Abstract
Because of its nutritious properties, the black soldier fly has emerged as one of the most popular species in advancing circular economy through the re-valorization of anthropogenic organic wastes to insect biomass. Black soldier fly frass accumulates as a major by-product in artificial rearing set-ups and harbors great potential to complement or replace commercial fertilizers. We applied frass from larvae raised on different diets in nitrogen-equivalent amounts as soil amendment, comparing it to NH4NO3 fertilizer as a control. While the soil properties did not reveal any difference between mineral fertilizer and frass, principal component analysis showed significant differences that are mainly attributed to nitrate and dissolved nitrogen contents. We did not find significant differences in the growth of perennial ryegrass between the treatments, indicating that frass serves as a rapidly acting fertilizer comparable to NH4NO3. While the abundance of coliform bacteria increased during frass maturation, after application to the soil, they were outcompeted by gram-negatives. We thus conclude that frass may serve as a valuable fertilizer and does not impair the hygienic properties of soils.
1. Introduction
In recent years, the use of saprobic insect larvae from the mealworm beetle (Tenebrio molitor), the black soldier fly (Hermetia illucens; BSF), or the house fly (Musca domestica) has attracted interest in the face of rising prices of animal feedstuff and accumulating amounts of waste [1,2]. In the European Union, green waste and food waste largely contribute to an annual amount of 118 to 138 million tons of organic wastes [3]. Especially BSF larvae (BSFL) have been shown to efficiently convert organic wastes into high quality fat and protein [4]. The economic potential and meaningful reintroduction of otherwise wasted nutrients into the biosphere via a circular economy enticed researchers, investors, and the public to contribute to a more efficient recycling of organic wastes by exploiting the potential of insect larvae on a large scale [5,6]. BSFL could also play a valuable role for smaller decentralized waste management systems operated by e.g., hobbyists or farmers in areas where the fly occurs naturally [7,8,9]. Additionally, the exploitation of BSF and its by-products could create an affordable opportunity for revenue generation by entrepreneurs and smallholder farmers in low-income countries [9,10,11]. The main by-product in the bioconversion of wastes into high quality protein for animal feedstuff is summarized as ‘frass’. Frass in general describes insect excretions, but in a commercial context it often refers to a mixture of mainly insect feces, substrate residues, and shed exoskeletons. It is an inevitable side-stream during the mass-rearing of insects that can add up to 75% of the fed substrate [12] and is often merchandised as a fertilizing product. In recent years, an increasing number of studies started focusing on meaningful applications of insect frass [9,13,14,15], and the first large-scale field studies provided promising perspectives for its application in agriculture, especially in terms of plant nutrient availability [10,11,16].
The substrate used to grow insects affects the properties of the frass, since undegradable residues remain unused, while the digested fraction is modified by the gut microbiota when passing through the gastrointestinal tract [17,18]. Wang et al. [19] used frass from T. molitor for subsequent rearing of BSFL to exploit leftover nutrients that T. molitor could not take up or digest. In substrates carrying a high bioburden like human feces and manure, BSFL have shown to reduce pathogenic bacteria such as Salmonella enterica [20,21] and Escherichia coli [20,22], which is attributed to their production of antimicrobial peptides [23]. In the wild, frass from various insects can help to increase the chances of survival and reproduction by either deterring [24,25] or attracting [26,27] conspecifics. Frass can act as a vector for phytopathogenic microorganisms [28,29] and as a source of probiotic yeasts [30]. Its effect on the insects’ environment can be observed in forests, where frass deposition goes hand in hand with insect canopy herbivory. It has been shown that frass has an impact on C and N dynamics, and has beneficial effects for tree growth by increasing soil total C, N, and NH4+, as well as microbial soil respiration [31,32]. In industrial environments, frass pyrolyzed to biochar has been successfully tested as a bioadsorbent for wastewater detoxification [33]. According to recent studies, frass’ agriculturally and economically most meaningful potential could lie in its application as fertilizer [9,34].
In this study, we assessed the fertilizing potential of process residues (frass) from three generic diets degraded by BSFL. Two of the diets represent major streams of organic waste, namely grass-cuttings (GC) and fruit/vegetable (FV) mix, while the chicken feed (CF) control diet is a commonly used insect breeding substrate. We hypothesized that (I) microbial colonization increases with frass maturation and (II) frass may serve as a valuable alternative to mineral fertilizer by inducing beneficial effects on plant growth.
2. Materials and Methods
2.1. Black Soldier Fly Frass Collection
The frass was collected from a preparatory feeding experiment conducted at 27 °C, 60% relative humidity (Figures S1 and S2, Table 1). The chicken feed (CF; Grünes Legekorn Premium, Unser Lagerhaus, Klagenfurt, Austria) was processed with a Fidibus flour mill (Komo Mills, Hopfgarten, Austria) and mixed with water in a 40:60 ratio.

Table 1.
Feeding experiment termination summary. The feeding experiment was terminated after a total of 23 days when more than 90% larvae from one treatment group transitioned to prepupal stage. Different lower-case letters indicate differences between treatments (p ≤ 0.05) according to the Tukey’s HSD test. (n = 4; average ± standard deviation; CF = Chicken feed diet, GC = Grass-cuttings diet, FW = Fruit/Vegetables diet).
The fruit/vegetable mix (FV; cucumber, tomato, apple, orange, in ratio 0.5:1:1:1) and fresh grass-cutting diet (GC) were shredded and homogenized using a Total Nutrition Center blender (Vitamix, Olmsted Township, United States). Feeding was done in organic content-equivalents (100, 250, and 370 mg larvae−1 day−1 for CF, GC, and FV). After termination of the feeding experiment, the black soldier fly frass (BSFF) from each treatment was collected in plastic bags and stored at room temperature until further use.2.2. Soil Preparation and Greenhouse Set Up
A greenhouse trial using soil collected from an agricultural site (47°15′54″ N, 11°20′20″ E; Table 2) was set up to evaluate the fertilizing effect of the BSFF on the soil. The neutral-to-slightly basic soil (pH 7.3 ± 0.4) had an electrical conductivity of 78.0 ± 2.7 µs cm−1 and a volatile solids content of 78.0 ± 26.6 g kg−1. In addition to a Ptotal content of 823 ± 190 mg kg−1 (Pbioavailable proportion 6.88 ± 1.28 mg kg−1), elemental analysis determined a C/N ratio of 24 (40 g Ctotal kg−1, 1.7 g Ntotal kg−1). The soil classified as a calcaric Fluvisol (IUSS Working Group WRB, 2015) was sieved (Ø < 4 mm) and homogenously mixed with a vermiculite/sand blend (1:1; v:v) at a ratio 2:1 w:w (soil:blend).

Table 2.
Characterization of the soil used for the greenhouse trial. Values expressed on a dry mass basis for n = 3 (average ± standard deviation). pH (pH CaCl2), EC (Electrical conductivity), VS (Volatile solids), Ctot (Total carbon), Ntot (Total nitrogen), Ptot (Total phosphorous), Pav (Plant available P).
The four experimental treatments were performed in 500 mL pots: soil was mixed with (1) mineral fertilizer (which served as control); (2) GC BSFF; (3) FV BSFF; (4) and CF BSFF. The mineral fertilizer (NH4NO3) and the different types of BSFF (Table 3) were added in an amount of 40 mg N kg−1 soil, which is equivalent to 80 kg N ha−1, considering the soil bulk density of 1 g cm−3 and a plough depth of 20 cm as described by Goberna et al. [35]. Thereby, all treatments received the same dose of total N.

Table 3.
Main properties of the three different black soldier fly frass fractions (CF-F: Chicken feed frass; GC-F: Grass-cuttings frass; FV-F: Fruit/Vegetables frass). Values expressed on a dry mass basis for n = 3 (average ± standard deviation). Different lower-case letters indicate differences between treatments (p ≤ 0.05) according to the Tukey´s HSD test. Different capital letters indicate significant differences between treatments (p ≤ 0.05) according to the Mann–Whitney test. EC (Electrical conductivity), VS (Volatile solids), Ctot (Total carbon content), Ntot (Total nitrogen content).
After an equilibration period of 16 h at 4 °C, pots were randomly placed in a greenhouse. Ryegrass (Lolium perenne; seed amount based on 30 kg seeds ha−1) was sown and left to develop. During the incubation period of 28 days, at an average temperature of 20 °C with a light/darkness cycle of 10/14 h, the soil moisture was kept at field capacity (moisture of the soil after drainage by gravity). All treatments were applied in four replicates, resulting in a total of 16 pots in this study. After the incubation period, plants were removed from the pots, and soil samples were sieved (Ø < 2mm) and immediately stored at +4 °C until analyses (Table 4).

Table 4.
Physicochemical and biological properties of the control (C-S: NH4NO3) and the frass amended soils (CF-S: Chicken feed frass + soil; GC-S: Grass-cuttings frass + soil and FV-S: Fruit/Vegetables frass + soil). Values expressed on a dry mass basis for n = 4 (average ± standard deviation). Different lower-case letters indicate differences between treatments (p ≤ 0.05) according to the Tukey´s HSD test. Different capital letters indicate significant differences between treatments (p ≤ 0.05) according to the Mann–Whitney test. EC (Electrical conductivity), VS (volatile solids), Ctot (Total carbon content), Ntot (Total nitrogen content), NH4+ (Ammonium content), NO3− (Nitrate content), DOC (Dissolved organic carbon), DC (Dissolved carbon), DN (Dissolved nitrogen), Pav (Plant available phosphorous content), Ptot (Total phosphorous content), BR (Basal respiration), qCO2 (Metabolic quotient).
2.2. Frass and Soil Analyses
Frass and soil samples (10 g fresh weight) were placed into a glass Petri dish and oven-dried (105 °C) for 24 h to determine the content of total solids. Volatile organic solid (VS) content was determined from the weight loss following ignition in a muffle furnace (CWF 1000, Carbolite, Neuhausen, Germany) at 550 °C for 5 h. Total C and N contents were analyzed in dried samples using a CN analyzer (TruSpec CHN, LECO, St. Joseph, MI, USA). EC and pH were determined in distilled water and 0.01 M CaCl2 extracts (1:2.5, w/v), respectively.
Soil inorganic nitrogen (NH4+ and NO3−) was determined in 0.0125 M CaCl2 extracts as described by Kandeler [36,37]. Soil total P (Ptot) and plant available P (Pav) were determined as described by Illmer et al. [38]. To estimate dissolved organic carbon (DOC), dissolved carbon (DC), and dissolved nitrogen (DN), 10 g of field-moist soil were shaken in 40 mL distilled water, filtered, and immediately measured using a TOC-L analyzer (Shimadzu, Kyōto, Japan). Soil basal respiration (BR) and microbial biomass (Cmic) were measured according to Heinemeyer et al. [39]. The metabolic quotient (qCO2) was calculated from BR and Cmic according to Anderson and Domsch [40]. At the end of the trial, aboveground plant biomass was determined by cutting plant shoots at the soil surface and drying them at 60 °C for 48 h. Samples were then re-weighted to determine the dry biomass.
2.3. Preparation of Media
For the assessment of the total cultivable bacterial colony forming units (CFUs), we used standard methods agar (0.5% peptone, 0.25% yeast extract, 0.1% glucose, 1.5% agar, pH adjusted to neutral). To determine the abundance of Salmonella sp., E. coli, coliforms, and other gram-negative bacteria, XLT-4 and ChromoCult® coliform agar (Merck, Darmstadt, Germany) were prepared according to the enclosed recipe.
2.4. Pathogen Quantification/Assessment of Microbial Colonization in Frass and Soil
An amount of 2 g frass or soil sample was added to 18 mL sterile saline solution (0.95% NaCl) and placed on a rotation shaker at 200 rpm for 15 min. Samples were diluted to 10−2 and 10−3 for soil, and 10−5 and 10−6 for frass using sterile 0.95% NaCl. From each dilution, 50 µL was plated using the spread plate technique. Plates were then incubated at 37 °C for 24 h, and the CFUs were counted.
2.5. Statistical Analyses
The effect of the BSFF application on soil parameters was tested with a one-way analysis of variance (ANOVA). In case of significant F-values, a Tukey’s HSD (honestly significant difference) post hoc test (p < 0.05) was performed. Prior to analysis, the homogeneity of the variances was tested (Levene’s test), and data were also tested for normality. Non-normal data were subjected to non-parametric tests for several independent samples (Kruskal–Wallis test), and pairwise comparisons between treatments were performed using the Mann–Whitney U test (p < 0.05). Statistical analyses were performed using the SPSS v. 23.0 Software (IBM, Armonk, NY, USA). Principal component analysis was performed in R [41] using the vegan package [42]. Analysis of similarity (ANOSIM) on the physicochemical data (999 permutations) was also conducted with vegan. All graphical representations of data were created with ggplot2 [43].
3. Results and Discussion
3.1. Assessment of Microbial Load in Frass and Frass-Amended Soils
The high moisture content of substrates and air, as well as the pleasantly warm temperature common in insect breeding, favor microbial growth. While the type of diet is known to directly influence the BSFL gut microbiome [17,44], the excrements in turn may influence the microbiome in the frass. It is likely that by agitating and mixing their surrounding substrate with feces and their inherent microorganisms, the larvae have an impact on their habitat. Similar effects are known from the widely used earthworms (Eisenia fetida), which can stabilize organic wastes and introduce ammonia-oxidizing microorganisms, thereby boosting nitrification and increasing nitrate concentrations in the resulting vermicompost [45]. Other insect species inoculate the soil with excreted microorganisms and provide beneficial effects for its quality both in wild and artificial settings [46,47,48].
Before and after applying frass as soil amendment, the number of cultivable E. coli, coliform, and other gram-negative bacteria were assessed (Figure 1). While frass counted up to 109 CFU g−1, the count in soil was down to 103–105 g−1. With the nutrient media used in this study, untreated soil contained no cultivable E. coli or coliforms, and only low abundances of cultivable gram-negatives with 102 CFUs g−1. In particular, frass from the CF treatment acted as a reservoir for coliforms with a CFU count of 1.9 × 109, thereby exceeding CFU counts recorded on larval surfaces (Figure S2). Gram-negative bacteria predominated the cultivable microbiota in frass-amended soil with highest CFU counts of up to 105 in soil treated with frass from a FV diet. High microbial load and dominance of coliforms in frass shifted to lower CFU numbers and predominantly gram-negative bacteria in the frass-soil mix, indicating that the autochthonous soil microbiota outcompeted allochthonous microorganisms introduced with frass [49,50,51].
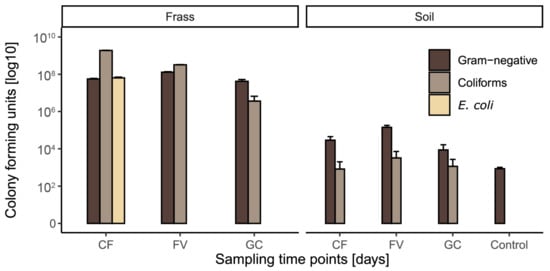
Figure 1.
Colony forming units counted for gram-negative, coliform, and Escherichia coli from frass samples after collection from the feeding experiment and soil samples after having mixed the soil with frass (n = 4). CF = Chicken feed, FV = Fruit/vegetable mix, GC = Grass-cuttings.
3.2. Black Soldier Fly Frass Properties, Soil Quality and Plant Performance
The physicochemical properties of frass were influenced by the larval diet (Table 3). Especially CF frass was more alkaline, had a higher EC, and a higher content of VS. While total C contents were similar in all types of frass, FV frass showed a C:N-ratio of 26.6, compared to 18.5 and 18.2 in CF frass and GC frass, respectively. Similar C:N ratios as found in CF and GC frass have been reported by other studies that used brewery spent grains as larval substrate [11,16]. A C:N-ratio > 20 bears the risk of soil N immobilization, which may favor plants with a more efficient N exploitation attributed to their rhizobiome [46,52]. The addition of biochar to the larval waste conversion process might further improve the frass’ N retention, while at the same time increasing larval biomass yield [10]. Moreover, larvae pass through six instars continuously shedding their exoskeleton. Chitin, an N-acetylglucosamine-based polymer (C8H13O5N)n, may influence not only the C:N ratio, but its degradation product chitosan may also provide underrated benefits for plant health and pathogen resistance [53,54]. The C:N ratio is one of the major parameters to consider when it comes to deciding whether frass should be used as soil amendment or as co-substrate in anaerobic digestion or composting [55,56]. Chitin utilization by insects is often associated with chitinolytic gut symbionts [57], which still needs to be further investigated in the context of BSF larvae. Chitin-containing fertilizers have previously been found to serve as splendid nitrogen sources [58].
Frass addition to the soil before planting Lolium perenne was adjusted on a basis of N-equivalence (80 kg N ha−1; Table 3 and Table 4). Soil amended with CF frass exhibited a higher Ptot content than the other frass-amended soils; however, Pav was not significantly different. Principal component analysis (Figure 2) highlighted the parameters that influenced the properties of the soil-frass mix the most, which was further confirmed by ANOSIM (R = 0.5061, p < 0.001). The three frass-amended soils clustered closely together, with Ptot and Pav, pH, DC, Ntot, C:N ratio, BR, and the qCO2 being the most influential parameters for their similarity.
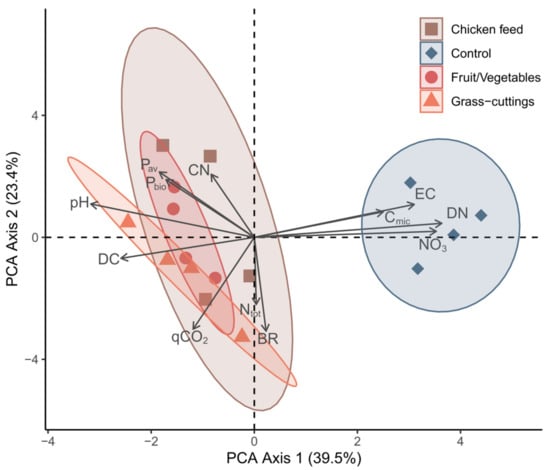
Figure 2.
Principal component analysis of samples from control soil and soil mixed with the three different frass types. Data points represent replicates, and arrows show the most influential parameters for the spread of the data. NO3 = Nitrate, DN = Dissolved nitrogen, CN = Carbon/Nitrogen ratio, Pbio = Phosphorus bioavailability, BR = Soil basal respiration, qCO2 = Metabolic quotient, Cmic = Microbial biomass, Ntot = Total nitrogen, Pav = Plant available phosphorus, DC = Dissolved carbon, EC = Electric conductivity.
The qCO2 describing the microbial soil respiration per unit Cmic is known to be tightly connected to the C:N ratio and increases when less N is available [59]. Higher qCO2 can indicate stress or disturbances within the soil because, although C sources are readily available, microbial metabolism and substrate decomposition are limited by N [60]. NO3 and DN, on the other hand, were the major drivers for the deviation of the control group from the frass treatment groups, since they were both significantly higher in control soil.
In our study, the frass treatments were compared with a control that received an equivalent of 80 kg ha−1 nitrogen in the form of NH4NO3. In a similar experiment, Ros et al. [61] found that such an amount of mineral N increased the maize yield by 33% compared with an unfertilized control, while N-equivalent additions of compost yielded only 15% increase. Recent observations at field-scale by Beesigamukama et al. showed that even at lower application rates of 30 kg N ha−1, BSFF exceeded the performance of mineral N fertilizer in terms of grain yield and nitrogen fertilizer replacement values when applied at the same rates [16]. Compared with commercial fertilizers, nitrogen recovery rates and nitrogen use efficiency of plants have been shown to be improved when amended with BSFF [11]. Additionally, the higher P concentrations in the frass could facilitate N accumulation in plants by improving N uptake, as P plays an important role in energy transfer [62,63].
Using BSFL instead of aerobic windrow composting has additionally been shown to reduce the global warming potential of treating organic wastes by 50% [64]. The addition of frass did not lead to significant differences in plant growth compared to the mineral fertilizer (Figure 3). In fact, the similar growth progress indicates that the nutrients from frass are readily available for uptake and have no detrimental impact on plant growth. These results, however, do not support the findings of Alattar et al. [13], who reported that the development of plant height and leaves in corn (Zea mays) was inhibited by the addition of BSFL frass. In their study, they attributed the negative effects to the low porosity of larval residues that may have created anaerobic conditions. The moisture content of the frass harvested from our preliminary feeding experiment was only 10% (Table 3), thereby facilitating aeration and miscibility in soil. Insufficient oxygen supply can occur when frass has a high moisture content and is not subjected to adequate post-processing. In an environment specialized on insect rearing, a multi-step treatment of frass could increase the efficiency of degradation. With additional downstream composting or anaerobic digestion [65,66], the recovery as soil amendment represents the economically most promising option.
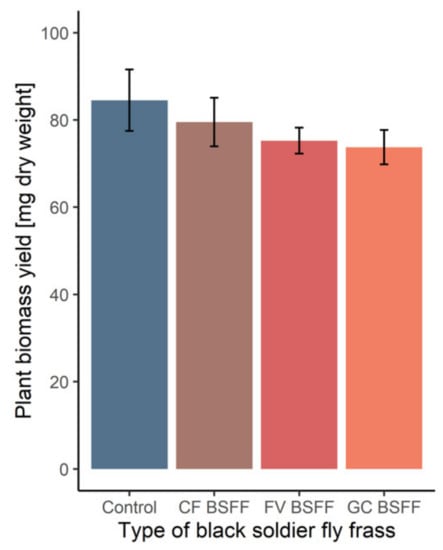
Figure 3.
Plant biomass yield of Lolium perenne after application of black soldier fly frass (BSFF) obtained from the degradation of various organic substrates. CF BSFF = Chicken feed frass, FV BSFF = Fruit/vegetables frass, GC BSFF = Grass-cuttings frass (n = 4).
4. Conclusions
The valorization of organic wastes by insect larvae generates frass as a side-product. From our study we conclude that frass may serve as a soil nutrient source and does not impair soil hygiene. In some cases, however, frass post-processing through anaerobic digestion or composting may be advised to avoid soil nitrogen deficiencies or impairing soil gas permeability. In the light of the increasing importance of insect rearing, the agricultural utilization of frass is demanding further research, in particular, long-term studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/10/1578/s1, Figure S1: Influence of three different diets on larval biomass increase, Figure S2: Microbial colonization of larval surfaces.
Author Contributions
Conceptualization, T.K. and M.F.-D.J.; formal analysis, V.T., T.K., and S.O.; funding acquisition, H.I., V.T., and T.K.; investigation, S.O. and V.T.; methodology, V.T. and M.F.-D.J.; resources, H.I.; supervision, T.K. and M.F.-D.J.; visualization, T.K.; writing—original draft, T.K. and M.F.-D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Austrian Science Fund (FWF; project number: P26444). Thomas Klammsteiner was supported by a PhD grant from the Vizerektorat für Forschung of the Universität Innsbruck (Doktoratsstipendium aus der Nachwuchsförderung). Veysel Turan was supported by a post-doctoral fellowship from the Scientific and Technological Research Council of Turkey (TUBITAK, grant number, 1059B191601133).
Acknowledgments
The authors show their gratitude to Carina D. Heussler for providing the black soldier fly larvae for this study. Open Access Funding by the Austrian Science Fund (FWF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; ISBN 978-92-5-107205-9. [Google Scholar]
- United Nations. World Population Prospects. The 2017 Revision; Department of Economic and Social Affairs—Population Division: New York, NY, USA, 2017; p. 53. [Google Scholar]
- European Commission. 811: Green Paper on the Management of Bio-Waste in the European Union; Commission of the European Communities: Brussels, Belgium, 2008. [Google Scholar]
- Pastor, B.; Velasquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Klocke, M.; Schluter, O. Insect biodiversity: Underutilized bioresource for sustainable applications in life sciences. Reg. Environ. Chang. 2017, 17, 1445–1454. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R.; Ashok, K.N.; Srinivas, K.; Arutchelvan, V.; Thota, K.R.; Ravi, S.N.; Sandeep, K.D.; Goutham, R.M. Black soldier fly larvae, a viable opportunity for entrepreneurship. Acta Sci. Agric. 2018, 2, 11–20. [Google Scholar]
- Klammsteiner, T.; Walter, A.; Pan, H.; Gassner, M.; Heussler, C.D.; Schermer, M.; Insam, H. On everyone’s lips: Insects for food and feed. In Proceedings of the 5th Austrian Citizen Science Conference, Obergurgl, Austria, 26—28 June 2019; Volume 366, p. 6. [Google Scholar]
- Quilliam, R.S.; Nuku-Adeku, C.; Maquart, P.; Little, D.; Newton, R.; Murray, F. Integrating insect frass biofertilisers into sustainable peri-urban agro-food systems. J. Insects Food Feed 2020, 1–8. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Dubois, T.; Subramanian, S.; Wangu, M.M.; Ekesi, S.; et al. Biochar and gypsum amendment of agro-industrial waste for enhanced black soldier fly larval biomass and quality frass fertilizer. PLoS ONE 2020, 15, e238154. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Musyoka, M.W.; Ekesi, S.; et al. Exploring Black Soldier Fly Frass as Novel Fertilizer for Improved Growth, Yield, and Nitrogen Use Efficiency of Maize Under Field Conditions. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. 2009, 27, 603–610. [Google Scholar] [CrossRef]
- Alattar, M.; Alattar, F.; Popa, R. Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant Sci. Today 2016, 3, 57–62. [Google Scholar] [CrossRef]
- Choi, Y.-C.; Choi, J.-Y.; Kim, J.-G.; Kim, M.-S.; Kim, W.-T.; Park, K.-H.; Bae, S.-W.; Jeong, G.-S. Potential usage of food waste as a natural fertilizer after digestion by Hermetia illucens (Diptera: Stratiomyidae). Int. J. Ind. Entomol. 2009, 19, 171–174. [Google Scholar]
- Sarpong, D.; Oduro-Kwarteng, S.; Gyasi, S.F.; Buamah, R.; Donkor, E.; Awuah, E.; Baah, M.K. Biodegradation by composting of municipal organic solid waste into organic fertilizer using the black soldier fly (Hermetia illucens) (Diptera: Stratiomyidae) larvae. Int. J. Recycl. Org. Waste Agric. 2019, 8, 45–54. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Mochoge, B.; Korir, N.; Musyoka, M.W.; Fiaboe, K.K.M.; Nakimbugwe, D.; Khamis, F.M.; Subramanian, S.; Dubois, T.; Ekesi, S.; et al. Nitrogen Fertilizer Equivalence of Black Soldier Fly Frass Fertilizer and Synchrony of Nitrogen Mineralization for Maize Production. Agronomy 2020, 10, 1395. [Google Scholar] [CrossRef]
- Klammsteiner, T.; Walter, A.; Bogataj, T.; Heussler, C.D.; Stres, B.; Steiner, F.M.; Schlick-Steiner, B.C.; Arthofer, W.; Insam, H. The core gut microbiome of black soldier fly (Hermetia illucens) larvae raised on low-bioburden diets. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Milanović, V.; Cardinali, F.; Garofalo, C.; Clementi, F.; Pasquini, M.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Loreto, N.; et al. The bacterial biota of laboratory-reared edible mealworms (Tenebrio molitor L.): From feed to frass. Int. J. Food Microbiol. 2018, 272, 49–60. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.; Liu, X.; Yang, Q.; Zheng, L.; Li, W.; Cai, M.; Li, Q.; Zhang, J.; Yu, Z. Insect biorefinery: A green approach for conversion of crop residues into biodiesel and protein. Biotechnol. Biofuels 2017, 10, 304. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli o157:h7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrugg, C.; Lindstrom, A.; Vinneras, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tomberlin, J.K.; Brady, J.A.; Sanford, M.R.; Yu, Z. Black soldier fly (Diptera: Stratiomyidae) larvae reduce Escherichia coli in dairy manure. Environ. Entomol. 2008, 37, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Zhang, J.; Bisch-Knaden, S.; Fandino, R.A.; Yan, S.; Obiero, G.F.; Grosse-Wilde, E.; Hansson, B.S.; Knaden, M. The olfactory co-receptor IR8a governs larval-frass mediated competition avoidance in a hawkmoth. Proc. Natl. Acad. Sci. USA 2019, 116, 21828–21833. [Google Scholar] [CrossRef]
- Zhang, X.G.; Li, X.; Gao, Y.L.; Liu, Y.; Dong, W.X.; Xiao, C. Oviposition deterrents in larval frass of potato tuberworm moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2019, 48, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lorenzana, L.R.J. Frass volatiles as attractant to the mango pulp weevil (Sternochetus frigidus (Fabr.) (Coleoptera: Curculionidae)). Philipp. Agric. Sci. 2014, 97, 385–390. [Google Scholar]
- Mitchell, R.F.; Hanks, L.M. Insect frass as a pathway for transmission of bacterial wilt of cucurbits. Environ. Entomol. 2009, 38, 395–403. [Google Scholar] [CrossRef]
- Roy, K.; Ewing, C.P.; Hughes, M.A.; Keith, L.; Bennett, G.M. Presence and viability of Ceratocystis lukuohia in ambrosia beetle frass from Rapid ʻŌhiʻa Death-affected Metrosideros polymorpha trees on Hawai’i Island. For. Pathol. 2019, 49, e12476. [Google Scholar] [CrossRef]
- Khisti, U.V.; Kathade, S.A.; Aswani, M.A.; Anand, P.K.; Bipinraj, N.K. Isolation and identification of saccharomyces cerevisiae from caterpillar frass and their probiotic characterization. Biosci. Biotechnol. Res. Asia 2019, 16, 179–186. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Recycling of nitrogen in herbivore feces: Plant recovery, herbivore assimilation, soil retention, and leaching losses. Oecologia 2007, 151, 42–53. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect canopy herbivory and frass deposition affect soil nutrient dynamics and export in oak mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Yang, S.-S.; Chen, Y.; Kang, J.-H.; Xie, T.-R.; He, L.; Xing, D.-F.; Ren, N.-Q.; Ho, S.-H.; Wu, W.-M. Generation of high-efficient biochar for dye adsorption using frass of yellow mealworms (larvae of Tenebrio molitor Linnaeus) fed with wheat straw for insect biomass production. J. Clean Prod. 2019, 227, 33–47. [Google Scholar] [CrossRef]
- Schmitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020. [Google Scholar] [CrossRef]
- Goberna, M.; Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; García, C.; Insam, H. Pathogenic bacteria and mineral N in soils following the land spreading of biogas digestates and fresh manure. Appl. Soil Ecol. 2011, 49, 18–25. [Google Scholar] [CrossRef]
- Kandeler, E. Nitrate. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 408–410. ISBN 978-3-642-60966-4. [Google Scholar]
- Kandeler, E. Ammonium. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 406–408. ISBN 978-3-642-60966-4. [Google Scholar]
- Illmer, P. Total, organic, inorganic and plant available phosphorus. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 412–416. ISBN 978-3-642-60966-4. [Google Scholar]
- Heinemeyer, O.; Insam, H.; Kaiser, E.A.; Walenzik, G. Soil microbial biomass and respiration measurements: An automated technique based on infra-red gas analysis. Plant Soil 1989, 116, 191–195. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as ph, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0.
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- De Smet, J.; Wynants, E.; Cos, P.; Campenhout, L.V. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and its impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e2722-17. [Google Scholar] [CrossRef]
- Huang, K.; Xia, H.; Cui, G.; Li, F. Effects of earthworms on nitrification and ammonia oxidizers in vermicomposting systems for recycling of fruit and vegetable wastes. Sci. Total Environ. 2017, 578, 337–345. [Google Scholar] [CrossRef]
- Fielding, D.J.; Trainor, E.; Zhang, M. Diet influences rates of carbon and nitrogen mineralization from decomposing grasshopper frass and cadavers. Biol. Fertil. Soils 2013, 49, 537–544. [Google Scholar] [CrossRef]
- McTavish, M.J.; Smenderovac, E.; Gunn, J.; Murphy, S.D. Insect defoliators in recovering industrial landscapes: Effects of landscape degradation and remediation near an abandoned metal smelter on gypsy moth (Lepidoptera: Lymantriidae) feeding, frass production, and frass properties. Environ. Entomol. 2019, 48, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Juárez, M.F.-D.; Zangerle, M.; Insam, H. Effects of digestate on soil chemical and microbiological properties: A comparative study with compost and vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef]
- Podmirseg, S.M.; Waldhuber, S.; Knapp, B.A.; Insam, H.; Goberna, M. Robustness of the autochthonous microbial soil community after amendment of cattle manure or its digestate. Biol. Fertil. Soils 2019, 55, 565–576. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef]
- Zhu, B.; Gutknecht, J.L.M.; Herman, D.J.; Keck, D.C.; Firestone, M.K.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol. Biochem. 2014, 76, 183–192. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Dioha, I.; Ikeme, C.H.; Nafiu, T. Effect of carbon to nitrogen ratio on biogas production. Int. Res. J. Nat. Sci. 2013, 1, 1–10. [Google Scholar]
- Wang, L.; Li, Y.; Prasher, S.O.; Yan, B.; Ou, Y.; Cui, H.; Cui, Y. Organic matter, a critical factor to immobilize phosphorus, copper, and zinc during composting under various initial C/N ratios. Bioresour. Technol. 2019, 289, 121745. [Google Scholar] [CrossRef] [PubMed]
- Borkott, H.; Insam, H. Symbiosis with bacteria enhances the use of chitin by the springtail, Folsomia candida (Collembola). Biol. Fertil. Soils 1990, 9, 126–129. [Google Scholar] [CrossRef]
- Insam, H.; Merschak, P. Nitrogen leaching from forest soil cores after amending organic recycling products and fertilizers. Waste Manag. Res. 1997, 15, 277–292. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Leita, L.; De Nobili, M.; Mondini, C.; Muhlbachova, G.; Marchiol, L.; Bragato, G.; Contin, M. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biol. Fertil. Soils 1999, 28, 371–376. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Tittonell, P.; Corbeels, M.; van Wijk, M.T.; Vanlauwe, B.; Giller, K.E. Combining Organic and Mineral Fertilizers for Integrated Soil Fertility Management in Smallholder Farming Systems of Kenya: Explorations Using the Crop-Soil Model FIELD. Agron. J. 2008, 100, 1511–1526. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient Interactions in Crop Plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Mertenat, A.; Diener, S.; Zurbrügg, C. Black soldier fly biowaste treatment—Assessment of global warming potential. Waste Manag. 2019, 84, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Bulak, P.; Proc, K.; Pawłowska, M.; Kasprzycka, A.; Berus, W.; Bieganowski, A. Biogas generation from insects breeding post production wastes. J. Clean Prod. 2020, 244, 118777. [Google Scholar] [CrossRef]
- Lalander, C.; Nordberg, A.; Vinneras, B. A comparison in product-value potential in four treatment strategies for food waste and faeces—Assessing composting, fly larvae composting and anaerobic digestion. Glob. Chang. Biol. Bioenergy 2018, 10, 84–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).