Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review
Abstract
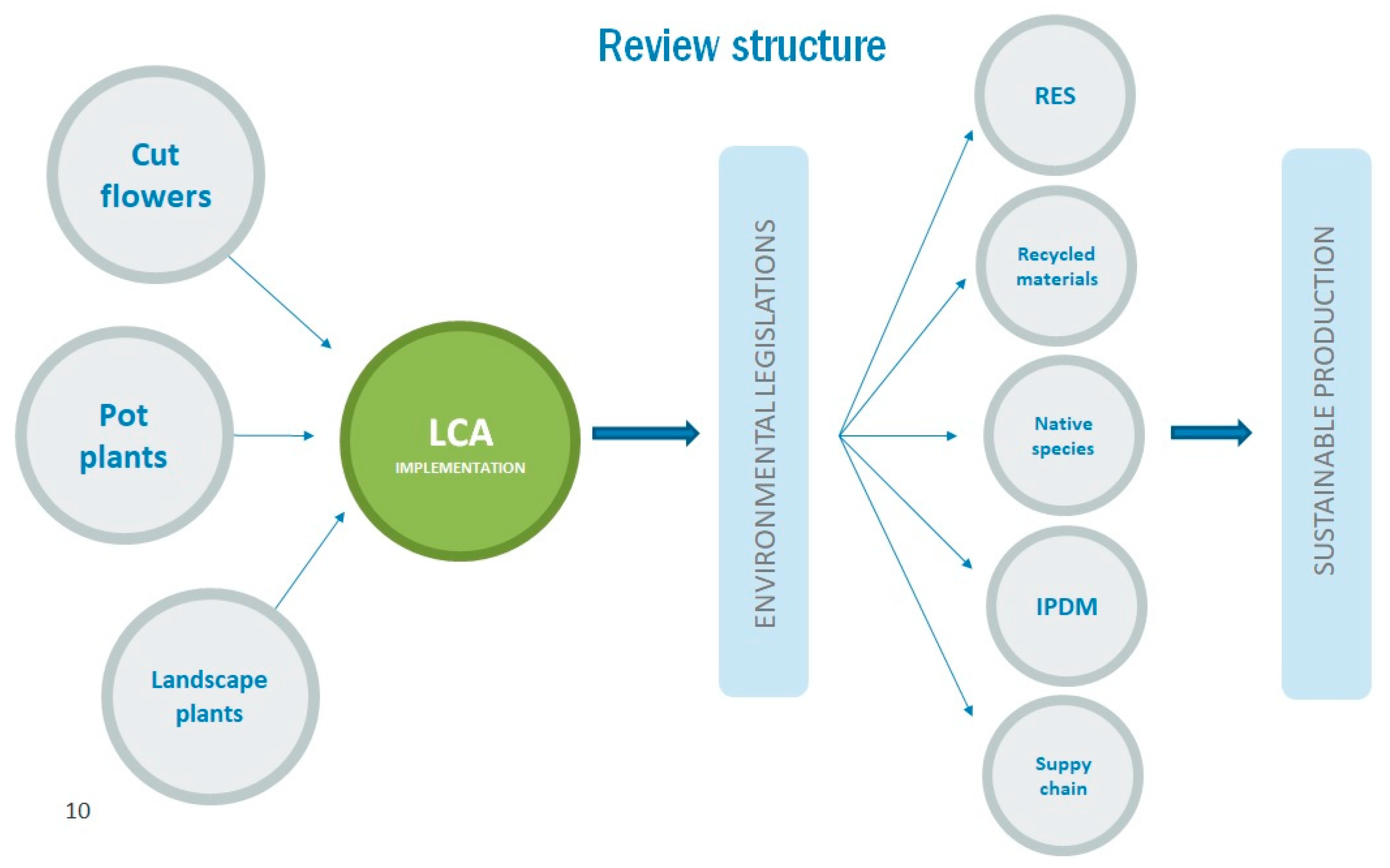
:1. Global Cut Flower and Pot-Plant Production
2. Life Cycle Assessment (LCA) of Floricultural Products
2.1. LCA of Cut Flowers and Foliage
2.2. LCA of Ornamental Pot-Plants
2.3. LCA of Landscape Plants
| Ornamental Species (Production Country) | Total CO2 Production (kg CO2 eq) | Contribution to GWP (%) | CO2 Sequestration (kg CO2 eq) | Reference | ||
|---|---|---|---|---|---|---|
| Cultivation | Handling | Transportation | ||||
| ||||||
| Roses (the Netherlands) | 24 | 96 | 3 | 0 | na | [14] |
| Roses (Austria) | 1.7 | 100 | ns | ns | na | [16] |
| Roses (Ethiopia) | ns | 72 | 22 | 6 | na | [11] |
| Roses (Germany) | 0.6–21 | 25–92 | ns | 7–21 | na | [19] |
| Chrysanthemum (Austria) | 0.4 | 100 | ns | ns | na | [16] |
| Calla (Austria) | 2.5 | 100 | ns | ns | na | [16] |
| Iris (Austria) | 1.9 | 100 | ns | ns | na | [16] |
| Lilium (Austria) | 1.6 | 100 | ns | ns | na | [16] |
| Spring flowers (Austria) | >0.1 | 100 | ns | ns | na | [16] |
| Summer flowers (Austria) | >0.1 | 100 | ns | ns | na | [16] |
| Carnations (Greece) | 0.316 | 19.4 | 65.6 | 15.0 | na | [9] |
| ||||||
| Phalaenopsis (the Netherlands) | 32.0 | 95 | 4 | 1 | na | [14] |
| Ficus (the Netherlands) | 11.5 | 49 | 49 | 2 | na | [14] |
| Poinsettia (the Netherlands) | 21.5 | 82 | 16 | 2 | na | [14] |
| Poinsetia (Austria) | 2.8 | 100 | ns | ns | na | [16] |
| Cyclamen (Italy) | ns | 100 | ns | ns | na | [13] |
| Cycalmen (Austria) | 5.7 | 100 | ns | ns | na | [16] |
| Begonia x seperflorens (USA) | 0.14 | 98.6 | ns | 1.4 | na | [17] |
| Chrysanthemum (USA) | 0.56 | 99.9 | ns | >0.1 | na | [17] |
| Orchids (Germany) | 4.2 | 18–98 | ns | >1 | na | [19] |
| Azalea (Austria) | 3.6 | 100 | ns | ns | na | [16] |
| ||||||
| ||||||
| Acer rubrum “October glory” | 8.98 | ns | 901 | [27] | ||
| Cercis canadensis | 13.71 | 165 | [20] | |||
| Picea glauca globosa | 1.01 | ns | ns | [22] | ||
| Taxus x media “Densiformis” | 0.28 | 2.45 | 12.55 | [25] | ||
| Viburnum x juddi | 1.62 | 0.916 | [23] | |||
| Picea pungens | 13.56 | 9.14 | [24] | |||
| Ilex aquifolium | 0.77 | ns | ns | [22] | ||
| Acer rubrum (Italy) | 0.61 | ns | ns | [22] | ||
| Robinia umbrellifera | 0.78 | ns | ns | [22] | ||
| ||||||
| Ilex crenata | 2.92 | 2.14 | [22] | |||
| Quercus fellus | 26.49 | ns | ns | [22] | ||
| Wisteria floribunda | 10.40 | ns | ns | [22] | ||
| Nandina domestica | 19.26 | ns | ns | [22] | ||
| Magnolia stellata | 10.26 | ns | ns | [22] | ||
| Cupressocyparis leylandii | 7.44 | ns | ns | [22] | ||
| Photinia x fraseri “Red robin” | 7.52 | ns | ns | [22] | ||
| Pinus pinea | 8.48 | ns | [22] | |||
3. Sustainable Ornamental Production and Trade Worldwide
3.1. Current Status in Certification and Legislation
3.2. Sustainability and Adaptation to Climate Change (CC)
3.3. Renewable Energy Sources (RES) and Other Innovative Automation Used in Ornamental Production
3.4. The Use of Recycled or Alternative Materials in Ornamental Production
3.5. Integrated Pest and Disease Management (IPDM)
3.6. Cultivation of Climate-Adopted or Native Ornamental Species
3.7. Sustainability within the Handling and Transport Chain
3.8. Sustainability Perception of Growers, Sellers, and Consumers
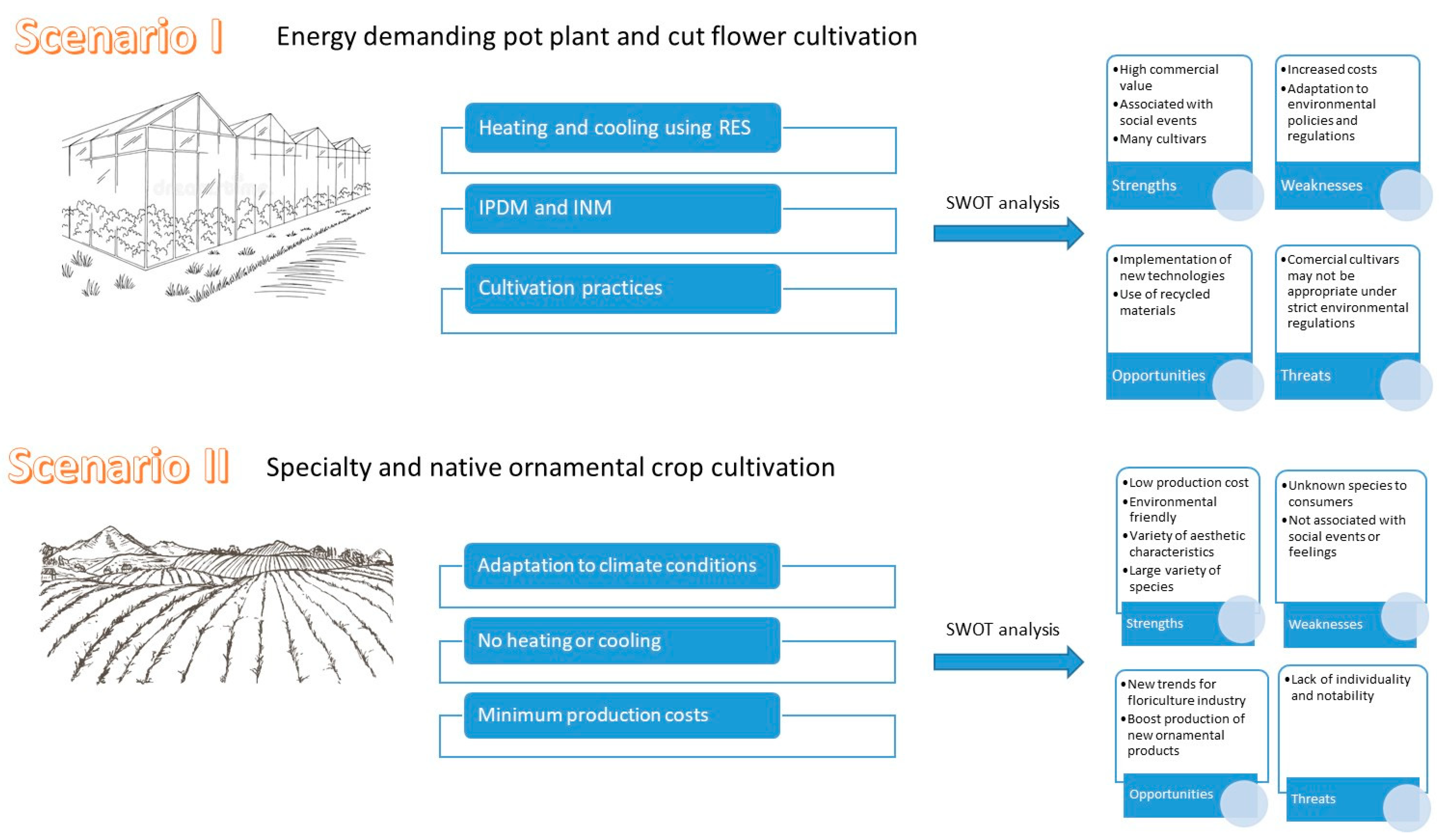
4. Implications for Sustainable Production—Two Possible Scenarios
5. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| LCA USDA | life cycle assessment US department of Agriculture |
| AD | abiotic depletion |
| AA | air acidification |
| EU | eutrophication |
| GW | global warming |
| GWP | global warming potential |
| PO | photochemical oxidation |
| GHG | greenhouse gas |
| OD | ozone depletion |
| HT | human toxicity |
| FAET | freshwater aquatic ecotoxicity |
| MAET | marine ecotoxicity |
| TE | terrestrial ecotoxicity |
| RES | renewable energy sources |
| CC | climate change |
| INM | integrated nutrient management |
| IPDM | integrated pest and disease management |
| TES: | thermal energy storage |
| MPS | Milieu Programma Sierteelt |
| OWC | olive waste compost |
| ASM | Acibenzolar-S-methyl |
| MeJA UV-C | methyl jasmonate ultraviolet-C |
| SWOT | strengths–weaknesses–opportunities–threats |
References
- Eurostat. Horticultural Products. Flowers and Ornamental Plants. Statistics 2006–2016; Eurostat: Luxembourg, 2017. [Google Scholar]
- USDA. Floriculture Crops 2015 Summary; USDA National Agricultural Statistics Service: Washington, DC, USA, 2016.
- Eurostat. Horticultural Products. Flowers and Ornamental Plants. Statistics 2017; Eurostat: Luxembourg, 2018. [Google Scholar]
- Xia, Y.; Deng, X.; Zhou, P.; Shima, K.; Da Silva, J.T. The world floriculture industry: Dynamics of production and markets. Flor. Ornam. Plant Biotech. 2006, 4, 336–347. [Google Scholar]
- Zhao, S.; Yue, C.; Meyer, M.H.; Hall, C.R. Factors affecting US consumer expenditures of fresh flowers and potted plants. HortTechnology 2016, 26, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Loyola, C.E.; Dole, J.M.; Dunning, R. North American Specialty Cut Flower Production and Postharvest Survey. HortTechnology 2019, 1, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Royal Floraholland Annual Report. 2019. Available online: www.floraholland.com (accessed on 5 May 2020).
- Royal Floraholland Annual Report. 2017. Available online: www.floraholland.com (accessed on 5 May 2020).
- Ingram, D.L.; Hall, C.R.; Knight, J. Understanding carbon footprint in production and use of landscape plants. HortTechnology 2019, 29, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.L.; Fernandez, R.T. Life cycle assessment: A tool for determining the environmental impact of horticultural crop production. HortTechnology 2012, 22, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Abeliotis, K.; Barla, S.A.; Detsis, V.; Malindretos, G. Life cycle assessment of carnation production in Greece. J. Clean. Prod. 2016, 112, 32–38. [Google Scholar] [CrossRef]
- Torrellas, M.; Antón, A.; Ruijs, M.; Victoria, N.G.; Stanghellini, C.; Montero, J.I. Environmental and economic assessment of protected crops in four European scenarios. J. Clean. Prod. 2012, 28, 45–55. [Google Scholar] [CrossRef]
- Sahle, A.; Potting, J. Environmental life cycle assessment of Ethiopian rose cultivation. Sci. Total Environ. 2013, 443, 163–172. [Google Scholar] [CrossRef]
- Franze, J.; Ciroth, A. A comparison of cut roses from Ecuador and the Netherlands. Int. J. Life Cycle Assessm. 2011, 16, 366–379. [Google Scholar] [CrossRef]
- Russo, G.; Buttol, P.; Tarantini, M. LCA (Life Cycle Assessment) of roses and cyclamens in greenhouse cultivation. Acta Hortic. 2008, 801, 359–366. [Google Scholar] [CrossRef]
- Blonk, H.; Kool, A.; Luske, B.; Ponsioen, T.; Scholten, J. Methodology for Assessing Carbon Footprints of Horticultural Products; Blonk Milieu Advies: Gouda, The Netherlands, 2010. [Google Scholar]
- Parrado, C.A.; Bojacá, C.R.; Schrevens, E. Exploring more sustainable technological alternatives for the greenhouse cut flowers industry in Colombia. Acta Hortic. 2011, 893, 1125–1132. [Google Scholar] [CrossRef]
- Wandl, M.T.; Haberl, H. Greenhouse gas emissions of small scale ornamental plant production in Austria—A case study. J. Clean. Prod. 2017, 141, 1123–1133. [Google Scholar] [CrossRef]
- Ingram, D.L.; Hall, C.R.; Knight, J. Global warming potential, variable costs, and water use of a model greenhouse production system for 11.4-cm annual plants using life cycle assessment. HortScience 2018, 53, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.L.; Hall, C.R.; Knight, J. Analysis of production system components of container-grown chrysanthemum for their impact on carbon footprint and variable costs using life cycle assessment. HortScience 2018, 53, 1139–1142. [Google Scholar] [CrossRef] [Green Version]
- Soode, E.; Weber-Blaschke, G.; Richter, K. Comparison of product carbon footprint standards with a case study on poinsettia (Euphorbia pulcherrima). Int. J. Life Cycle Assess. 2013, 18, 1280–1290. [Google Scholar] [CrossRef]
- Ingram, D.L.; Hall, C.R. Carbon footprint and related production costs of system components of a field-grown Cercis canadensis L. ‘Forest Pansy’ using life cycle assessment. J. Environ. Hort. 2013, 31, 169–176. [Google Scholar] [CrossRef]
- Lazzerini, G.; Lucchetti, S.; Nicese, F.P. Analysis of greenhouse gas emissions from ornamental plant production: A nursery level approach. Urban For. Urban Green. 2014, 13, 517–525. [Google Scholar] [CrossRef]
- Lazzerini, G.; Lucchetti, S.; Nicese, F.P. Green House Gases (GHG) emissions from the ornamental plant nursery industry: A Life Cycle Assessment (LCA) approach in a nursery district in central Italy. J. Clean. Prod. 2016, 112, 4022–4030. [Google Scholar] [CrossRef]
- Ingram, D.L.; Hall, C.R. Carbon footprint and related production costs of system components for a field-grown Viburnum× juddi using life cycle assessment. J. Env. Hort. 2014, 32, 175–181. [Google Scholar]
- Ingram, D.L. Life cycle assessment to study the carbon footprint of system components for Colorado blue spruce field production and use. J. Am. Soc. Hort. Sci. 2013, 138, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.R.; Ingram, D.L. Carbon footprint and production costs associated with varying the intensity of production practices during field-grown shrub production. HortScience 2015, 50, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.L.; Hall, C.R. Comparison of carbon footprint and variable costs of selected nursery production systems for a 5-cm-caliper red maple. HortScience 2016, 51, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Ingram, D.L. Life cycle assessment of a field-grown red maple tree to estimate its carbon footprint components. Int. J. Life Cycle Assessm. 2012, 17, 453–462. [Google Scholar] [CrossRef]
- Riisgaard, L. How the Market for Standards Shapes Competition in the Market for Goods: Sustainability Standards in the Cut Flower Industry. 2009. DIIS Working Paper. Available online: https://www.econstor.eu/handle/10419/44693 (accessed on 13 July 2020).
- Gruda, N.; Bisbis, M.; Tanny, J. Influence of climate change on protected cultivation: Impacts and sustainable adaptation strategies–A review. J. Clean. Prod. 2019, 225, 481–495. [Google Scholar] [CrossRef]
- Raza, A.; Ashraf, F.; Zou, X.; Zhang, X.; Tosif, H. Plant Adaptation and Tolerance to Environmental Stresses: Mechanisms and Perspectives. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 117–145. [Google Scholar]
- Corner, D. Positioning the Queensland Cut Flower Industry to Strategically Respond to the Impact of Climate Change. 2011. Available online: https://knowledge.flowersmagazine.com.au/wp-content/uploads/2018/11/pdf_file_3632.pdf (accessed on 22 June 2020).
- Surówka, E.; Rapacz, M.; Janowiak, F. Climate Change Influences the Interactive Effects of Simultaneous Impact of Abiotic and Biotic Stresses on Plants. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 1–50. [Google Scholar]
- Niu, G.; Rodriguez, D.S.; Wang, Y.T. Impact of drought and temperature on growth and leaf gas exchange of six bedding plant species under greenhouse conditions. HortScience 2006, 41, 1408–1411. [Google Scholar] [CrossRef]
- Vaid, T.M.; Runkle, E.S. Developing flowering rate models in response to mean temperature for common annual ornamental crops. Sci. Hort. 2013, 161, 15–23. [Google Scholar] [CrossRef]
- Hicklenton, P.R.; Newman, S.M.; Davies, L.J. Night temperature, photosynthetic photon flux, and long days affect Gypsophila paniculata flowering. HortScience 1993, 28, 888–890. [Google Scholar] [CrossRef] [Green Version]
- Warner, R.M.; Erwin, J.E. Prolonged high temperature exposure and daily light integral impact growth and flowering of five herbaceous ornamental species. J. Amer. Soc. Hort. Sci. 2005, 130, 319–325. [Google Scholar] [CrossRef]
- Blanchard, M.G.; Runkle, E.S. Quantifying the thermal flowering rates of eighteen species of annual bedding plants. Sci. Hort. 2011, 128, 30–37. [Google Scholar] [CrossRef]
- Hegde, S.; Umekawa, Y.; Watanabe, E.; Kasajima, I. High-Temperature Tolerance of Flowers. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 343–371. [Google Scholar]
- Tubby, K.V.; Webber, J.F. Pests and diseases threatening urban trees under a changing climate. For. Int. J. For. Res. 2010, 83, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.; Kapoor, D.; Sharma, P.; Bakshi, P.; Sharma, P.; Saini, P.; Ohri, P.; Mir, B.A.; Kaur, R.; Bhardwaj, R. Plant-Microbe Interactions under Adverse Environment. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Springer: Singapore, 2020; pp. 717–751. [Google Scholar]
- Bisaglia, C.; Cutini, M.; Romano, E.; Fedrizzi, M.; Menesatti, P.; Santoro, G.; Frangi, P.; Minuto, G.; Tinivella, F.; Miccolis, V.; et al. Trends and perspectives for the optimal use of energy in ornamental plant production and distribution in Italy. Acta Hortic. 2008, 801, 795–802. [Google Scholar] [CrossRef]
- Vadiee, A.; Martin, V. Energy management in horticultural applications through the closed greenhouse concept, state of the art. Renew. Sustain. Energy Rev. 2012, 16, 5087–5100. [Google Scholar] [CrossRef]
- Mugnozza, G.S.; Russo, G.; De Lucia Zeller, B. LCA methodology application in flower protected cultivation. Acta Hortic. 2007, 761, 625–632. [Google Scholar] [CrossRef]
- Russo, G.; Scarascia Mugnozza, G.; De Lucia Zeller, B. Environmental improvements of greenhouse flower cultivation by means of LCA methodology. Acta Hortic. 2008, 801, 301–308. [Google Scholar] [CrossRef]
- Papafotiou, M.; Phsyhalou, M.; Kargas, G.; Chatzipavlidis, I.; Chronopoulos, J. Olive-mill wastes compost as growing medium component for the production of poinsettia. Sci. Hort. 2004, 102, 167–175. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kargas, G.; Lytra, I. Olive-mill waste compost as a growth medium component for foliage potted plants. HortScience 2005, 40, 1746–1750. [Google Scholar] [CrossRef] [Green Version]
- Grigatti, M.; Giorgioni, M.E.; Ciavatta, C. Compost-based growing media: Influence on growth and nutrient use of bedding plants. Biores. Technol. 2007, 98, 3526–3534. [Google Scholar] [CrossRef]
- Tzavara, S.; Darras, A.; Assimakopoulou, A. Tobacco dust waste as an alternative medium to grow geranium (Pelargonium x hortorum) plants. Adv. Hort. Sci. 2019, 33, 295–298. [Google Scholar]
- Papafotiou, M.; Chronopoulos, J.; Kargas, G.; Voreakou, M.; Leodaritis, N.; Lagogiani, O.; Gazi, S. Cotton gin trash compost and rice hulls as growing medium components for ornamentals. J. Hort. Sci. Biotech. 2001, 76, 431–435. [Google Scholar] [CrossRef]
- Younis, A.; Riaz, A.; Mushtaq, N.; Tahir, Z.; Siddique, M.I. Evaluation of the suitability of sewage and recycled water for irrigation of ornamental plants. Commun. Soil Sci. Plant Anal. 2015, 46, 62–79. [Google Scholar] [CrossRef]
- Bonaguro, J.E.; Coletto, L.; Samuele, B.; Zanin, G.; Sambo, P. Environmental impact in floriculture: LCA approach at farm level. Acta Hortic. 2016, 1112, 419–424. [Google Scholar] [CrossRef]
- Toumi, K.; Vleminckx, C.; van Loco, J.; Schiffers, B. Pesticide residues on three cut flower species and potential exposure of florists in Belgium. Int. J. Environ. Res. Pub. Health 2016, 13, 943. [Google Scholar] [CrossRef] [Green Version]
- Ruett, M.; Whitney, C.; Luedeling, E. Model-based evaluation of management options in ornamental plant nurseries. J. Clean. Prod. 2020, 12, 26–53. [Google Scholar] [CrossRef]
- Gardener, B.B.M.; Fravel, D.R. Biological control of plant pathogens: Research, commercialization and application in the USA. Plant Health Prog. 2002, 10, 1094. [Google Scholar] [CrossRef] [Green Version]
- Thacker, J.R. An Introduction to Arthropod Pest Control; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Darras, A.I. Postharvest Disease Management. In Handbook of Florists’ Crop Diseases (HFCD); McGovern, R., Elmer, W., Eds.; Springer Publishing: New York, NY, USA, 2018. [Google Scholar]
- Cook, R.J. Making greater use of introduced microorganisms for biological control of plant pathogens. Annu. Rev. Phytopathol. 1993, 31, 53–80. [Google Scholar] [CrossRef]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instr. 2006. [Google Scholar] [CrossRef] [Green Version]
- Darras, A.Ι. Novel elicitors induce defence responses in cut flowers. In Plant Pathology; Cumagan, C.J.R., Ed.; InTech Publishing: Rijeka, Croatia, 2012. [Google Scholar]
- Weiss, D. Introduction of new cut flowers: Domestication of new species and introduction of new traits not found in commercial varieties. In Breeding for Ornamentals: Classical and Molecular Approaches; Springer: Dordrecht, The Netherlands, 2002; pp. 129–137. [Google Scholar]
- Heywood, V. Conservation and sustainable use of wild species as sources of new ornamentals. Acta Hortic. 2003, 598, 43–53. [Google Scholar] [CrossRef]
- Maloupa, E.; Grigoriadou, K.; Zervaki, D.; Papanastassi, K. Management of the Balkan native flora for sustainable floricultural commercial use. Acta Hortic. 2005, 683, 189–196. [Google Scholar] [CrossRef]
- Johnston, M.E.; Joyce, D. The centre for native floriculture: Progress and opportunities. Acta Hortic. 2009, 813, 279–284. [Google Scholar] [CrossRef]
- Reinten, E.; van Wyk, B.E. Floriculture industry benefits from southern African floral biodiversity. Acta Hortic. 2018, 1201, 659–664. [Google Scholar] [CrossRef]
- De Pascale, S.; Romano, D. Potential use of wild plants in floriculture. Acta Hortic. 2019, 1240, 87–98. [Google Scholar] [CrossRef]
- Ratnayake, K.; Joyce, D. Native Australian Acacias: Unrealised ornamental potential. Chron. Hortic. 2010, 50, 19–22. [Google Scholar]
- Cunningham, A.B.; Garnett, S.T.; Gorman, J. Policy lessons from practice: Australian bush products for commercial markets. GeoJournal 2009, 74, 429. [Google Scholar] [CrossRef]
- Halevy, A.H. Introduction of native Israeli plants as new cut flowers. Acta Hortic. 2000, 541, 79–82. [Google Scholar] [CrossRef]
- Chimonidou, D.; Vlahos, J.C.; Odysseos, M.; Georgiou, K.; Della, A. Evaluation of species from Cyprus flora for sustainable use in commercial floriculture. Acta Hortic. 2005, 683, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Darras, A.I.; Kargakou, V. Postharvest physiology and handling of cut Spartium junceum inflorescences. Sci. Hort. 2019, 252, 130–137. [Google Scholar] [CrossRef]
- Royal Flora Holland. Facts and Figures. Available online: www.floraholland.com (accessed on 22 June 2020).
- Behe, B.K.; Campbell, B.L.; Hall, C.R.; Khachatryan, H.; Dennis, J.H.; Yue, C. Consumer preferences for local and sustainable plant production characteristics. HortScience 2013, 48, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.; Khachatryan, H.; Behe, B.; Dennis, J.; Hall, C. Consumer perceptions of eco-friendly and sustainable terms. Agric. Res. Econ. Rev. 2015, 44, 21–34. [Google Scholar] [CrossRef]
- Berki-Kiss, D.; Menrad, K. Consumer preferences of sustainability labeled cut roses in Germany. Sustainability 2019, 11, 3358. [Google Scholar] [CrossRef] [Green Version]
- Dennis, J.H.; Lopez, R.G.; Behe, B.K.; Hall, C.R.; Yue, C.; Campbell, B.L. Sustainable production practices adopted by greenhouse and nursery plant growers. HortScience 2010, 45, 1232–1237. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Type of Cultivation or Process * | CO2 Output (kg CO2 eq kg−1) | Contribution to CO2 Output (%) | ||
|---|---|---|---|---|
| >20% | 5–20% | <5% | ||
| Protected, heated with air transport | 3–32 | Energy use in the greenhouse; Peat substrate; Air transport | Substrate materials; Packaging materials; Transport; Cooling and storage; Propagating material | Pesticides; Fertilizers |
| Protected, heated without air transport | 1–32 | Energy use in the greenhouse; Peat substrate | Substrate materials (non-peat); packing materials; Transport; cooling and storage; Propagation material | Pesticides; Fertilizers |
| Field with land transport | 0.1–27 | Substrate materials (peat and non-peat); Transport; Propagation material | Pesticides; Fertilizers | Liner; irrigation; Preparation and planting |
| Protected, unheated in soil, with air transport | 0.1–2.5 | Peat substrate; Propagation material; Air transport; | Packaging; Energy use on farm; Transport; Cooling and storage | Pesticides; Fertilizers |
| Protected, unheated in soil, without air transport | 0.2–2.5 | Cooling and storage; Peat substrate; | Packaging materials; Energy; Transport; Propagation materials; Pesticides; Fertilizers | |
| Field, without air transport, processed | 0.1–0.3 | Transport; Energy use on farm | Packaging materials; Cooling and storage; Pesticides; Fertilizers | |
| Plant Material | Production Stage | Inputs | Outputs * |
|---|---|---|---|
| Production and Delivery | Construction materials, fertilizers, agrochemicals, substrates, propagation materials | AD, AA, EU, GW, PO |
| Cultivation | Fuel, electricity, water, fertilizers, agrochemicals | AD, AA, EU, GW, PO | |
| Postharvest Handling | Water, electricity, packaging materials | AD, GW, PO | |
| Transportation | Fuel | AA, GW, PO | |
| Production and Delivery | Construction materials, fertilizers, agrochemicals, substrates, propagation materials | AD, AA, EU, GW, PO |
| Cultivation | Fuel, electricity, water, fertilizers, agrochemicals | AD, AA, EU, GW, PO | |
| Packaging and Handling | Packaging materials, fuel | AD, GW, PO | |
| Transportation | Fuel | AA, GW, PO | |
| Production and delivery | Construction materials, fertilizers, agrochemicals, growing materials, propagation materials | AD, AA, EU, GW, PO |
| Cultivation | Electricity, water, fertilizers, agrochemicals | AD, AA, EU, GW, PO | |
| Transportation | Fuel | AA, GW, PO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darras, A.I. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy 2020, 10, 1570. https://doi.org/10.3390/agronomy10101570
Darras AI. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy. 2020; 10(10):1570. https://doi.org/10.3390/agronomy10101570
Chicago/Turabian StyleDarras, Anastasios I. 2020. "Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review" Agronomy 10, no. 10: 1570. https://doi.org/10.3390/agronomy10101570




