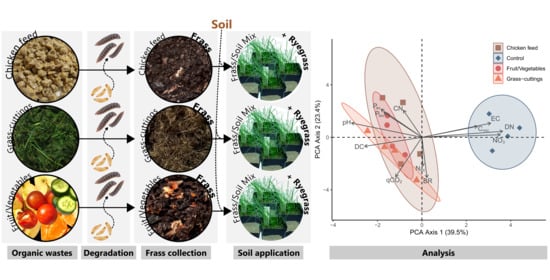
Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization
Abstract
1. Introduction
2. Materials and Methods
2.1. Black Soldier Fly Frass Collection
2.2. Frass and Soil Analyses
2.3. Preparation of Media
2.4. Pathogen Quantification/Assessment of Microbial Colonization in Frass and Soil
2.5. Statistical Analyses
3. Results and Discussion
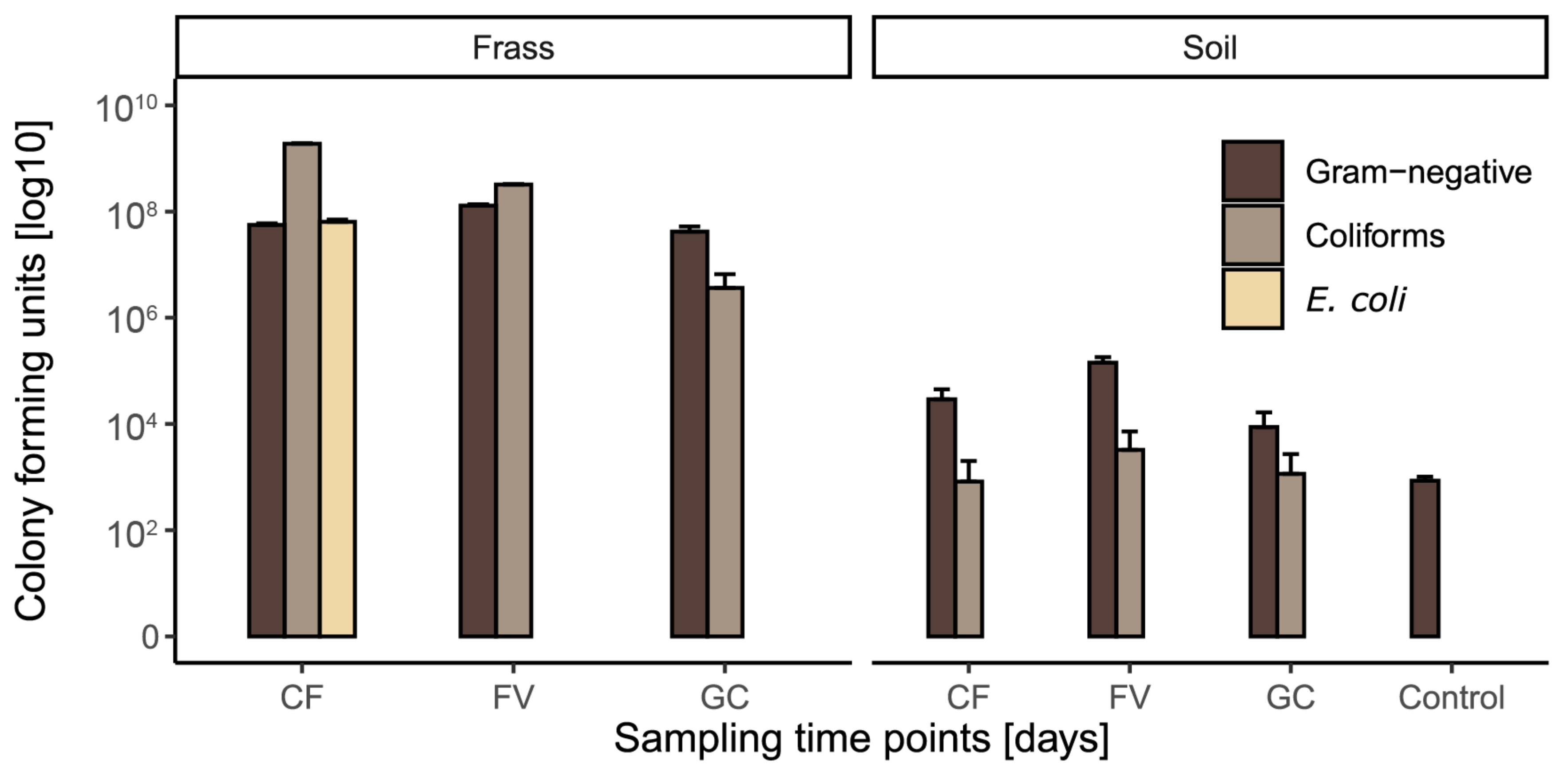
3.1. Assessment of Microbial Load in Frass and Frass-Amended Soils
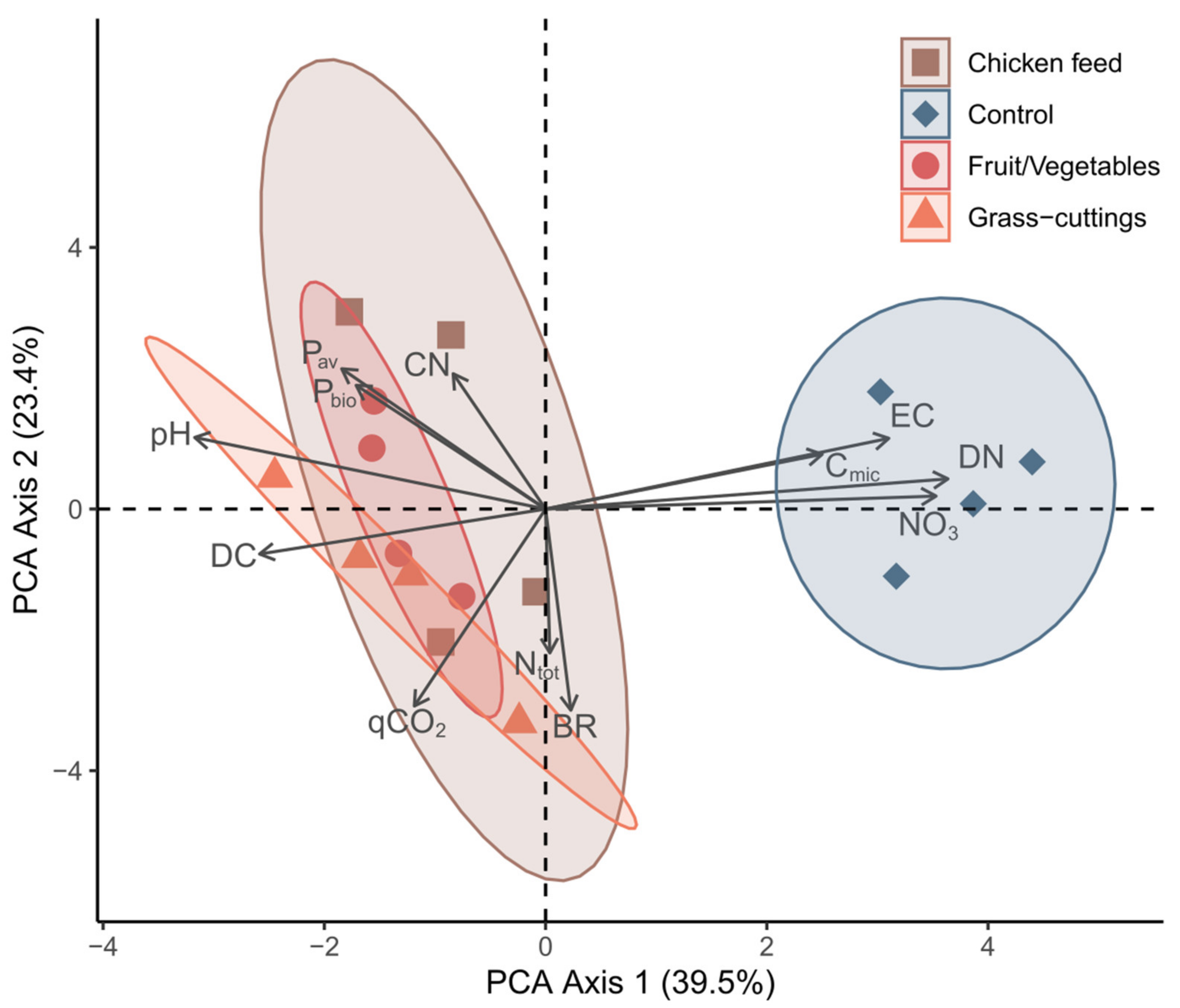
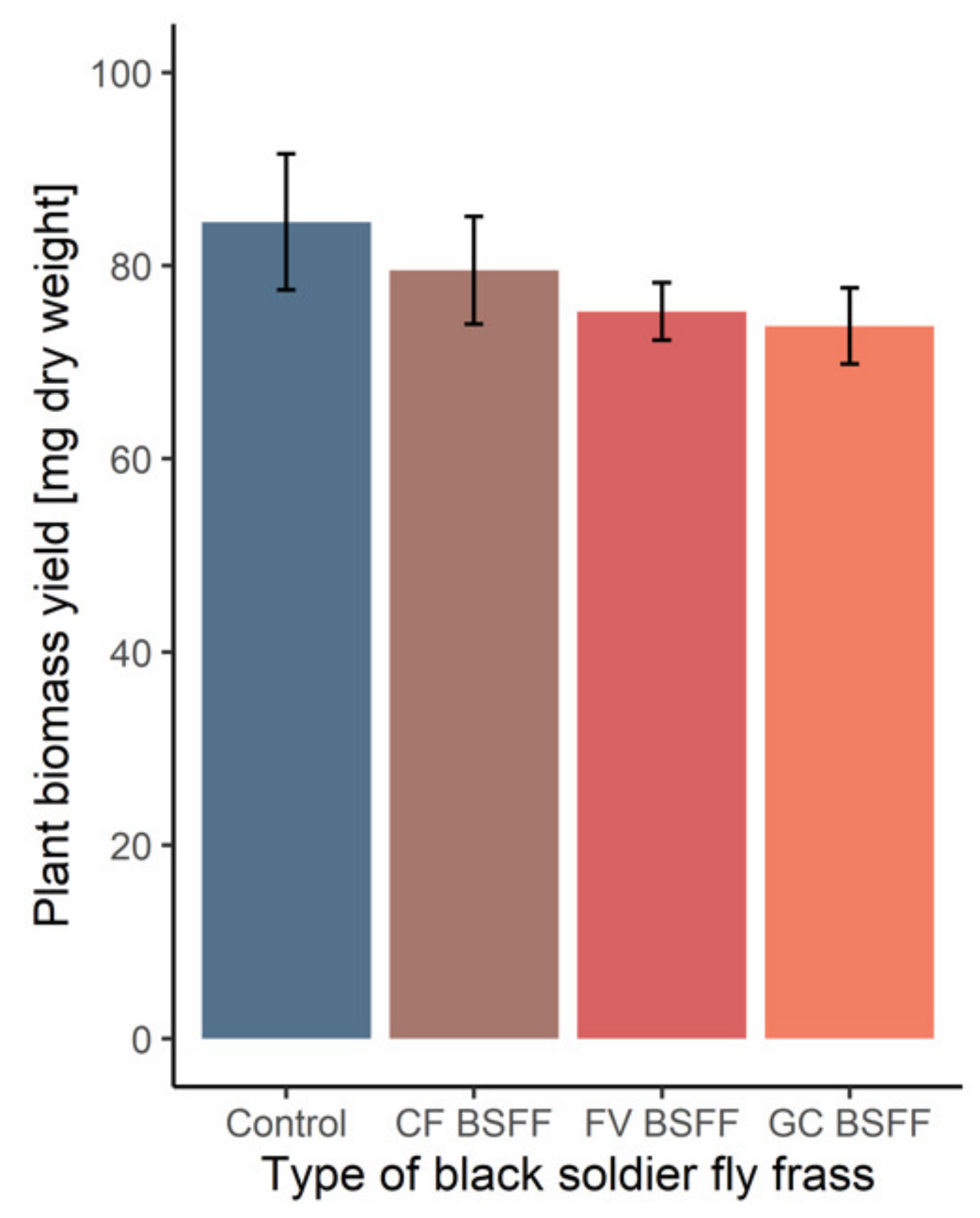
3.2. Black Soldier Fly Frass Properties, Soil Quality and Plant Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- United Nations. World Population Prospects. The 2017 Revision; Department of Economic and Social Affairs—Population Division: New York, NY, USA, 2017; p. 53. [Google Scholar]
- European Commission. 811: Green Paper on the Management of Bio-Waste in the European Union; Commission of the European Communities: Brussels, Belgium, 2008. [Google Scholar]
- Pastor, B.; Velasquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Klocke, M.; Schluter, O. Insect biodiversity: Underutilized bioresource for sustainable applications in life sciences. Reg. Environ. Chang. 2017, 17, 1445–1454. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R.; Ashok, K.N.; Srinivas, K.; Arutchelvan, V.; Thota, K.R.; Ravi, S.N.; Sandeep, K.D.; Goutham, R.M. Black soldier fly larvae, a viable opportunity for entrepreneurship. Acta Sci. Agric. 2018, 2, 11–20. [Google Scholar]
- Klammsteiner, T.; Walter, A.; Pan, H.; Gassner, M.; Heussler, C.D.; Schermer, M.; Insam, H. On everyone’s lips: Insects for food and feed. In Proceedings of the 5th Austrian Citizen Science Conference, Obergurgl, Austria, 26—28 June 2019; Volume 366, p. 6. [Google Scholar]
- Quilliam, R.S.; Nuku-Adeku, C.; Maquart, P.; Little, D.; Newton, R.; Murray, F. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed 2020, 1–8. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Dubois, T.; Subramanian, S.; Wangu, M.M.; Ekesi, S.; et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e238154. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring Black Soldier Fly Frass as Novel Fertilizer for Improved Growth, Yield, and Nitrogen Use Efficiency of Maize Under Field Conditions. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Alattar, M.; Alattar, F.; Popa, R. Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant Sci. Today 2016, 3, 57–62. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Choi, J.-Y.; Kim, J.-G.; Kim, M.-S.; Kim, W.-T.; Park, K.-H.; Bae, S.-W.; Jeong, G.-S. Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Sarpong, D.; Oduro-Kwarteng, S.; Gyasi, S.F.; Buamah, R.; Donkor, E.; Awuah, E.; Baah, M.K. Biodegradation by composting of municipal organic solid waste into organic fertilizer using the black soldier fly (Hermetia illucens) (Diptera: Stratiomyidae) larvae. Int. J. Recycl. Org. Waste Agric. 2019, 8, 45–54. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S.; et al. Nitrogen Fertilizer Equivalence of Black Soldier Fly Frass Fertilizer and Synchrony of Nitrogen Mineralization for Maize Production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The core gut microbiome of black soldier fly (Hermetia illucens) larvae raised on low-bioburden diets. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli o157:h7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrugg, C.; Lindstrom, A.; Vinneras, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z. Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environ. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory co-receptor IR8a governs larval-frass mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, X.; Gao, Y.L.; Liu, Y.; Dong, W.X.; Xiao, C. Oviposition deterrents in larval frass of potato tuberworm moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2019, 48, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lorenzana, L.R.J. Frass volatiles as attractant to the mango pulp weevil (Sternochetus frigidus (Fabr.) (Coleoptera: Curculionidae)). Philipp. Agric. Sci. 2014, 97, 385–390. [Google Scholar]
- Mitchell, R.F.; Hanks, L.M. Insect frass as a pathway for transmission of bacterial wilt of cucurbits. Environ. Entomol. 2009, 38, 395–403. [Google Scholar] [CrossRef]
- Roy, K.; Ewing, C.P.; Hughes, M.A.; Keith, L.; Bennett, G.M. Presence and viability of Ceratocystis lukuohia in ambrosia beetle frass from Rapid ʻŌhiʻa Death-affected Metrosideros polymorpha trees on Hawai’i Island. For. Pathol. 2019, 49, e12476. [Google Scholar] [CrossRef]
- Khisti, U.V.; Kathade, S.A.; Aswani, M.A.; Anand, P.K.; Bipinraj, N.K. Isolation and identification of saccharomyces cerevisiae from caterpillar frass and their probiotic characterization. Biosci. Biotechnol. Res. Asia 2019, 16, 179–186. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Recycling of nitrogen in herbivore feces: Plant recovery, herbivore assimilation, soil retention, and leaching losses. Oecologia 2007, 151, 42–53. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Kang, J.-H.; Xie, T.-R.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Kandeler, E. Nitrate. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 408–410. ISBN 978-3-642-60966-4. [Google Scholar]
- Kandeler, E. Ammonium. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 406–408. ISBN 978-3-642-60966-4. [Google Scholar]
- Illmer, P. Total, organic, inorganic and plant available phosphorus. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 412–416. ISBN 978-3-642-60966-4. [Google Scholar]
- Heinemeyer, O.; Insam, H.; Kaiser, E.A.; Walenzik, G. Soil microbial biomass and respiration measurements: An automated technique based on infra-red gas analysis. Plant Soil 1989, 116, 191–195. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- De Smet, J.; Wynants, E.; Cos, P.; Campenhout, L.V. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and its impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e2722-17. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef]
- Fielding, D.J.; Trainor, E.; Zhang, M. Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadavers. Biol. Fertil. Soils 2013, 49, 537–544. [Google Scholar] [CrossRef]
- McTavish, M.J.; Smenderovac, E.; Gunn, J.; Murphy, S.D. Insect defoliators in recovering industrial landscapes: Effects of landscape degradation and remediation near an abandoned metal smelter on gypsy moth (Lepidoptera: Lymantriidae) feeding, frass production, and frass properties. Environ. Entomol. 2019, 48, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Juárez, M.F.-D.; Zangerle, M.; Insam, H. Effects of digestate on soil chemical and microbiological properties: A comparative study with compost and vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef]
- Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; Insam, H.; Goberna, M. Robustness of the autochthonous microbial soil community after amendment of cattle manure or its digestate. Biol. Fertil. Soils 2019, 55, 565–576. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Dioha, I.; Ikeme, C.H.; Nafiu, T. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wang, L.; Li, Y.; Prasher, S.O.; Yan, B.; Ou, Y.; Cui, H.; Cui, Y. Organic matter, a critical factor to immobilize phosphorus, copper, and zinc during composting under various initial C/N ratios. Bioresour. Technol. 2019, 289, 121745. [Google Scholar] [CrossRef] [PubMed]
- Borkott, H.; Insam, H. Symbiosis with bacteria enhances the use of chitin by the springtail, Folsomia candida (Collembola). Biol. Fertil. Soils 1990, 9, 126–129. [Google Scholar] [CrossRef]
- Insam, H.; Merschak, P. Nitrogen leaching from forest soil cores after amending organic recycling products and fertilizers. Waste Manag. Res. 1997, 15, 277–292. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertil. Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Tittonell, P.; Corbeels, M.; van Wijk, M.T.; Vanlauwe, B.; Giller, K.E. Combining Organic and Mineral Fertilizers for Integrated Soil Fertility Management in Smallholder Farming Systems of Kenya: Explorations Using the Crop-Soil Model FIELD. Agron. J. 2008, 100, 1511–1526. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient Interactions in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Mertenat, A.; Diener, S.; Zurbrügg, C. Black soldier fly biowaste treatment—Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas generation from insects breeding post production wastes. J. Clean Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- Lalander, C.; Nordberg, A.; Vinneras, B. A comparison in product-value potential in four treatment strategies for food waste and faeces—Assessing composting, fly larvae composting and anaerobic digestion. Glob. Chang. Biol. Bioenergy 2018, 10, 84–91. [Google Scholar] [CrossRef]
| CF | GC | FV | |
|---|---|---|---|
| Pupation rate [%] | 55.2 ± 5.3 b | 98.7 ± 2.0 c | 22.4 ± 2.0 a |
| Prepupae fresh weight [mg] | 198 ± 10 | 167 ± 11 | 165 ± 31 |
| Prepupae dry weight [%] | 32.6 ± 1.9 | 29.6 ± 1.9 | 32.2 ± 9.3 |
| Prepupae water content [%] | 67.4 ± 3.7 | 70.4 ± 5.6 | 67.8 ± 9.9 |
| Prepupae organic content [%] | 27.6 ± 1.6 | 26.5 ± 1.8 | 31.4 ± 8.4 |
| Prepupae inorganic content [%] | 5.1 ± 0.3 c | 3.1 ± 0.2 b | 0.9 ± 0.9 a |
| Frass residues [%] | 43.7 ± 1.0 c | 46.0 ± 1.5 b | 28.5 ± 0.5 a |
| Parameter | Value |
|---|---|
| pH | 7.3 ± 0.4 |
| EC [µs cm-1] | 78.1 ± 2.7 |
| VS [g kg-1] | 78.5 ± 26.6 |
| Ctot [g kg-1] | 40 |
| Ntot [g kg-1] | 1.7 |
| Ptot [mg kg-1] | 823 ± 190 |
| Pav [mg kg-1] | 6.88 ± 1.28 |
| CF-F | GC-F | FV-F | |
|---|---|---|---|
| pH | 6.22 ± 0.14 C | 5.40 ± 0.03 A | 5.58 ± 0.01 B |
| EC [mS cm-1] | 5.67 ± 0.27 c | 3.06 ± 0.03 b | 2.36 ± 0.11 a |
| Dry matter [%] | 90.9 ± 0.0 | 89.9 ± 0.0 | 90.4 ± 0.0 |
| Ctot [g kg-1] | 479 ± 8 B | 443 ± 6 A | 488 ± 4 B |
| Ntot [g kg-1] | 25.9 ± 0.9 b | 24.4 ± 0.2 b | 18.3 ± 1.2 a |
| C:N ratio | 18.5 ± 0.3 a | 18.2 ± 0.4 a | 26.6 ± 1.7 b |
| VS [g kg-1] | 910 ± 7 c | 825 ± 9 a | 873 ± 4 b |
| C-S | CF-S | GC-S | FV-S | |
|---|---|---|---|---|
| pH CaCl2 | 7.53 ± 0.02 a | 7.57 ± 0.01 ab | 7.58 ± 0.03 b | 7.58 ± 0.02 b |
| EC [µS cm-1] | 95.5 ± 1.8 B | 79.8 ± 6.4 A | 77.3 ± 2.9 A | 81.5 ± 7.8 A |
| VS [g kg-1] | 37.3 ± 1.1 | 35.4 ± 1.3 | 38.4 ± 2.0 | 37.8 ± 2.1 |
| Ctot [g kg-1] | 17.9 ± 3.2 | 21.7 ± 4. | 22.5 ± 6.1 | 20.6 ± 5.2 |
| Ntot [g kg-1] | 0.98 ± 0.35 | 0.99 ± 0.51 | 1.17 ± 0.39 | 0.98 ± 0.44 |
| C:N ratio | 20.3 ± 8.1 | 26.2 ± 11.7 | 21.0 ± 9.8 | 22.1 ± 9.0 |
| NH4+ [mg kg-1] | 0.57 ± 0.13 | 0.58 ± 0.06 | 0.58 ± 0.08 | 0.61 ± 0.14 |
| NO3- [mg kg-1] | 45.2 ± 4.1 b | 15.4 ± 3.3 a | 17.0 ± 3.5 a | 12.1 ± 4.4 a |
| DOC [mg kg-1] | 48.3 ± 3.8 | 50.2 ± 1.5 | 48.5 ± 1.9 | 51.8 ± 1.3 |
| DC [mg kg-1] | 95.6 ± 1.6 a | 104.7 ± 1.4 b | 103.5 ±4.0 b | 112.1 ± 2.0 c |
| DN [mg kg-1] | 35.6 ± 3.9 C | 17.0 ± 1.5 B | 15.3 ± 1.8 AB | 15.0 ± 0.6 A |
| Pav [mg kg-1] | 5.2 ± 0.4 | 6.1 ± 1.0 | 6.1 ± 1.4 | 5.8 ± 0.9 |
| Ptot [mg kg-1] | 783 ± 46 ab | 866 ± 35 b | 757 ± 33 a | 721 ± 45 a |
| P bioavailability [%] | 67 ± 8 | 70 ± 12 | 81 ± 23 | 80 ± 12 |
| BR [µg CO2 g-1 dw h-1] | 5.6 ± 0.15 | 4.6 ± 1.5 | 6.7 ± 0.7 | 5.6 ± 0.5 |
| Cmic [µg CO2 g-1 dw soil] | 416.1 ± 103.5 | 276.4 ± 38.7 | 279.0 ± 22.7 | 336.2 ± 16.1 |
| qCO2 [µg CO2-C h-1/µg-1 C mic] | 14.7 ± 4.7 | 16.4 ± 4.3 | 24.5 ± 4.5 | 16.6 ± 1.4 |
| Plant biomass [mg dw] | 85 ± 7 | 80 ± 6 | 74 ± 4 | 75 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klammsteiner, T.; Turan, V.; Fernández-Delgado Juárez, M.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. https://doi.org/10.3390/agronomy10101578
Klammsteiner T, Turan V, Fernández-Delgado Juárez M, Oberegger S, Insam H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy. 2020; 10(10):1578. https://doi.org/10.3390/agronomy10101578
Chicago/Turabian StyleKlammsteiner, Thomas, Veysel Turan, Marina Fernández-Delgado Juárez, Simon Oberegger, and Heribert Insam. 2020. "Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization" Agronomy 10, no. 10: 1578. https://doi.org/10.3390/agronomy10101578
APA StyleKlammsteiner, T., Turan, V., Fernández-Delgado Juárez, M., Oberegger, S., & Insam, H. (2020). Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy, 10(10), 1578. https://doi.org/10.3390/agronomy10101578