Oviposition and Development of Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) on Different Types of Flour
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Flours Tested
2.3. Oviposition
2.4. Development
2.5. Analyses
3. Results
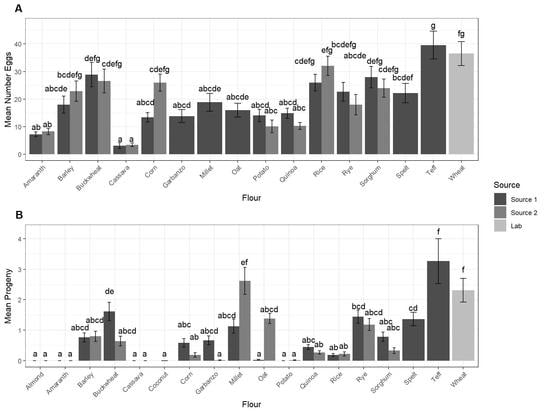
3.1. Oviposition and Development
3.2. Association between Flour Nutrition and Oviposition and Development
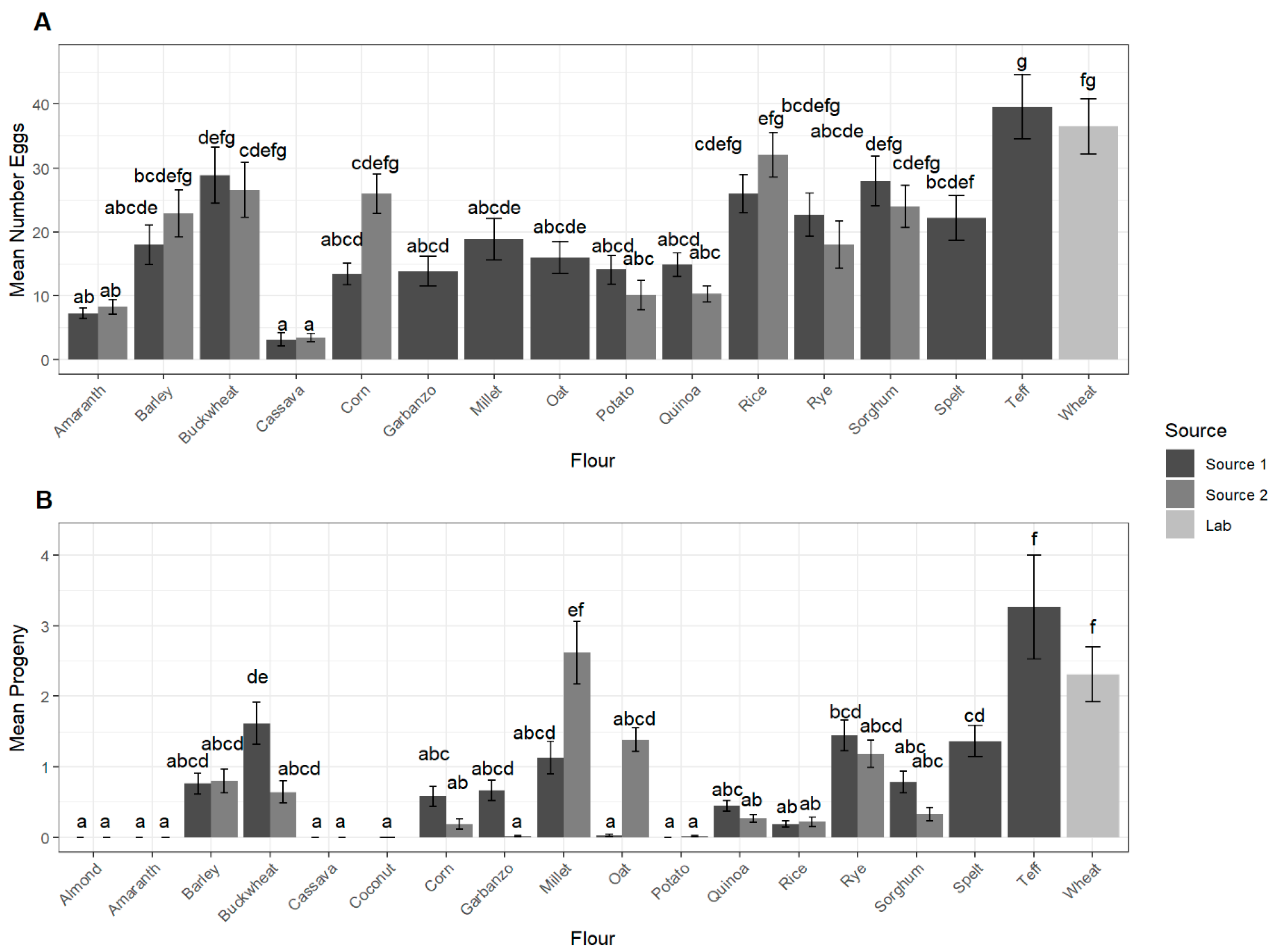
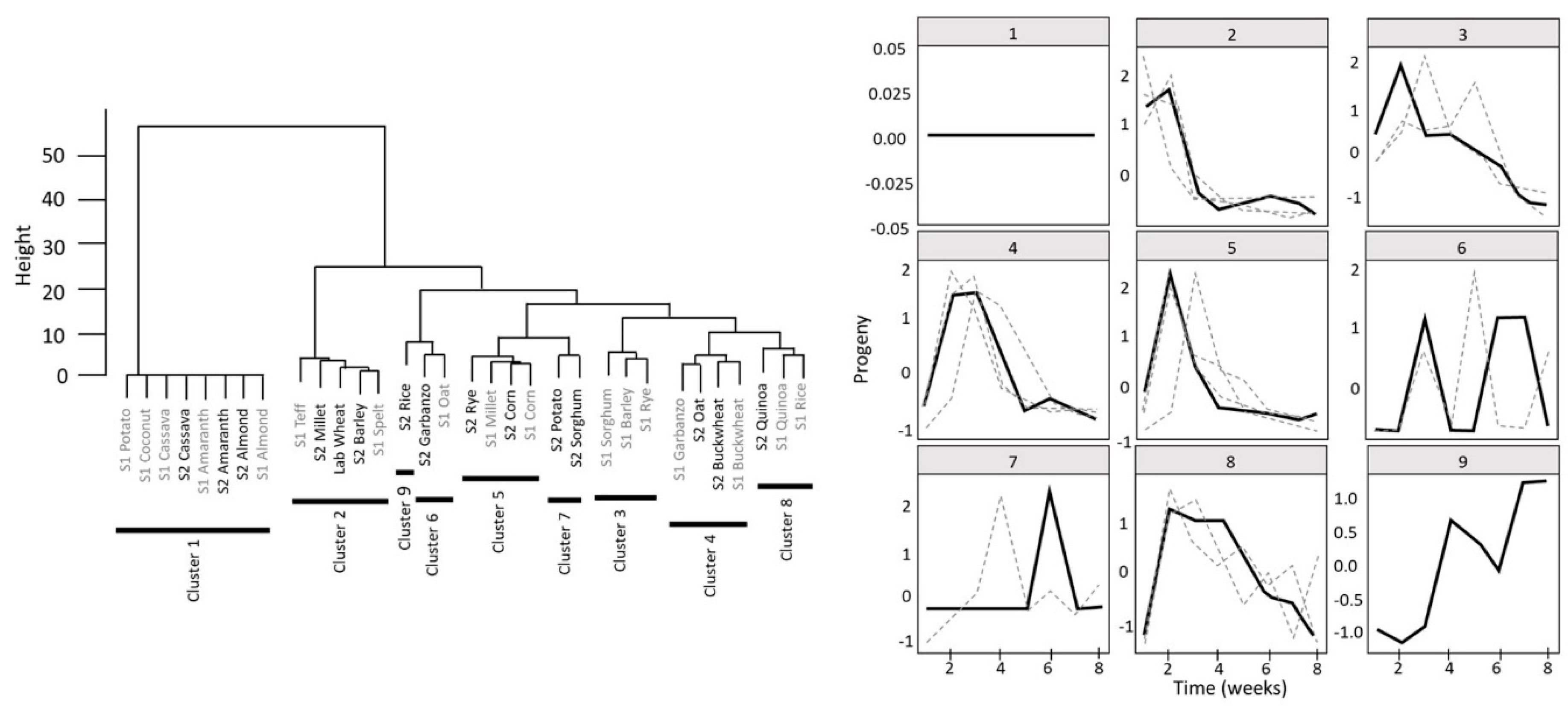
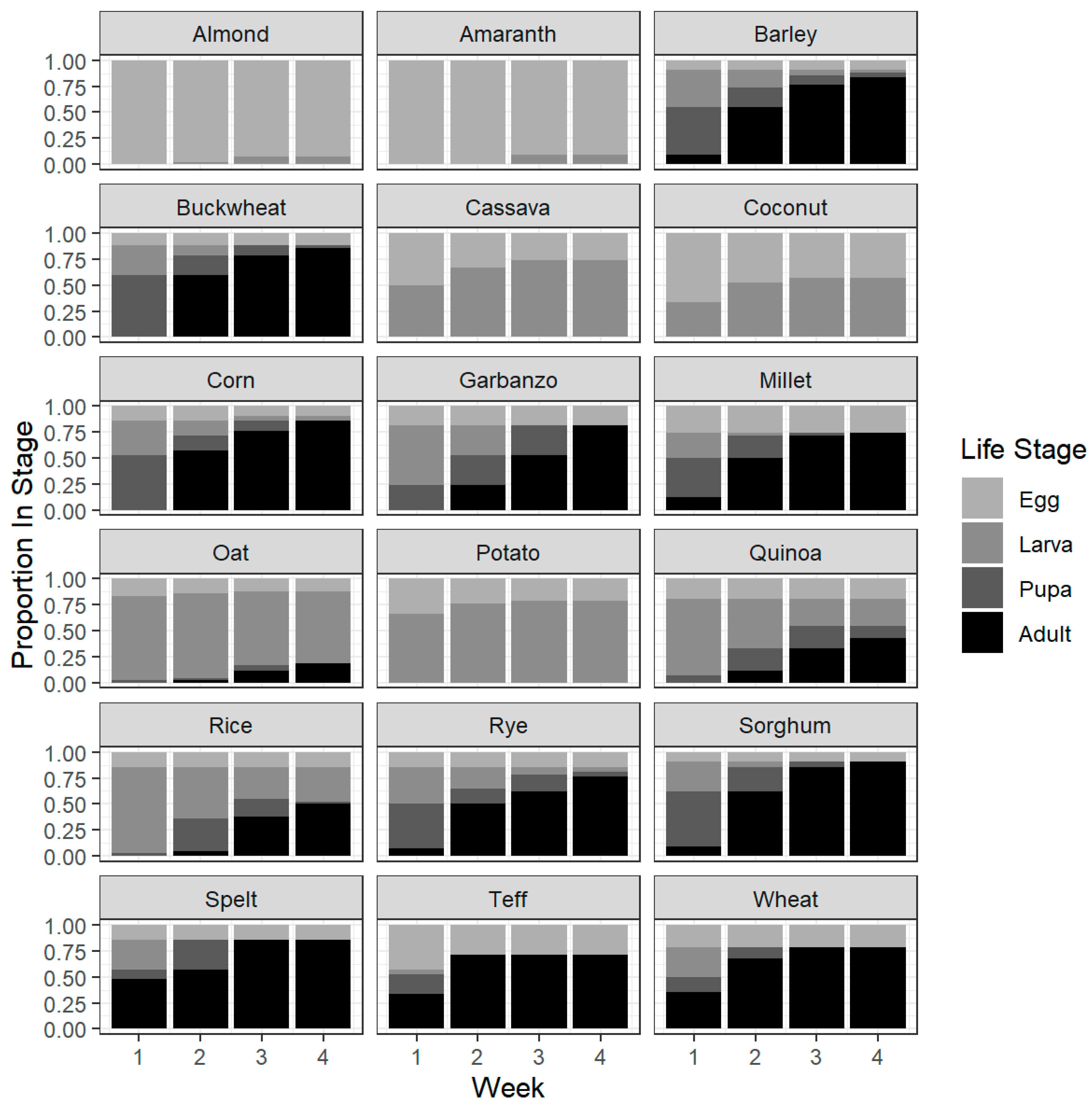
3.3. Adult Progeny Emergence over Time
3.4. Development Success
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heller, L. Commercial Aspects of Gluten-Free Products. In Gluten Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Chichester, UK, 2009; pp. 99–106. [Google Scholar]
- Cureton, P.; Fasano, A. The Increasing Incidence of Celiac Disease and the Range of Gluten-Free Products in the Marketplace. In Gluten Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Chichester, UK, 2009; pp. 1–15. [Google Scholar]
- Bogue, J.; Sorenson, D. The marketing of gluten-free cereal products. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Dal Bello, F., Eds.; Elsevier: Burlington, MA, USA, 2008; pp. 393–412. [Google Scholar]
- Gluten-Free Products Market Size, Share & Trends Analysis Report by Product (Bakery Products, Dairy/Dairy Alternatives), by Distribution Channel (Grocery Stores, Mass Merchandiser), by Region, and Segment Forecasts, 2020–2027. Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/gluten-free-products-market (accessed on 6 July 2020).
- Arthur, F.H.; Starkus, L.A.; Gerken, A.R.; Campbell, J.F.; McKay, T. Growth and development of Tribolium castaneum (Herbst) on rice flour and brown rice as affected by time and temperature. J. Stored Prod. Res. 2019, 83, 73–77. [Google Scholar] [CrossRef]
- Arthur, F.H.; Bean, S.R.; Smolensky, D.; Gerken, A.R.; Siliveru, K.; Scully, E.D.; Baker, N. Development of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) on sorghum milling fractions. J. Stored Prod. Res. 2020, 87, 101606. [Google Scholar] [CrossRef]
- Campbell, J.F.; Toews, M.D.; Arthur, F.H.; Arbogast, R.T. Long-term monitoring of Tribolium castaneum in two flour mills: Seasonal patterns and impact of fumigation. J. Econ. Entomol. 2010, 103, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.; Bowombe-Toko, M.P.; Starkus, L.A.; Arthur, F.H.; Campbell, J.F. Monitoring of Tribolium castaneum (Coleoptera: Tenebrionidae) in rice mills using pheromone-baited traps. J. Econ. Entomol. 2019, 112, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Sarwar, M. Evaluating resistance of wheat germplasms to attack by red flour beetle, Tribolium castaneum (Herbst) (Coleoptera). Pak. J. Zool. 2011, 43, 793–797. [Google Scholar]
- Fraenkel, G.; Blewett, M. The importance of folic acid and unidentified members of the vitamin B complex in the nutrition of certain insects. Biochem. J. 1947, 41, 469–475. [Google Scholar] [CrossRef]
- Chaudhary, K.D.; Lemonde, A. Phosphorus in the nutrition of Tribolium confusum Duval. Can. J. Zool. 1962, 40, 375–380. [Google Scholar] [CrossRef]
- Naseri, B.; Borzoui, E.; Majd, S.; Mozaffar Mansouri, S. Influence of different food commodities on life history, feeding efficiency, and digestive enzymatic activity of Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2017, 110, 2263–2268. [Google Scholar] [CrossRef]
- Ajayi, F.A.; Rahman, S.A. Susceptibility of some staple processed meals to red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Pak. J. Biol. Sci. 2006, 9, 1744–1748. [Google Scholar]
- Sokoloff, A.; Franklin, I.R.; Lakhanpal, R.K. Comparative studies with Tribolium (Coleoptera, Tenebrionidae)—II: Productivity of T. castaneum (Herbst) and T. confusum Duv. on natural, semi-synthetic and synthetic diets. J. Stored Prod. Res. 1966, 1, 313–324. [Google Scholar] [CrossRef]
- Fields, P.G. Effect of Pisum sativum fractions on the mortality and progeny production of nine stored-grain beetles. J. Stored Prod. Res. 2006, 42, 86–96. [Google Scholar] [CrossRef]
- Wong, N.; Lee, C.Y. Relationship between population growth of the red flour beetle Tribolium castaneum and protein and carbohydrate content in flour and starch. J. Econ. Entomol. 2001, 104, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, A.; Franklin, I.R.; Overton, L.F.; Ho, F.K. Comparative studies with Tribolium (Coleoptera, Tenebrionidae)—I: Productivity of T. castaneum (Herbst) and T. confusum Duv. on several commercially-available diets. J. Stored Prod. Res. 1966, 1, 295–311. [Google Scholar] [CrossRef]
- Fabres, A.; da Silva, J.D.C.M.; Fernandes, K.V.; Xavier-Filho, J.; Rezende, G.L.; Oliveira, A.E.A. Comparative performance of the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae) on different plant diets. J. Pest Sci. 2014, 87, 495–506. [Google Scholar] [CrossRef]
- Namin, F.R.; Naseri, B.; Nouri-Ganbalani, G.; Razmjou, J. Demographic studies of Tribolium castaneum (Coleoptera: Tenebrionidae) on various barley cultivars. J. Stored Prod. Res. 2018, 79, 60–65. [Google Scholar] [CrossRef]
- Fardisi, M.; Mason, L.J.; Ileleji, K.E. The susceptibility of animal feed containing Dried Distiller’s Grains with Solubles to Tribolium castaneum (Herbst) infestation. J. Stored Prod. Res. 2017, 72, 59–63. [Google Scholar] [CrossRef]
- Fardisi, M.; Mason, L.J.; Ileleji, K.E.; Richmond, D.S. Effect of chemical and physical properties of Dried Distillers Grains with solubles (DDGS) on Tribolium castaneum (Herbst) development. J. Stored Prod. Res. 2019, 80, 57–64. [Google Scholar] [CrossRef]
- Astuti, L.P.; Rizali, A.; Firnanda, R.; Widjayanti, T. Physical and chemical properties of flour products affect the development of Tribolium castaneum. J. Stored Prod. Res. 2020, 86, 101555. [Google Scholar] [CrossRef]
- Cambron, L.D.; Thapa, G.; Greenlee, K.J. Effects of high-fat diet on feeding and performance in the tobacco hornworm, Manduca sexta. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 236, 110526. [Google Scholar] [CrossRef]
- Haemaelaeinen, M.K.; Loschiavo, S.R. Effect of synthetic b-vitamin and natural enrichment of flour on larval development and fecundity of Tribolium confusum and T. castaneum 1. Entomol. Exp. Appl. 1977, 21, 29–37. [Google Scholar] [CrossRef]
- Begum, M.; Hasan, M.; Saifullah, A.S.M.; Howlader, M.A. Effects of sodium chloride on oviposition and development of blowfly, Lucilia cuprina, (Wiedmann) (Diptera: Calliphoridae). Dhaka Univ. J. Biol. Sci. 2014, 23, 47–51. [Google Scholar] [CrossRef]
- Gitonga, N.K. Use of Salt to Reduce Post-Harvest Losses of Cured Fish Due to Blowfly (Lucilia and Chrysomya spp.) Infestation in KENYA. In Food and Agriculture Organization of the United Nations, Proceedings of the Expert Consultation on Fish Technology in Africa; FAO: Saly-Mbour, Republic of Senegal, 2001. [Google Scholar]
- Fields, P.G.; Xie, Y.S.; Hou, X. Repellent effect of pea (Pisum sativum) fractions against stored-product insects. J. Stored Prod. Res. 2001, 37, 359–370. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Boukouvala, M.C. Population growth of the khapra beetle, Trogoderma granarium Everts (Coleoptera: Dermestidae) on different commodities. J. Stored Prod. Res. 2016, 69, 72–77. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Boukouvala, M.C.; Nika, E.P. Influence of commodity on the population growth of the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrychidae). J. Stored Prod. Res. 2017, 73, 129–134. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Throne, J.E.; Nakas, C.T. Competition among species of stored-product psocids (Psocoptera) in stored grain. PLoS ONE 2014, 9, e102867. [Google Scholar] [CrossRef]
- Quellhorst, H.; Athanassiou, C.G.; Bruce, A.; Scully, E.D.; Morrison III, W.R. Temperature-Mediated Competition Between the Invasive Larger Grain Borer (Coleoptera: Bostrichidae) and the Cosmopolitan Maize Weevil (Coleoptera: Curculionidae). Environ. Entomol. 2020, 49, 255–264. [Google Scholar] [CrossRef]
- Gerken, A.R.; Scully, E.D.; Campbell, J.F. Red flour beetle (Coleoptera: Tenebrionidae) response to volatile cues varies with strain and behavioral assay. Environ. Entomol. 2018, 47, 1252–1265. [Google Scholar] [CrossRef]
- Holloway, G.J.; Smith, R.H. Inheritance of the ability of Sitophilus oryzae (L.) (Coleoptera: Curculionidae) to feed and breed on yellow split-pea (Pisum sativum). Bull. Entomol. Res. 1985, 75, 367–375. [Google Scholar] [CrossRef]
| Flour | Plant Family | Domesticated, Years Ago | Gluten Level | Flour From | Specific Flour Detail | Pre-sieved for Oviposition Willingness | Weight (Grams) for Oviposition Study | Weight (Grams) for Egg Development Study | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |||||
| Almond | Rosaceae | 5000 | None | Seed | Almond Meal | No | No | 1.34 ± 0.04 | 1.23 ± 0.03 | 0.22 ± 0.02 | 0.25 ± 0.02 | |
| Amaranth | Amaranthaceae | 1000 | None | Seed | Yes | Yes | 1.36 ± 0.04 | 1.58 ± 0.03 | 0.29 ± 0.01 | 0.32 ± 0.02 | ||
| Barley | Poaceae | 9000 | Low | Grain | Yes | Yes | 1.47 ± 0.04 | 1.29 ± 0.02 | 0.30 ± 0.02 | 0.25 ± 0.01 | ||
| Buckwheat | Polygonaceae | 8000 | None | Seed | Organic | Whole | Yes | Yes | 1.63 ± 0.02 | 1.63 ± 0.03 | 0.36 ± 0.02 | 0.35 ± 0.02 |
| Cassava | Euphorbiaceae | 10,000 | None | Vegetable | Yes | Yes | 1.85 ± 0.02 | 1.24 ± 0.02 | 0.38 ± 0.02 | 0.27 ± 0.01 | ||
| Coconut | Arecaceae | None | Seed | Organic | No | Yes | 1.25 ± 0.02 | 1.46 ± 0.03 | 0.25 ± 0.02 | 0.28 ± 0.02 | ||
| Corn | Poaceae | 9000 | None | Grain | Snow White | Yes | Yes | 1.34 ± 0.03 | 1.30 ± 0.04 | 0.28 ± 0.02 | 0.29 ± 0.02 | |
| Garbanzo | Fabaceae | 5500 | None | Legume | Yes | No | 1.29 ± 0.04 | 1.32 ± 0.03 | 0.31 ± 0.02 | 0.27 ± 0.01 | ||
| Millet | Poaceae | 10,000 | None | Seed | Yes | No | 1.46 ± 0.03 | 1.38 ± 0.04 | 0.31 ± 0.01 | 0.28 ± 0.02 | ||
| Oat | Poaceae | 4500 | None | Grain | Whole Grain | Yes | No | 1.37 ± 0.04 | 1.80 ± 0.02 | 0.25 ± 0.02 | 0.34 ± 0.01 | |
| Potato | Solanaceae | 8000 | None | Vegetable | Yes | Yes | 2.03 ± 0.02 | 1.45 ± 0.03 | 0.43 ± 0.02 | 0.27 ± 0.02 | ||
| Quinoa | Amaranthaceae | 4000 | None | Seed | Organic | Yes | Yes | 1.56 ± 0.04 | 1.90 ± 0.04 | 0.28 ± 0.02 | 0.40 ± 0.02 | |
| Rice | Poaceae | 9000 | None | Grain | Brown | Brown | Yes | Yes | 1.83 ± 0.05 | 1.34 ± 0.03 | 0.36 ± 0.01 | 0.25 ± 0.02 |
| Rye | Poaceae | 10,000 | Low | Grain | Organic Dark | Yes | Yes | 1.41 ± 0.04 | 1.46 ± 0.04 | 0.31 ± 0.02 | 0.30 ± 0.02 | |
| Sorghum | Poaceae | 6000 | None | Grain | Yes | 1.83 ± 0.03 | 0.34 ± 0.02 | |||||
| Spelt | Poaceae | 7000 | High | Grain | Organic | Yes | 1.70 ± 0.03 | 0.34 ± 0.02 | ||||
| Teff | Poaceae | 6000 | None | Seed | Yes | 1.54 ± 0.03 | 0.30 ± 0.02 | |||||
| Wheat (Lab) | Poaceae | 10,000 | High | Grain | Yes (Lab) | 1.77 ± 0.04 | 0.35 ± 0.02 | |||||
| Eggs | Progeny | |||||
|---|---|---|---|---|---|---|
| Flour | Estimate ± SE | t-Ratio | p-Value | Estimate ± SE | t-Ratio | p-Value |
| Almond | Both sources not counted | 0 ± 3.3 | 0 | 1.00 | ||
| Amaranth | 0.9 ± 4.3 | 0.20 | 1.00 | 0 ± 3.3 | 0 | 1.00 |
| Barley | 4.9 ± 4.3 | 1.15 | 0.94 | 0.09 ± 3.3 | 0.029 | 1.00 |
| Buckwheat | −2.3 ± 4.3 | −0.55 | 1.00 | −7.9 ± 3.3 | −2.39 | 0.21 |
| Cassava | 0.3 ± 4.3 | 0.067 | 1.00 | 0 ± 3.3 | 0 | 1.00 |
| Corn | 12.6 ± 4.3 | 2.96 | 0.03 | −3.3 ± 3.3 | −1.015 | 0.99 |
| Garbanzo | Source 2 not counted | −5.1 ± 3.3 | −1.57 | 0.83 | ||
| Millet | Source 2 not counted | 11.8 ± 3.3 | 3.59 | 0.0049 | ||
| Oat | Source 2 not counted | 10.6 ± 3.3 | 3.22 | 0.018 | ||
| Potato | −3.9 ± 4.3 | −0.93 | 0.99 | 0.09 ± 3.3 | 0.029 | 1.00 |
| Quinoa | −4.6 ± 4.3 | −1.07 | 0.96 | −1.4 ± 3.3 | −0.44 | 1.00 |
| Rice | 6.1 ± 4.3 | 1.44 | 0.80 | 0.05 ± 3.3 | 0.014 | 1.00 |
| Rye | −4.7 ± 4.3 | −1.10 | 0.96 | −2.1 ± 3.3 | −0.62 | 1.00 |
| Sorghum | −4.0 ± 4.3 | −0.94 | 0.99 | −3.3 ± 3.3 | −1.02 | 0.99 |
| Eggs | Progeny | |||
|---|---|---|---|---|
| Flour | LS-mean ± SE | DF | LS-mean ± SE | DF |
| Almond | Not calculated | 0 ± 1.9 a | 137.4 | |
| Amaranth | 8.8 ± 3.6 ab | 13.8 | 0 ± 1.9 a | 137.4 |
| Cassava | 4.2 ± 3.6 a | 13.5 | 0 ± 1.9 a | 137.4 |
| Coconut | Not counted | 0 ± 2.5 ab | 419.1 | |
| Corn | 26.9 ± 4.2 defg | 28.3 | 3.1 ± 1.9 abc | 137.4 |
| Barley | 21.4 ± 3.6 cde | 13.5 | 6.1 ± 1.9 abc | 137.4 |
| Buckwheat | 28.7 ± 3.6 efg | 13.5 | 9.0 ± 1.9 bc | 137.4 |
| Garbanzo | 14.9 ± 4.2 abcd | 30 | 2.7 ± 1.9 abc | 137.4 |
| Millet | 19.8 ± 4.2 bcde | 28.3 | 20.9 ± 2.5 de | 419.1 |
| Oat | 17.2 ± 4.2 abcde | 30 | 10.8 ± 2.5 bcd | 419.1 |
| Potato | 13.1 ± 3.6 abc | 13.5 | 0.05 ± 1.9 a | 137.4 |
| Quinoa | 13.5 ± 3.6 abc | 13.5 | 2.7 ± 1.9 abc | 137.4 |
| Rice | 29.9 ± 3.6 efg | 13.5 | 1.7 ± 1.9 ab | 137.4 |
| Rye | 21.3 ± 3.6 cde | 13.5 | 10.4 ± 1.9 c | 137.4 |
| Spelt | 23.1 ± 4.2 cdef | 28.3 | 11.1 ± 2.5 bcd | 419.1 |
| Sorghum | 26.9 ± 3.6 def | 13.5 | 4.5 ± 1.9 abc | 137.4 |
| Teff | 40.6 ± 4.2 g | 28.3 | 26.1 ± 2.5 e | 419.1 |
| Wheat | 36.7 ± 3.9 fg | 20.4 | 19.7 ± 1.9 e | 8.42 |
| Nutrition Information | t-Value | p-Value | Correlation Coefficient | t-Value | p-Value | Correlation Coefficient | t-Value | p-Value | Correlation Coefficient |
|---|---|---|---|---|---|---|---|---|---|
| Eggs | Sources Combined | Source 1 | Source 2 | ||||||
| Calories | −0.77 | 0.44 | −0.035 | 3.65 | 0.0003 | 0.21 | −1.11 | 0.27 | −0.077 |
| Calories from Fat | 0.70 | 0.49 | 0.036 | −1.57 | 0.12 | −0.12 | 1.97 | 0.05 | 0.14 |
| Saturated Fat (g) | −0.82 | 0.41 | −0.037 | −3.31 | 0.001 | −0.19 | 1.64 | 0.10 | 0.11 |
| Sodium (mg) | −5.85 | <0.0001 | −0.25 | −1.22 | 0.22 | −0.07 | −6.63 | <0.0001 | −0.42 |
| Carbohydrates (g) | −0.89 | 0.37 | −0.04 | 1.59 | 0.11 | 0.093 | −1.07 | 0.28 | −0.074 |
| Fiber (g) | 2.65 | 0.0083 | 0.12 | 2.57 | 0.01 | 0.15 | 1.71 | 0.088 | −0.12 |
| Protein (g) | 2.43 | 0.015 | 0.11 | 3.55 | 0.005 | 0.20 | 1.2 | 0.23 | 0.083 |
| Progeny | Sources Combined | Source 1 | Source 2 | ||||||
| Calories | −0.74 | 0.46 | −0.029 | 6.96 | <0.0001 | 0.36 | −1.42 | 0.16 | −0.083 |
| Calories from Fat | −2.95 | 0.0033 | −0.14 | −0.55 | 0.58 | −0.04 | −2.52 | 0.012 | −0.14 |
| Saturated Fat (g) | −6.07 | <0.0001 | −0.24 | −4.32 | <0.0001 | −0.23 | −3.87 | 0.00013 | −0.22 |
| Sodium (mg) | −5.68 | <0.0001 | −0.22 | −2.05 | 0.041 | −0.11 | −5.5 | <0.0001 | −0.31 |
| Carbohydrates (g) | 0.17 | 0.87 | 0.0067 | 5.30 | <0.0001 | 0.28 | −0.64 | 0.52 | −0.037 |
| Fiber (g) | 4.55 | <0.0001 | 0.18 | 5.30 | <0.0001 | 0.28 | 2.59 | 0.01 | 0.15 |
| Protein (g) | 1.49 | 0.14 | 0.059 | 4.29 | <0.0001 | 0.23 | 0.19 | 0.85 | 0.011 |
| Effect | χ2 | DF | p-Value |
|---|---|---|---|
| Egg Life Stage | |||
| Flour | 230.39 | 17 | <0.0001 |
| Source | 0 | 1 | 1.00 |
| Week | 0 | 2 | 1.00 |
| Flour-by-Source | 65.03 | 14 | <0.0001 |
| Flour-by-Week | 13.34 | 51 | 1.00 |
| Source-by-Week | 1.34 | 3 | 0.72 |
| Flour-by-Source-by-Week | 10.88 | 42 | 1.00 |
| Larval Life Stage | |||
| Flour | 42.60 | 17 | <0.0001 |
| Source | 13.56 | 1 | 0.00023 |
| Week | 4.85 | 3 | 0.18 |
| Flour-by-Source | 23.13 | 13 | 0.04 |
| Flour-by-Week | 49.40 | 35 | 0.05 |
| Source-by-Week | 6.28 | 3 | 0.09 |
| Flour-by-Source-by-Week | 14.84 | 22 | 0.87 |
| Pupal Life Stage | |||
| Flour | 141.24 | 12 | <0.0001 |
| Source | 15.34 | 1 | <0.0001 |
| Week | 30.53 | 3 | <0.0001 |
| Flour-by-Source | 29.84 | 8 | 0.00023 |
| Flour-by-Week | 222.01 | 25 | <0.0001 |
| Source-by-Week | 28.77 | 3 | <0.0001 |
| Flour-by-Source-by-Week | 62.96 | 9 | <0.0001 |
| Adult Life Stage | |||
| Flour | 55.50 | 12 | <0.0001 |
| Source | 0.059 | 1 | 0.81 |
| Week | 22.18 | 3 | <0.0001 |
| Flour-by-Source | 41.60 | 9 | <0.0001 |
| Flour-by-Week | 38.67 | 30 | 0.13 |
| Source-by-Week | 1.34 | 3 | 0.72 |
| Flour-by-Source-by-Week | 4.94 | 18 | 0.99 |
| Time to Hatch | Time to Adult Emergence | |||
|---|---|---|---|---|
| Flour | Predicted Weeks to 50% ± Standard Error | Predicted Weeks to 90% ± Standard Error | Predicted Weeks to 50% ± Standard Error | Predicted Weeks to 90% ± Standard Error |
| Almond | 12.6 ± 3.6 | 23.9 ± 4.4 | >500 ± 500 | >500 ± 500 |
| Amaranth | 9.8 ± 3.3 | 14.9 ± 3.6 | >500 ± 500 | >500 ± 500 |
| Barley | 3.7 ± 3.1 | 5.1 ± 3.1 | 5.1 ± 3.0 | 7.2 ± 3.1 |
| Buckwheat | 3.7 ± 3.1 | 5.4 ± 3.1 | 5.1 ± 3.0 | 6.7 ± 3.1 |
| Cassava | 4.5 ± 3.1 | 8.4 ± 3.2 | >500 ± 500 | >500 ± 500 |
| Coconut | 5.5 ± 3.1 | 12.5 ± 3.4 | >500 ± 500 | >500 ± 500 |
| Corn | 3.7 ± 3.1 | 5.3 ± 3.1 | 5.2 ± 3.1 | 6.8 ± 3.1 |
| Garbanzo | 3.9 ± 3.1 | 6.6 ± 3.2 | 5.9 ± 3.1 | 7.7 ± 3.1 |
| Millet | 4.1 ± 3.1 | 8.1 ± 3.2 | 5.3 ± 3.1 | 8.1 ± 3.1 |
| Oat | 3.8 ± 3.1 | 5.6 ± 3.1 | 8.7 ± 3.2 | 13.1 ± 3.4 |
| Potato | 4.1 ± 3.1 | 7.3 ± 3.1 | >500 ± 500 | >500 ± 500 |
| Quinoa | 3.9 ± 3.1 | 6.6 ± 3.1 | 7.1 ± 3.1 | 10.7 ± 3.2 |
| Rice | 3.8 ± 3.1 | 5.8 ± 3.1 | 6.8 ± 3.1 | 9.2 ± 3.1 |
| Rye | 3.8 ± 3.1 | 5.8 ± 3.1 | 5.4 ± 3.1 | 8.1 ± 3.1 |
| Sorghum | 3.7 ± 3.1 | 5.1 ± 3.1 | 4.9 ± 3.1 | 6.4 ± 3.1 |
| Spelt | 3.8 ± 3.1 | 5.8 ± 3.2 | 4.5 ± 3.1 | 7.0 ± 3.2 |
| Teff | 4.4 ± 3.1 | 8.7 ± 3.3 | 4.8 ± 3.1 | 8.4 ± 3.2 |
| Wheat | 3.9 ± 3.1 | 7.0 ± 3.2 | 4.6 ± 3.1 | 7.5 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerken, A.R.; Campbell, J.F. Oviposition and Development of Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) on Different Types of Flour. Agronomy 2020, 10, 1593. https://doi.org/10.3390/agronomy10101593
Gerken AR, Campbell JF. Oviposition and Development of Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) on Different Types of Flour. Agronomy. 2020; 10(10):1593. https://doi.org/10.3390/agronomy10101593
Chicago/Turabian StyleGerken, Alison R., and James F. Campbell. 2020. "Oviposition and Development of Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) on Different Types of Flour" Agronomy 10, no. 10: 1593. https://doi.org/10.3390/agronomy10101593
APA StyleGerken, A. R., & Campbell, J. F. (2020). Oviposition and Development of Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae) on Different Types of Flour. Agronomy, 10(10), 1593. https://doi.org/10.3390/agronomy10101593