Protection of Highbush Blueberry Plants against Phytophthora cinnamomi Using Serendipita indica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of S. indica Inoculum and Peat Substrate Inoculation
2.3. Preparation of the Substrate Infected with P. cinnamomi
2.4. Inoculation of Highbush Blueberry Plants with S. indica and P. cinnamomi
2.5. Assessment of Colonisation of Highbush Blueberry Roots by S. indica
2.6. Re-Isolation of P. cinnamomi from Infected Plants and Substrates
2.7. Assessment of Plant Growth and Tracking of Disease
3. Results and Discussion
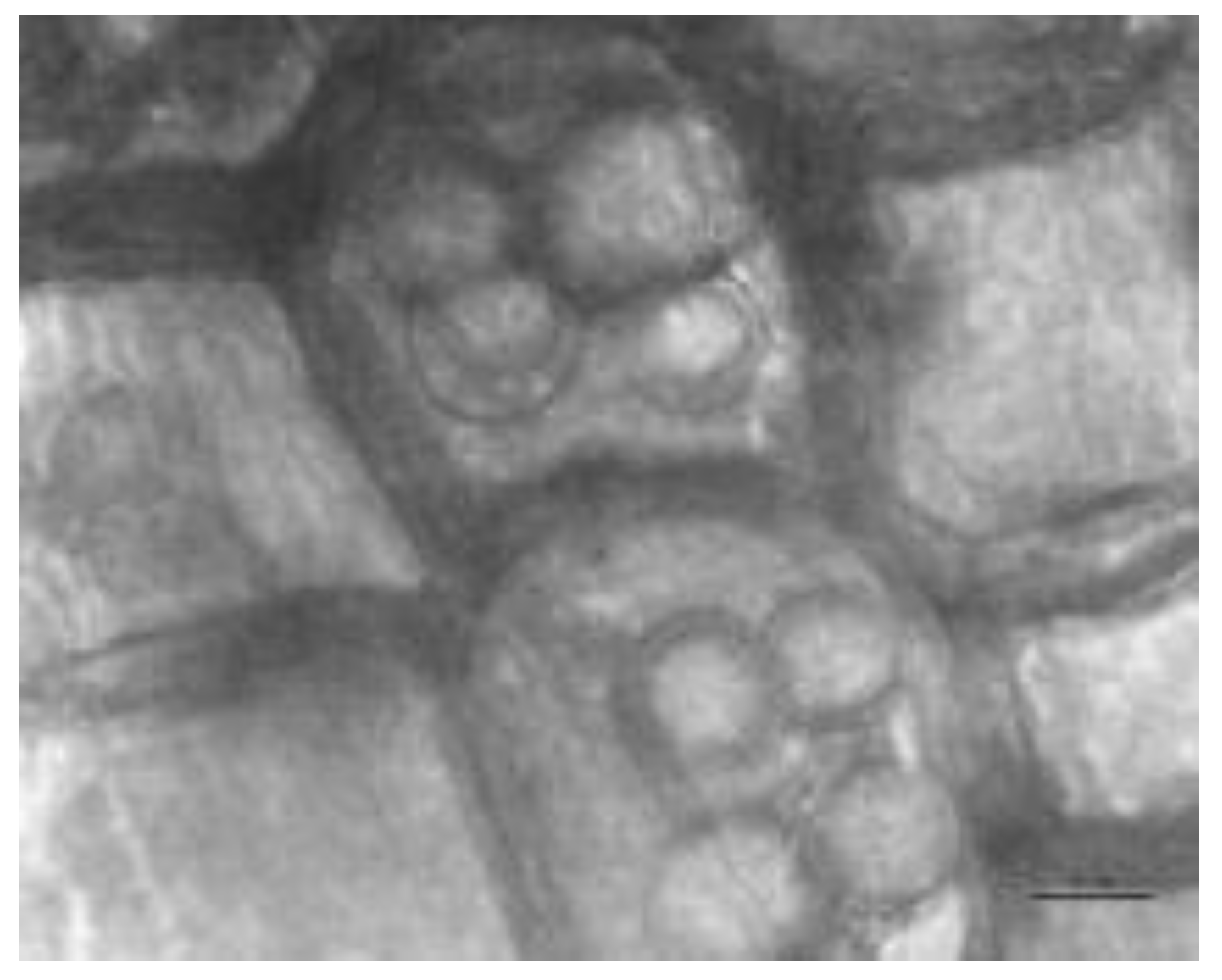
3.1. Colonisation of Roots with S. indica
3.2. Influence of S. indica and P. cinnamomi on Growth of Plants
3.3. Influence of S. indica on Protection of Blueberry Plants against P. cinnamomi
3.4. Re-Isolation of Pathogen from Plants and Substrate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neto, C. Cranberry and blueberry: Evidence for protective effects against cancer and vascular diseases. Mol. Nutr. Food Res. 2007, 51, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Može, S.; Polak, T.; Gašperlin, L.; Koron, D.; Vanzo, A.; Poklar, U.N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancook, J.F. Blueberry, 2nd ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Brazelton, C.; Young, K. 2016 World Blueberry Statistics and Global Market Analysis. International Blueberry Organization. 2017. Available online: https://www.internationalblueberry.org/2017/05/03/2016-global-blueberry-statistics-and-intelligence-report-is-now-available-at-the-ibos-library/ (accessed on 3 May 2017).
- Pluta, S.; Żurawicz, E. The High-Bush Blueberry (Vaccinium corymbosum L.) breeding programme in Poland. Acta Hortic. 2014, 1017, 178–180. [Google Scholar] [CrossRef]
- Tooley, P.W.; Kyde, K.I.; Englander, L. Susceptibility of selected Ericaceous host species to Phytophthora ramorum. Plant Dis. 2004, 88, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Shearer, B.L.; Crane, C.E.; Cochrane, J.A.; Dunne, C.P. Variation in susceptibility of threatened flora to Phytophthora cinnamomi. Austr. Plant Pathol. 2013, 42, 491–502. [Google Scholar] [CrossRef]
- Bryla, D.R.; Linderman, R.G. Incidence of Phytophthora and Pythium infection and the relation to cultural conditions in commercial blueberry fields. HortScience 2008, 43, 260–263. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.J. Survival of southern highbush blueberry cultivars in Phytophthora root rot-infested fields in South Mississipi. Int. J. Fruit Sci. 2012, 12, 146–155. [Google Scholar] [CrossRef]
- Larach, A.; Besoain, X.; Salgado, E. Crown and root rot of highbush blueberry caused by Phytophthora cinnamomi and P. citrophthora and cultivar susceptibility. Cien. Inv. Agr. 2009, 36, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Orlikowski, L.B.; Ptaszek, M.; Meszka, B. Phytophthora cinnamomic—New pathogen of high blueberry in Poland. Prog. Plant Prot. 2015, 55, 472–477. [Google Scholar]
- Smith, B.J. Cultural practices and chemical treatments affect Phytophthora root rot severity of blueberries grown in south Mississippi. Int. J. Fruit Sci. 2008, 8, 173–181. [Google Scholar] [CrossRef]
- Yeo, J.R.; Weiland, J.E.; Sullivan, D.M.; Bryla, D.R. Nonchemical, cultural management strategies to suppress Phytophthora root rot in Northern highbush blueberry. HortScience 2017, 52, 725–731. [Google Scholar] [CrossRef]
- Oelmüller, R.; Sherameti, I.; Tripathi, S.; Varma, A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 2009, 49, 1–17. [Google Scholar] [CrossRef]
- Franken, P. The plant strenghthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biotechnol. 2012, 96, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- Varma, A.; Sherameti, I.; Tripathi, S.; Prasad, R.; Das, A.; Sharma, M.; Bakshi, M.; Johnson, J.M.; Bhardwaj, S.; Arora, M.; et al. The symbiotic fungus Pirimospora indica. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–260. [Google Scholar]
- Johnson, J.M.; Alex, T.; Oelmueller, R. Piriformospora indica: The versatile and multifunctional root endophytic fungus for enhanced yield and tolerance to biotic and abiotic stress in crop plants. J. Trop. Agric. 2014, 52, 103–122. [Google Scholar]
- Rabiey, M.; Ullah, I.; Shaw, L.J.; Shaw, M.W. Potential ecological effects of Piriformospora indica, a possible biocontrol agent, in UK agricultural system. Biol. Control. 2017, 104, 1–9. [Google Scholar] [CrossRef]
- Panda, S.; Busatto, N.; Hussain, K.; Kamble, A. Pirimospora indica-primed transcriptional reprogramming induces defense response against early blight in tomato. Sci. Hortic. 2019, 255, 209–219. [Google Scholar] [CrossRef]
- Trzewik, A.; Maciorowski, R.; Klocke, E.; Orlikowska, T. The influence of Piriformospora indica on the resistance of two rhododendron cultivars to Phytophthora cinnamomi and P. plurivora. Biol. Control 2020, 140, 104121. [Google Scholar] [CrossRef]
- Hill, T.W.; Keafer, E. Improved protocols for Aspergillus medium: Trace elements and minimum medium salt stock solutions. Fungal Genet. Newsl. 2001, 48, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.M.; Sherameti, I.; Ludwig, A.; Nongbri, P.L.; Sun, C.; Lou, B.; Varma, A.; Oelmüller, R. Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation—A model system to study plant beneficial traits. J. Endocytobiosis Cell Res. 2011, 21, 101–113. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Disease Worldwide; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA for phytogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Whitw, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Themann, K.; Werres, S.; Diener, H.A.; Luttmann, R. Comparison of different methods to detect Phytophthora spp. in recycling water from nurseries. J. Plant Pathol. 2002, 84, 41–50. [Google Scholar]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 2009, 155, 780–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannwischer, M.E.; Mitchell, D.J. The influence of a fungicide on the epidemiology of black shank of tobacco. Phytopathology 1978, 68, 1760–1765. [Google Scholar] [CrossRef]
- Varma, A.; Verma, S.; Sahay, N.; Bütehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environment. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzewik, A.; Kowalczyk, W.; Maciorowski, R.; Marasek-Ciolakowska, A.; Klocke, E.; Orlikowska, T. Stimulation of ex vitro growth of Rhododendron hybrids ‘Nova Zembla’ and ‘Alfred’ by inoculation of roots with Serendipita indica. Hort. Sci. 2020, in press. [Google Scholar]
- Yeo, J.R.; Weiland, J.E.; Sullivan, D.M.; Bryla, D.R. Susceptibility of highbush blueberry cultivars to Phytophthora root rot. HortScience 2016, 51, 74–78. [Google Scholar] [CrossRef]
- De Silva, A.; Patterson, K.; Rothrock, C.; Moore, J. Growth promotion of highbush blueberry by fungal and bacterial inoculants. HortScience 2000, 35, 1228–1230. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Gómez, F.J.; Pérez-de-Logue, A.; Nawarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Curr. Forestry Reports 2019, 5, 251–266. [Google Scholar]
- Saeed-ur-Rahman, M.K.; Huang, D. Molecular mechanism underlying Piriformospora indica-mediated plant improvement/protection for sustainable agriculture. ABBS 2019, 51, 229–242. [Google Scholar]




| Cultivar | Percentage of Colonised Roots of Plants Inoculated with S. indica | Percentage of Colonised Roots of Plants Inoculated with S. indica + P. cinnamomi | Percentage of Colonised Roots of Plants Inoculated with S. indica | Percentage of Colonised Roots of Plants Inoculated with S. indica + P. cinnamomi |
| 9 Months (5 Plants × 10 Root Fragments × 10 Field s of View = 500 Observations) | 24 Months (10 Plants × 10 Root Fragments × 10 Fields of View n = 1000 Observations) | |||
| ‘Chandler’ | 48 b * | 42 b | 51 b | 43 b |
| ‘Darrow’ | 53 c | 46 c | 55 c | 48 c |
| ‘Spartan’ | 37 a | 33 a | 40 a | 36 a |
| Treatment | Average Increase in Length of the Longest Shoot (cm) | Average Increase in Length of All Shoots (cm) | No. of Plants with Infection Symptoms | No. of Dead Plants |
|---|---|---|---|---|
| ‘Chandler’ | ||||
| Control | 20.6 c * | 93.1 d | 0 | 0 |
| S. indica | 20.6 c | 89.2 c (−4%) | 0 | 0 |
| P. cinnamomi | 8.4 a | 26.9 a (n = 26) | 17 (59%) | 3 (10%) |
| S. indica/P. cinn | 16.1 b (+92%) | 46.8 b (n = 28) (+74%) | 17 (59%) | 1 (3%) |
| ‘Darrow’ | ||||
| Control | 18.9 b | 76.6 ab | 0 | 0 |
| S. indica | 20.3 c (+11%) | 84.8 b (+11%) | 0 | 0 |
| P. cinnamomi | 10.4 a | 71.0 a (n = 28) | 24 (83%) | 1 (3%) |
| S. indica/P. cinn | 18.1 b (+74%) | 73.6 a (+4%) | 8 (28%) | 0 |
| ‘Spartan’ | ||||
| Control | 23.0 c | 92.4 c | 0 | 0 |
| S. indica | 27.7 d (+12%) | 95.7 d (+10%) | 0 | 0 |
| P. cinnamomi | 6.8 a | 34.0 a (n = 10) | 29 (100%) | 19 (66%) |
| S. indica/P. cinn | 19.2 b (+182%) | 74.7 b (n = 25) (+120%) | 26 (90%) | 4 (14%) |
| Cultivar | Control | P. cinnamomi | S. indica + P. cinnamomi | Control | P. cinnamomi | S. indica + P. cinnamomi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Term I after 9 Months | Term II after 24 Months | |||||||||||
| RH | HB | RH | HB | RH | HB | RH | HB | RH | HB | RH | HB | |
| ‘Chandler’ | − | − | + | − | + | − | − | − | + | − | + | − |
| ‘Darrow’ | − | − | + | − | + | − | − | − | + | − | + | − |
| ‘Spartan’ | − | − | + | + | + | + | − | − | + | + | + | + |
| Cultivar | P. cinnamomi | S. indica + P. cinnamomi |
|---|---|---|
| ‘Chandler’ | 70 a * | 71 a |
| ‘Darrow’ | 69 a | 66 a |
| ‘Spartan’ | 77 b | 83 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzewik, A.; Marasek-Ciolakowska, A.; Orlikowska, T. Protection of Highbush Blueberry Plants against Phytophthora cinnamomi Using Serendipita indica. Agronomy 2020, 10, 1598. https://doi.org/10.3390/agronomy10101598
Trzewik A, Marasek-Ciolakowska A, Orlikowska T. Protection of Highbush Blueberry Plants against Phytophthora cinnamomi Using Serendipita indica. Agronomy. 2020; 10(10):1598. https://doi.org/10.3390/agronomy10101598
Chicago/Turabian StyleTrzewik, Aleksandra, Agnieszka Marasek-Ciolakowska, and Teresa Orlikowska. 2020. "Protection of Highbush Blueberry Plants against Phytophthora cinnamomi Using Serendipita indica" Agronomy 10, no. 10: 1598. https://doi.org/10.3390/agronomy10101598
APA StyleTrzewik, A., Marasek-Ciolakowska, A., & Orlikowska, T. (2020). Protection of Highbush Blueberry Plants against Phytophthora cinnamomi Using Serendipita indica. Agronomy, 10(10), 1598. https://doi.org/10.3390/agronomy10101598





