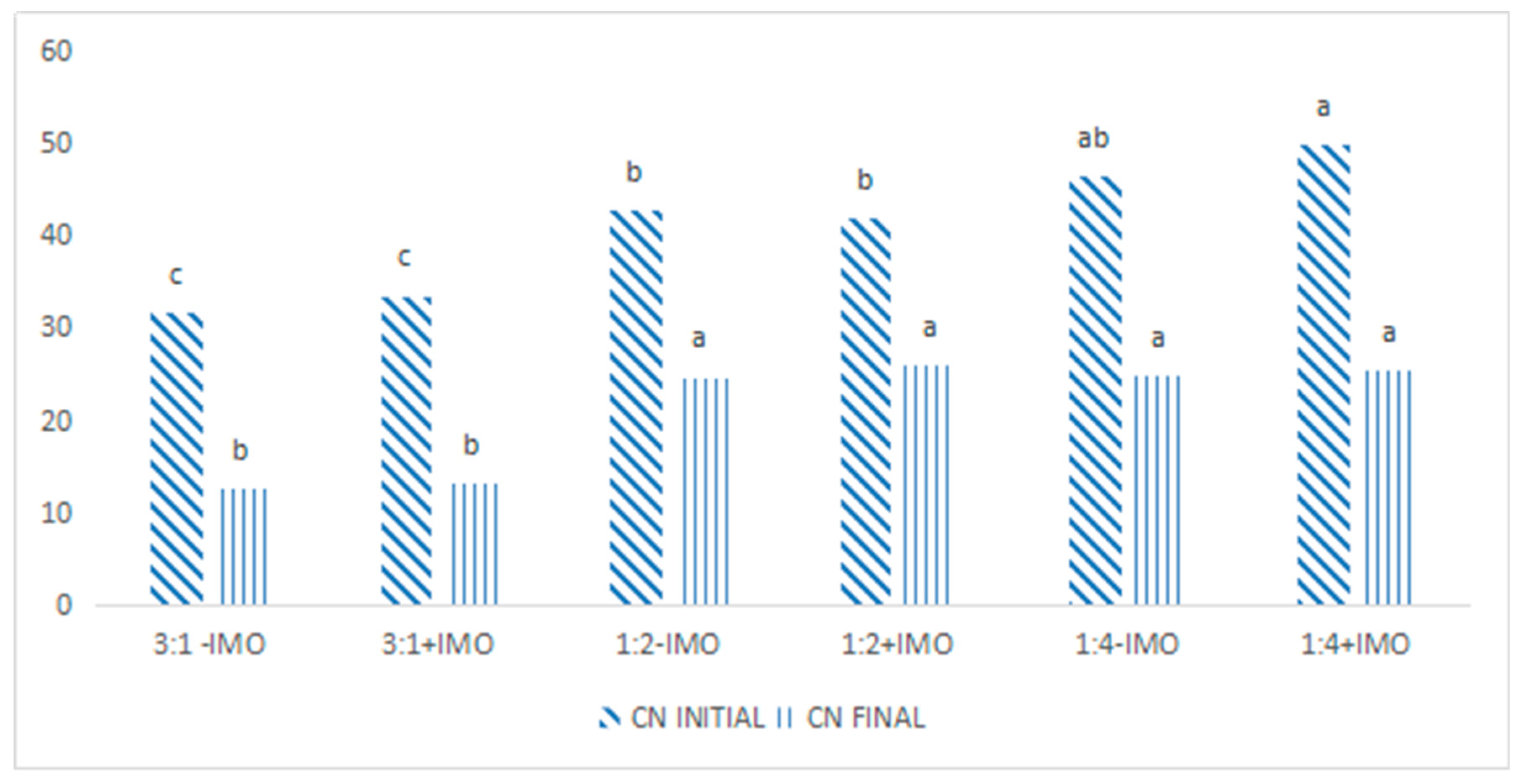
3.1. Co-Composting Materials
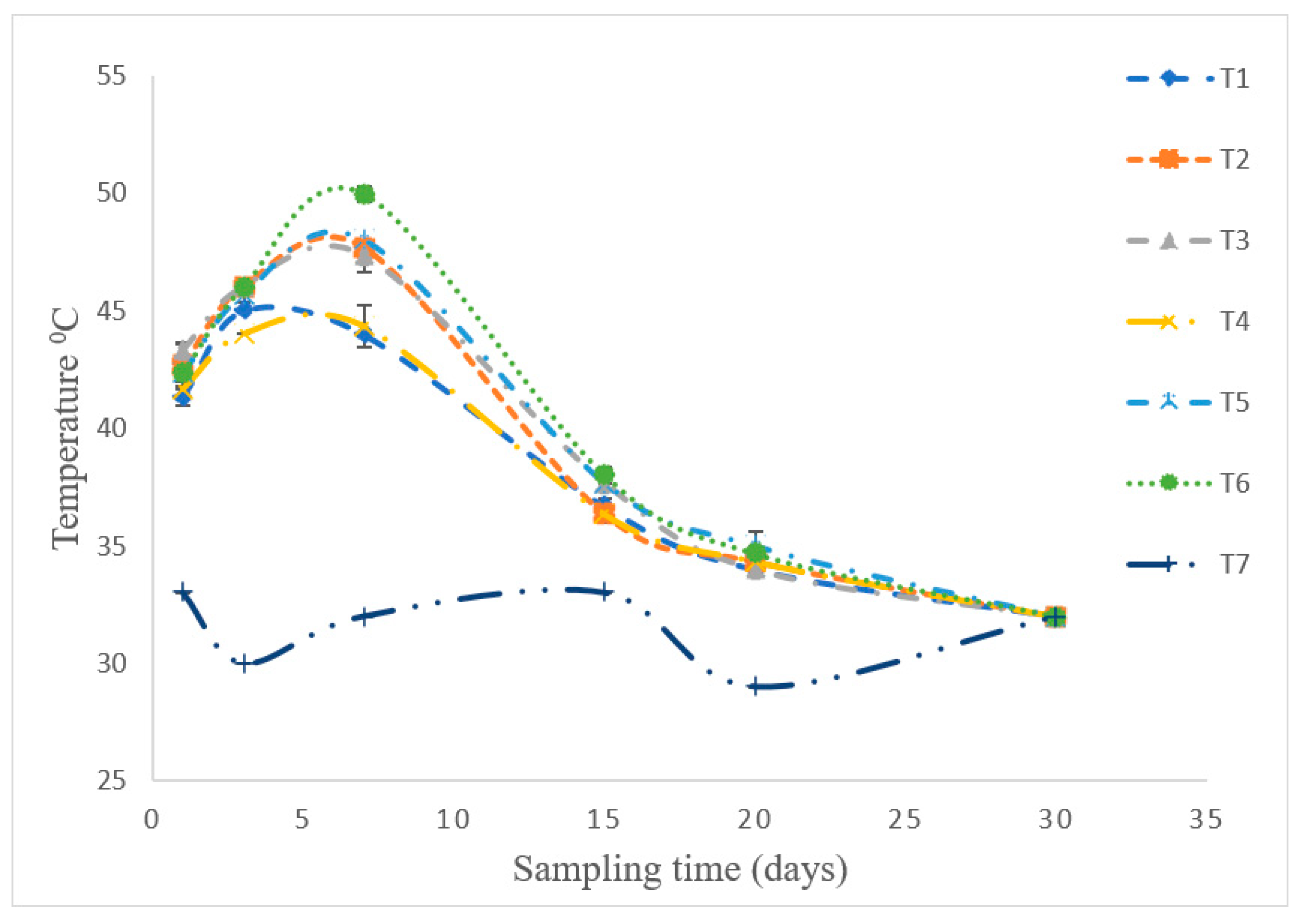
3.2. Temperature Profile during Co-Composting for 30 Days
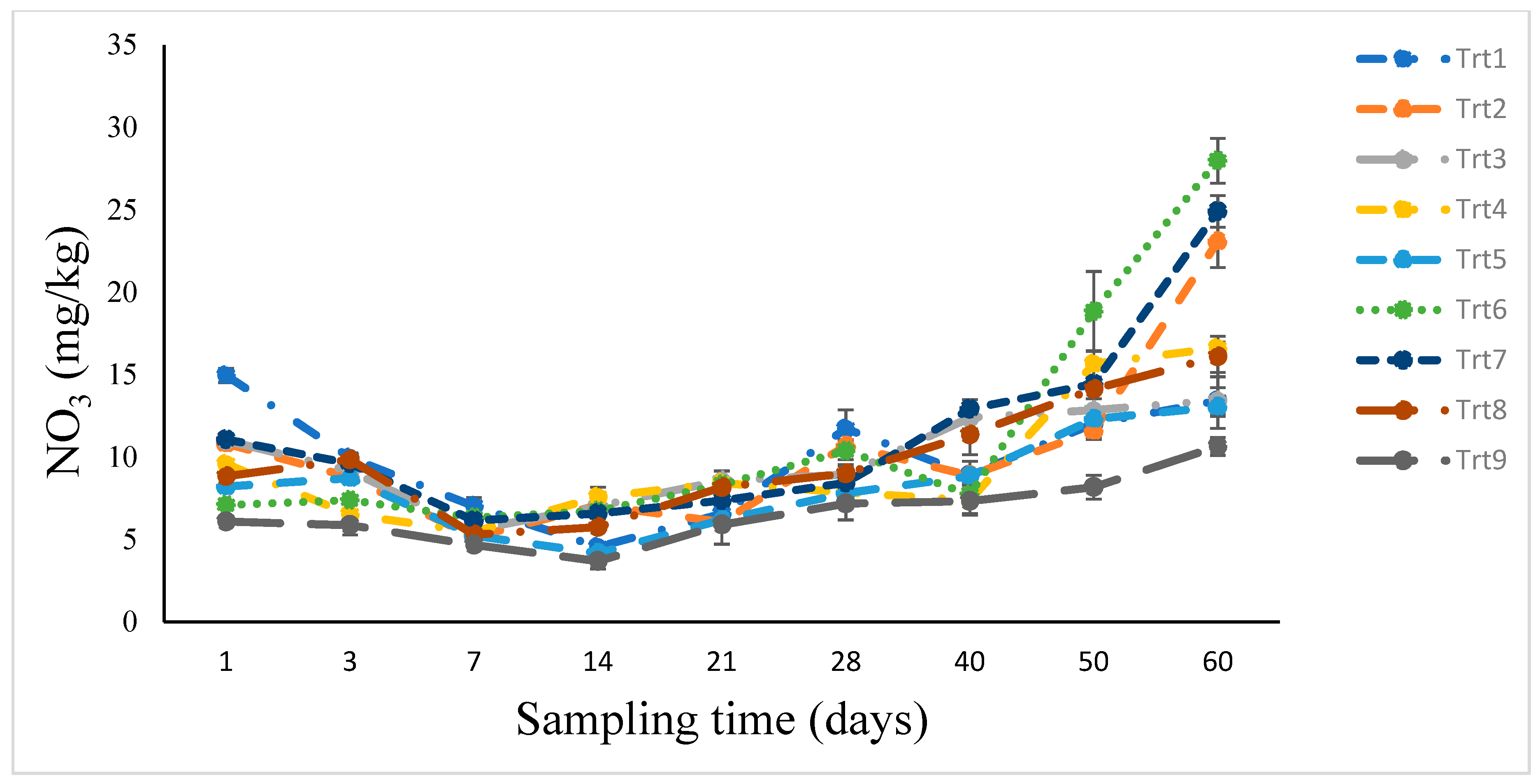
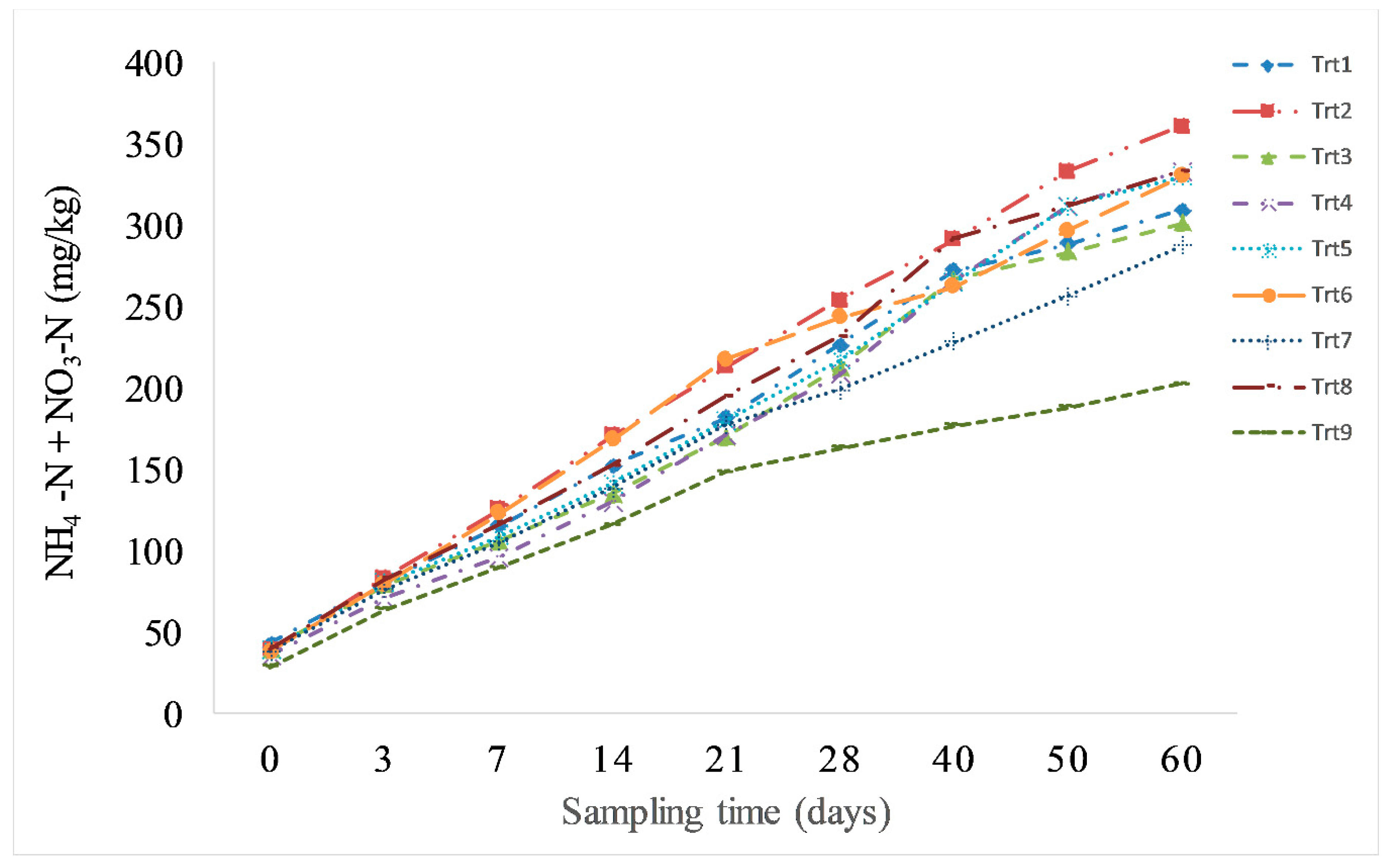
3.3. Nitrogen Mineralization: Soil Incubation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management; Urban development series; knowledge papers no. 15; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Vilariño, M.V.; Franco, C.; Quarrington, C. Food loss and waste reduction as an integral part of a circular economy. Front. Environ. Sci. 2017, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.; Panesar, P.S.; Bera, M.B. Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour. Bioprocess. 2019, 6, 26. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- Aminah, S.; Muttalib, A.; Norkhadijah, S.; Ismail, S.; Praveena, S.M. Application of Effective Microorganism (EM) in Food Waste Composting: A review. Asia Pac. Environ. Occup. Health J. 2016, 2, 37–47. [Google Scholar]
- López-González, J.A.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; Jurado, M.M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality, and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Tratsch, M.V.M.; Ceretta, C.A.; da Silva, L.S.; Ferreira, P.A.A.; Brunetto, G. Composition and mineralization of organic compost derived from composting of fruit and vegetable waste. Rev. Ceres 2019, 66, 307–315. [Google Scholar] [CrossRef]
- Jara-Samaniego, J.; Pérez-Murcia, M.D.; Bustamante, M.A.; Paredes, C.; Pérez-Espinosa, A.; Gavilanes-Terán, I.; López, M.; Marhuenda-Egea, F.C.; Brito, H.; Moral, R. Development of organic fertilizers from food market waste and urban gardening by composting in Ecuador. PLoS ONE 2017, 12, 0181621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawoteea, S.A.; Mudhoo, A.; Kumar, S. Co-composting of vegetable wastes and carton: Effect of carton composition and parameter variations. Bioresour. Technol. 2017, 227, 171–178. [Google Scholar] [CrossRef]
- Katre, N.H. Use of vegetable waste through aerobic composting of village bamhani, district: -Gondia (Maharashta state), India. Int. J. Life Sci. Biotechnol. Pharma Res. 2012, 1, 134–142. [Google Scholar]
- Zhang, Y.; Xu, W.; Duan, P.; Cong, Y.; An, T.; Yu, N.; Zou, H.; Dang, X.; An, J.; Fan, Q.; et al. Evaluation and simulation of nitrogen mineralization of paddy soils in Mollisols area of Northeast China under waterlogged incubation. PLoS ONE 2017, 12, e0171022. [Google Scholar] [CrossRef]
- Antonious, G.F. Recycling Organic Waste for Enhancing Soil Urease and Invertase Activity. Int. J. Waste Resour. 2016, 6, 219. [Google Scholar] [CrossRef]
- Tognetti, C.; Mazzarino, M.J.; Laos, F. Improving the quality of municipal organic waste compost. Bioresour. Technol. 2007, 98, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and Biological Characterization of Organic Matter during Composting of Municipal Solid Waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis, Agronomy Monograph 9, Part 2; ASA, SSSA: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Kim, E.Y.; Hong, Y.K.; Lee, C.H.; Oh, T.K.; Kim, S.C. Effect of organic compost manufactured with vegetable waste on nutrient supply and phytotoxicity. Appl. Biol. Chem. 2018, 61, 509–521. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Li, G.; Jia, X.; Zhao, X.; Lin, Q. Net nitrogen mineralization delay due to microbial regulation following the addition of granular organic fertilizer. Geoderma 2020, 359, 113994. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Gabhane, J.; William, S.P.; Bidyadhar, R.; Bhilawe, P.; Anand, D.; Vaidya, A.N.; Wate, S.R. Additives aided composting ofgreen waste: Effects on organic matter degradation, compost maturity, and quality of the finished compost. Bioresour. Technol. 2012, 114, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38, 17. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Khan, J.; Bundela, P.S.; Wong, J.W.C.; Selvam, A. Evaluation of thermophilic fungal consortium for organic municipal solid waste composting. Bioresour. Technol. 2014, 168, 214–221. [Google Scholar] [CrossRef]
- Mohee, R.; Boojhawon, A.; Sewhoo, B.; Rungasamy, S.; Somaroo, G.D.; Mudhoo, A. Assessing the potential of coal ash and bagasse ash as inorganic amendments during composting of municipal solid wastes. J. Environ. Manag. 2015, 159, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.K.; Shafiq, T.; Ahmed, S.; Ahmed, K. Effect of carbon nitrogen ratio, ammonia nitrogen in food waste composting using different techniques. In World Environment Day; Pakistan Engineering Congress (PEC): Punjab, Pakistan, 2010; pp. 149–153. [Google Scholar]
- Wang, C.; Wang, X.; Pei, G.; Xia, Z.; Peng, B.; Sun, L.; Wang, J.; Gao, D.; Chen, S.; Liu, D.; et al. Stabilization of microbial residues in soil organic matter after two years of decomposition. Soil Biol. Biochem. 2020, 141, 107687. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Yee, L.L.; Tin, L.C.; Shiun, L.J.; Klemeš, J.J.; Siong, H.C.; Mansor, N.N.A. Feedstock amendment for the production of quality compost for soil amendment & heavy metal immobilisation. Chem. Eng. Trans. 2017, 56, 499–504. [Google Scholar]
- Horrocks, A.; Curtin, D.; Tregurtha, C.; Meenken, E. Municipal compost as a nutrient source for organic crop production in New Zealand. Agronomy 2016, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Ansong Omari, R.; Bellingrath-Kimura, D.; Fujii, Y.; Sarkodee-Addo, E.; Appiah Sarpong, K.; Oikawa, Y. Nitrogen Mineralization and Microbial Biomass Dynamics in Different Tropical Soils Amended with Contrasting Organic Resources. Soil Syst. 2018, 2, 63. [Google Scholar] [CrossRef] [Green Version]
- Butterly, C.; Baldock, J.; Tang, C. Chemical mechanisms of soil pH change by agricultural residues. In 19th World Congress of Soil Science, Soil Solutions for a Changing World; DVD: Brisbane, Australia, 2010. [Google Scholar]
- Xu, J.M.; Tang, C.; Chen, Z.L. The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. Biochem. 2006, 38, 709–719. [Google Scholar] [CrossRef]
- Xu, R.K.; Coventry, D.R. Soil pH changes associated with lupin and wheat plant materials incorporated in a red-brown earth soil. Plant Soil 2003, 250, 113–119. [Google Scholar] [CrossRef]
- Escudero, A.; González-Arias, A.; del Hierro, O.; Pinto, M.; Gartzia-Bengoetxea, N. Nitrogen dynamics in soil amended with manures composted in dynamic and static systems. J. Environ. Manag. 2012, 108, 66–72. [Google Scholar] [CrossRef]
- Mokolobate, M.S.; Haynes, R.J. Increases in pH and soluble salts influence the effect that additions of organic residues have on concentrations of exchangeable and soil solution aluminium. Eur. J. Soil Sci. 2002, 53, 481–489. [Google Scholar] [CrossRef]
- Tang, C.; Yu, Q. Impact of chemical composition of legume residues and initial soil pH on pH change of a soil after residue incorporation. Plant Soil 1999, 215, 29–38. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W. Nitrogen mineralization potential of three animal manures applied on a sandy clay loam soil. Bioresour. Technol. 2010, 101, 5645–5651. [Google Scholar] [CrossRef]
- Calderón, F.J.; McCarty, G.W.; Van Kessel, J.A.S.; Reeves, J.B. Carbon and Nitrogen Dynamics during Incubation of Manured Soil. Soil Sci. Soc. Am. J. 2004, 68, 1592–1599. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]



| Materials | MC FW | C (%) DW | N (%) DW | C/N DW | P (%) DW | K (%) DW | Ca (%) DW | Mg (%) DW |
|---|---|---|---|---|---|---|---|---|
| Spinach | 89.26 | 29.82 | 4.73 | 6.31 | 0.22 | 4.06 | 1.95 | 0.24 |
| Cabbage | 87.93 | 35.56 | 2.88 | 12.36 | 0.17 | 2.73 | 0.73 | 0.18 |
| Mustard | 91.89 | 31.30 | 6.12 | 5.11 | 0.27 | 3.92 | 3.87 | 0.39 |
| Carrot | 89.93 | 34.99 | 1.91 | 18.35 | 0.13 | 2.59 | 0.23 | 0.10 |
| Cucumber | 96.71 | 34.55 | 2.00 | 16.46 | 0.07 | 2.31 | 0.28 | 0.12 |
| Orange | 81.27 | 35.25 | 0.67 | 53.01 | 0.03 | 0.98 | 0.63 | 0.13 |
| Pineapple | 84.31 | 36.37 | 0.78 | 46.75 | 0.06 | 2.35 | 1.57 | 0.26 |
| Apple | 84.60 | 35.91 | 0.39 | 90.92 | 0.07 | 1.80 | 0.22 | 0.07 |
| Banana | 70.99 | 34.33 | 0.56 | 60.97 | 0.05 | 2.10 | 0.05 | 0.24 |
| Watermelon | 89.72 | 30.14 | 2.67 | 11.31 | 0.20 | 3.83 | 0.45 | 0.33 |
| Sweet melon | 90.58 | 32.79 | 1.97 | 16.62 | 0.15 | 3.15 | 0.36 | 0.32 |
| Material | MC (%) | C (%) | N (%) | C/N | P (%) | K (%) | Ca (%) | Mg (%) |
|---|---|---|---|---|---|---|---|---|
| 70.47 | 37.36 | 1.60 | 23.33 | 0.08 | 0.90 | 0.54 | 0.09 | |
| 1.51 | 30.93 | 0.18 | 169.96 | 0.01 | 0.87 | 2.77 | 0.06 | |
| 0.93 | 41.25 | 1.35 | 30.60 | 0.05 | 0.42 | 1.06 | 0.09 |
| Material Used | 3:1 | 1:2 | 1:4 | |
|---|---|---|---|---|
| Fruit | 70% | 5.25 | 2.33 | 1.4 |
| Vegetable | 30% | 2.25 | 1.00 | 0.6 |
| Paper | 30% | 0.75 | 2.00 | 2 |
| Rice | 50% | 1.25 | 3.33 | 4 |
| Yard wastes | 20% | 0.5 | 1.33 | 1.6 |
| Total (kg) | 10 kg | 10 kg | 10 kg |
| TRT | 3:1 − IMO | 1:2 − IMO | 1:4 − IMO | 3:1 + IMO | 1:2 + IMO | 1:4 + IMO | Standard Compost |
|---|---|---|---|---|---|---|---|
| N (%) | 2.76 a | 1.65 b | 1.65 b | 2.70 a | 1.52 b | 1.58 b | 0.6 |
| P (%) | 0.17 a | 0.05 b | 0.05 b | 0.18 a | 0.06 b | 0.05 b | 0.22% |
| K (%) | 3.23 a | 1.48 b | 1.15 b,c | 3.04 a | 1.50 b | 1.03 c | 0.25% |
| Ca (%) | 4.02 a | 2.66 c | 2.54 c | 3.76 a,b | 2.88 b,c | 2.49 c | 1.42 |
| Mg (%) | 0.36 a | 0.19 b | 0.17 b | 0.31 a | 0.20 b | 0.15 b | 0.18 |
| EC (mS cm) | 2.29 a | 2.33 a | 2.30 a | 2.32 a | 2.37 a | 2.28 a | <3 |
| Germ (%) | 68.55 a | 87.14 a | 86.59 a | 96.63 a | 80.12 a | 75.8 a | >70% * |
| MC (%) | 44.93 a | 48.71 a | 44.45 a | 43.85 a | 40.45 a | 43.67 a | variable |
| Cu (mg kg−1) | 14.40 a | 7.27 a | 41.23 a | 23.47 a | 8.13 a | 6.60 a | 300 |
| Zn (mg kg−1) | 71.2 a | 44.3 a | 93.6 a | 29.2 a | 53.3 a | 41.00 a | 1000 |
| Cd (mg kg−1) | 0.02 c | 0.02 d | 0.01 e | 0.07 a | 0.06 b | 0.07 a | <5 ** |
| As (mg kg−1) | 0.53 b | 0.43 c | 0.53 a | 0.28 f | 0.34 d | 0.29 e | <50 ** |
| Cr (mg kg−1) | 5.88 d | 4.68 e | 7.15 c | 8.13 b | 1.23 f | 8.95 a | <200 ** |
| Ni (mg kg−1) | 1.19 e | 0.73 f | 2.29 d | 3.53 b | 4.25 a | 2.71 c | <150 ** |
| pH | NH4-N | NO3-N | NH4-N + NO3-N | |
|---|---|---|---|---|
| pH | 1 | |||
| NH4-N | 0.3349 | 1 | ||
| <0.0001 | ||||
| NO3-N | −0.2596 | −0.3801 | 1 | |
| <0.0001 | <0.0001 | |||
| NH4-N + NO3-N | 0.2278 | 0.88168 | 0.1013 | 1 |
| 0.0003 | < 0.0001 | 0.1152 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MUSA, A.M.; ISHAK, C.F.; KARAM, D.S.; Md JAAFAR, N. Effects of Fruit and Vegetable Wastes and Biodegradable Municipal Wastes Co-Mixed Composts on Nitrogen Dynamics in an Oxisol. Agronomy 2020, 10, 1609. https://doi.org/10.3390/agronomy10101609
MUSA AM, ISHAK CF, KARAM DS, Md JAAFAR N. Effects of Fruit and Vegetable Wastes and Biodegradable Municipal Wastes Co-Mixed Composts on Nitrogen Dynamics in an Oxisol. Agronomy. 2020; 10(10):1609. https://doi.org/10.3390/agronomy10101609
Chicago/Turabian StyleMUSA, Aishatu Mala, Che Fauziah ISHAK, Daljit Singh KARAM, and Noraini Md JAAFAR. 2020. "Effects of Fruit and Vegetable Wastes and Biodegradable Municipal Wastes Co-Mixed Composts on Nitrogen Dynamics in an Oxisol" Agronomy 10, no. 10: 1609. https://doi.org/10.3390/agronomy10101609
APA StyleMUSA, A. M., ISHAK, C. F., KARAM, D. S., & Md JAAFAR, N. (2020). Effects of Fruit and Vegetable Wastes and Biodegradable Municipal Wastes Co-Mixed Composts on Nitrogen Dynamics in an Oxisol. Agronomy, 10(10), 1609. https://doi.org/10.3390/agronomy10101609






