Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endophytic Bacteria
2.2. Plant Material
2.3. Pot Experiment
2.4. Plant Growth Parameters
2.5. Determination of Proline and Hydrogen Peroxide Levels
2.6. Statistical Analysis
3. Results
3.1. Salinity Tolerance of Bacterial Strains
3.2. Inoculation Effect on Germination and Growth Parameters
3.3. Inoculation Effect on Biochemical Parameters
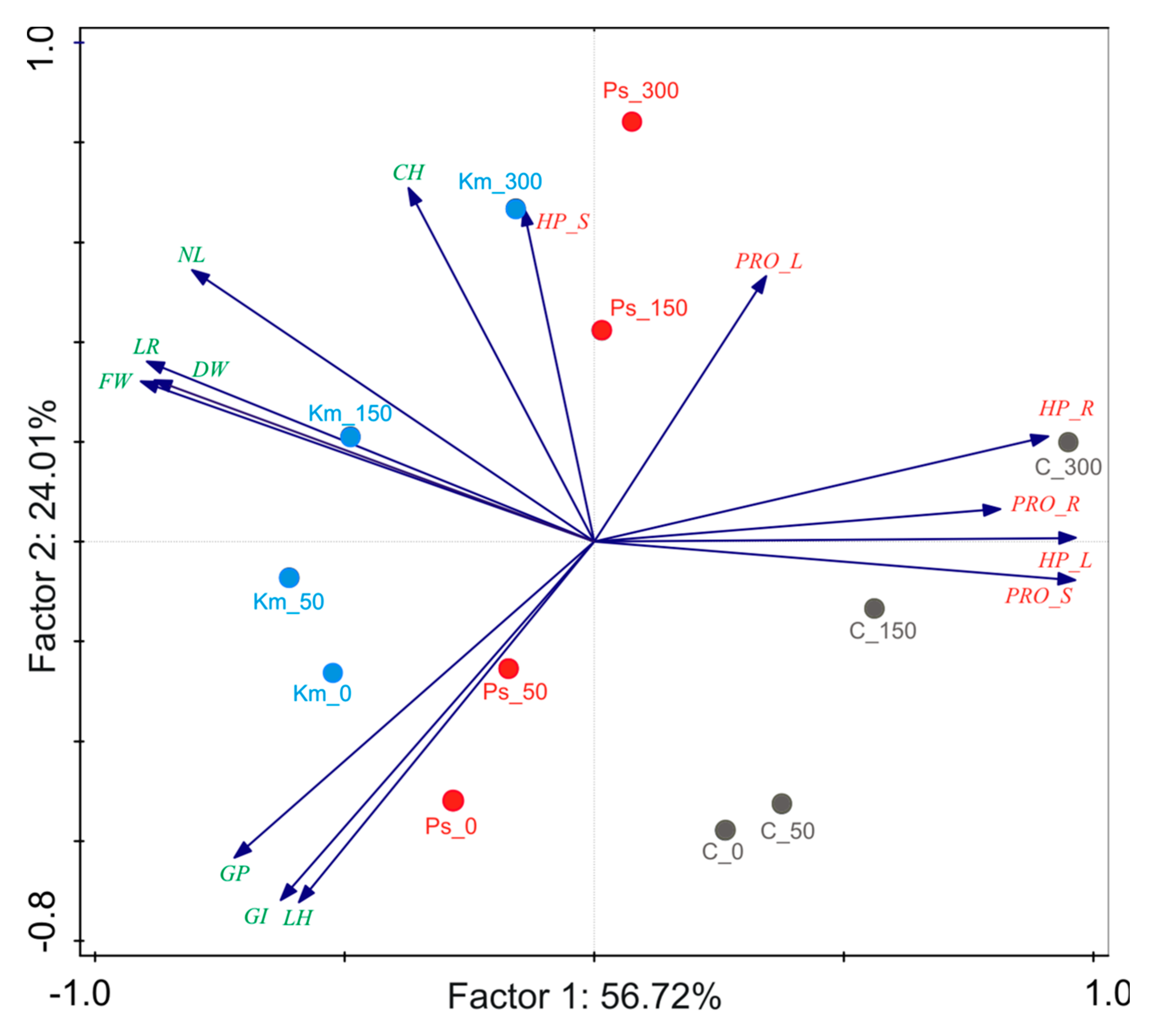
3.4. Comparison of the Strain Effects
4. Discussion
4.1. Effect of Salinity on the Growth and Biochemical Parameters of B. vulgaris
4.2. Effect of K. marisflavi CSE9 and P. stutzeri ISE12 Bioaugmentation on the Growth and Biochemical Parameters of B. vulgaris
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [PubMed] [Green Version]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AOB Plants 2016, 8, plw055. [Google Scholar] [PubMed] [Green Version]
- Abd Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interac. 2018, 13, 37–44. [Google Scholar]
- Kumar, M.; Kumar, R.; Jain, V.; Jain, S. Differential behavior of the antioxidant system in response to salinity induced oxidative stress in salt-tolerant and salt-sensitive cultivars of Brassica juncea L. Biocatal. Agric. Biotechnol. 2018, 13, 12–19. [Google Scholar]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar]
- Shin, W.; Siddikee, A.; Joe, M.M.; Benson, A.; Kim, K.; Selvakumar, G.; Kang, Y.; Jeon, S.; Samaddar, S.; Chatterjee, P.; et al. Halotolerant plant growth promoting bacteria mediated salinity stress amelioration in plants. Korean J. Soil Sci. Fert. 2016, 49, 355–367. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, V.P.; Prasad, S.M. Plant tolerance mechanizm against salt stress: The nutrient management approach. Biochem. Pharm. 2014, 3, 5. [Google Scholar]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Bioph. Res. Commun. 2018, 495, 286–291. [Google Scholar]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar]
- Kapadia, G.J.; Rao, G.S. Anticancer effects of red beet pigments. In Red Beet Biotechnology: Metabolites for Food and Pharmaceutical Applications, 1st ed.; Neelwarne, B., Ed.; Springer: New York, NY, USA, 2013; pp. 124–154. [Google Scholar]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Piernik, A.; Hrynkiewicz, K.; Wojciechowska, A.; Szymańska, S.; Lis, M.I.; Muscolo, A. Effect of halotolerant endophytic bacteria isolated from Salicornia europaea L. on the growth of fodder beet (Beta vulgaris L.) under salt stress. Arch. Agron. Soil Sci. 2017, 63, 1404–1418. [Google Scholar] [CrossRef]
- Sairam, R.; Tyagi, A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004, 86, 407–421. [Google Scholar]
- Bybordi, A. The influence of salt stress on seed germination, growth and yield of canola cultivars. Not. Bot. Horti Agrob. 2010, 38, 128–133. [Google Scholar]
- Mane, A.V.; Deshpande, T.V.; Wagh, V.B.; Karadge, B.A.; Samant, J.S. A critical review on physiological changes associated with reference to salinity. IJEST 2011, 6, 1192–1216. [Google Scholar]
- Alavi, M.H.; Ranjbar, G.A. Effects of different levels of salinity on germination, proline contents and a-, b- chlorophylls in rapessed (Brassica napus L.). IJACS 2012, 4, 1055–1059. [Google Scholar]
- Maurya, V.K.; Gothandam, K.M. Factors influencing the salt stress tolerance in plants—An overview. Res. J. Biotech. 2014, 9, 79–88. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Schmitt, F.J.; Renger, G.; Friedrich, T.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Los, D.A.; Kuznetsov, V.V.; Allakhverdiev, S.I. Reactive oxygen species: Reevaluation of generation, monitoring and role in stress-signalingin phototrophic organisms. BBA 2014, 1837, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omicstechnologies; Iqbal, N., Nazar, R., Khan, N.A., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011, 33, 797–802. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Wu, G.H.; Tian, C.Y. Isolation of endophytic plant growth-promoting bacteria associated with the halophyte Salicornia europaea and evaluation of their promoting activity under salt stress. Curr. Microbiol. 2016, 73, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, S.; Dąbrowska, G.; Tyburski, J.; Niedojadło, K.; Piernik, A.; Hrynkiewicz, K. Boosting the Brassica napus L. tolerance to salinity by the halotolerant strain Pseudomonas stutzeri ISE12. Environ. Exp. Bot. 2019, 163, 55–68. [Google Scholar] [CrossRef]
- Batra, P.; Barkodia, M.; Ahlawat, U.; Sansanwal, R.; Sharma, T.; Wati, L. Endophytes: An environmental friendly bacteria for plant growth promotion. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1899–1911. [Google Scholar] [CrossRef] [Green Version]
- Reyad, A.M.M.; Radwan, T.E.E.; Hemida, K.A.; Al-Qassem, N.A.A.; Ali, R.M. Salt tolerant endophytic bacteria from carthamus tinctorius and their role in plant salt tolerance improvement. Int. J. Curr. Sci. Res. 2017, 3, 1467–1488. [Google Scholar]
- Szymańska, S.; Płociniczak, T.; Piotrowska-Seget, Z.; Hrynkiewicz, K. Endophytic and rhizosphere bacteria associated with the roots of the halophyte Salicornia europaea L.—Community structure and metabolic potential. Microbiol. Res. 2016, 192, 37–51. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 36–39. [Google Scholar]
- Richardson, A.D.; Duigan, P.S.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Method for detrmination of proline in plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar] [PubMed]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Anderson, J.A. Catalase activity, hydrogen peroxide content and thermotolerance of pepper leaves. Sci. Hortic. 2002, 95, 277–284. [Google Scholar] [CrossRef]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Rahneshan, Z.; Nasibi, F.; Moghadam, A.A. Effects of salinity stress on some growth, physiological, biochemical parameters and nutrients in two pistachio (Pistacia vera L.) rootstocks. J. Plant Interact. 2018, 1, 73–82. [Google Scholar]
- Ashraf, M.; Athar, H.R.; Harris, P.J.C.; Kwon, T.R. Some prospective strategies for improving crop salt tolerance. Adv. Agron. 2008, 97, 45–110. [Google Scholar]
- Bhatt, M.J.; Patel, A.D.; Bhatti, P.M.; Pandey, A.N. Effect of soil salinity on growth, water status and nutrient accumulation in seedlings of Ziziphus mauritiana (Rhamnaceae). J. Fruit Ornam. Plant Res. 2008, 16, 383–401. [Google Scholar]
- Bacarin, M.A.; Deuner, S.; Silva, F.S.P.; Cassol, D.; Silva, D.M. Chlorophyll a fluorescence as indicative of the salt stress on Brassica napus L. Braz. J. Plant Physiol. 2011, 23, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Ambede, J.G.; Netondo, G.W.; Mwai, G.N.; Musyimi, D.M. NaCl salinity affects germination, growth, physiology, and biochemistry of bambara groundnut. Braz. J. Plant Physiol. 2012, 24, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). BJM 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozema, J.; Cornelisse, D.; Zhang, Y.; Li, H.; Bruning, B.; Katschnig, D.; Broekman, R.; Ji, B.; van Bodegom, P. Comparing salt tolerance of beet cultivars and their halophytic ancestor: Consequences of domestication and breeding programmes. Aob Plants 2015, 7, plu083. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.O.; de Silva, Ê.F.; Klar, A.E. Yield of beet cultivars under fertigation management and salinity control in a protected environment. Chil. J. Agric. Res. 2016, 76, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Saeidi, M.; Abdoili, M.; Azhand, M. Effect of foliar application of indole-3-acetic acid (IAA) at the beginning of grain growth (cell division) stage on agronomic characteristics and seedling growth parameters of two bread wheat under water and salinity stresses. Int. J. Biosci. 2014, 5, 244–255. [Google Scholar]
- Ghoulam, C.; Fares, K. Effect of salinity on seed germination and early seedling growth of sugar beet (Beta vulgaris L.). Seed Sci. Technol. 2001, 29, 357–364. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance—Current assessment. J. Irrig. Drain. Div. ASCE 1977, 103, 115–134. [Google Scholar]
- Ramoliya, P.J.; Patel, H.M.; Pandey, A.N. Effect of salinization of soil on growth and nutrient accumulation in seedlings of Prosopis Cineraria. J. Plant Nutr. 2006, 29, 283–303. [Google Scholar] [CrossRef]
- Wu, G.Q.; Liang, N.; Feng, R.J.; Zhang, J.J. Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol. Plant. 2013, 35, 2665–2674. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wheeler, R.M.; Levine, L.H.; Stutte, G.W. Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J. Plant Physiol. 2001, 158, 767–776. [Google Scholar] [CrossRef]
- Harinasut, P.; Srisunak, S.; Pitukchaisopol, S.; Charoensataporn, R. Mechanisms of adaptation to increasing salinity of mulberry: Proline content and ascorbate peroxidase activity in leaves of multiple stems. Sci. Asia 2000, 26, 207–211. [Google Scholar] [CrossRef]
- Nazarbeygi, E.; Yazdi, H.L.; Naseri, R.; Soleimani, R. The Effects of different levels of salinity on proline and a-, b- chlorophylls in canola. AEJAES 2011, 10, 70–74. [Google Scholar]
- Mehr, Z.S. Salt-induced changes in germination and vegetative stages of Anethum graveolens L. J. Stress Physiol. Biochem. 2013, 9, 189–198. [Google Scholar]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt Stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C. Change in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [Green Version]
- Kong-ngern, K.; Bunnag, S.; Theerakulpisut, P. Proline, hydrogen peroxide, membrane stability and antioxidant enzyme activity as potential indicators for salt tolerance in rice (Oryza sativa L.). Int. J. Bot. 2012, 8, 54–65. [Google Scholar]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Hernandez, M.; Fernandez-Garcia, N.; Diaz-Vivancos, P.; Olmos, E. A different role for hydrogen peroxide and the antioxidative system under short and long salt stress in Brassica oleracea roots. J. Exp. Bot. 2010, 61, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2018, 38, 650–668. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Egamberdieva, D. The role of phytohormone producing bacteria in alleviating salt stress in crop plants. In Biotechnological Techniques of Stress Tolerance in Plants; Miransari, M., Ed.; Stadium Press: Houston, TX, USA, 2013; pp. 21–39. [Google Scholar]
- El-Metwally, I.M.; Ali, O.A.M.; Abdelhamid, M.T. Response of wheat (Triticum aestivum L.) and associated grassy weeds grown in salt-affected soil to effects of graminicides and indole acetic acid. Agriculture 2015, 61, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Torre, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Isolation of plant-growth-promoting and metal-resistant cultivable bacteria from Arthrocnemum macrostachyum in the odiel marshes with potential use in phytoremediation. Mar. Pollut. Bull. 2016, 110, 133–142. [Google Scholar] [CrossRef]
- Yasmin, H.; Naeem, S.; Bakhtawar, M.; Jabeen, Z.; Nosheen, A.; Naz, R.; Keyani, R.; Mumtaz, S.; Hassan, M.N. Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. PLoS ONE 2020, 15, e0231348. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial soil bacterium Pseudomonas frederiksbergensis OS261 augments salt tolerance and promotes red pepper plant growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Ros Barceló, A. Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann. Bot. 1998, 82, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Bacilio, M.; Moreno, M.; Bashan, Y. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil Ecol. 2016, 107, 394–404. [Google Scholar] [CrossRef]
- Kumar, K.; Amaresan, N.; Madhuri, K. Alleviation of the adverse effect of salinity stress by inoculation of plant growth promoting rhizobacteria isolated from hot humid tropical climate. Ecol. Eng. 2017, 102, 361–366. [Google Scholar] [CrossRef]
- Deepalaxmi, R.K.; Gayathri, C. Screening of halophilic microorganisms (Oceanobacillus oncorhynchi and Pseudomonas stutzeri) for the effect of plant growth promotion and its formulation as a biofertilizer. SSRG Int. J. Agric. Environ. Sci. 2018, 5, 2394–2568. [Google Scholar]
- Lami, M.J.; Adler, C.; Caram-Di Santo, M.C.; Zenoff, A.M.; de Cristobal, R.E.; Espinosa-Urgel, M.; Vincent, P.A. Pseudomonas stutzeri MJL19, a rhizosphere-colonizing bacterium that promotes plant growth under saline stress. J. Appl. Microbiol. 2020, 1364–5072. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Marasco, R.; Rolli, E.; Barbato, M.; Cherif, H.; Guesmi, A.; Ouzari, I.; Daffonchio, D.; Borin, S. Potential for plant growth promotion of rhizobacteria associated with Salicornia growing in Tunisian hypersaline soils. BioMed Res. Int. 2013, 2013, 248078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Ruiz, M.D.R.; Font-Verdera, F.; Diaz-Gil, C.; Urdiain, M.; Rodríguez-Valdecantos, G.; González, B.; Orfila, A.; Rosselló-Móra, R. Moderate halophilic bacteria colonizing the phylloplane of halophytes of the subfamily Salicornioideae (Amaranthaceae). Syst. Appl. Microbiol. 2015, 38, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and characterization of a phosphate-solubilizing halophilic bacterium Kushneria sp. YCWA18 from Daqiao Saltern on the coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, 615032. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymańska, S.; Tyburski, J.; Piernik, A.; Sikora, M.; Mazur, J.; Katarzyna, H. Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes. Agronomy 2020, 10, 1571. https://doi.org/10.3390/agronomy10101571
Szymańska S, Tyburski J, Piernik A, Sikora M, Mazur J, Katarzyna H. Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes. Agronomy. 2020; 10(10):1571. https://doi.org/10.3390/agronomy10101571
Chicago/Turabian StyleSzymańska, Sonia, Jarosław Tyburski, Agnieszka Piernik, Marcin Sikora, Justyna Mazur, and Hrynkiewicz Katarzyna. 2020. "Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes" Agronomy 10, no. 10: 1571. https://doi.org/10.3390/agronomy10101571
APA StyleSzymańska, S., Tyburski, J., Piernik, A., Sikora, M., Mazur, J., & Katarzyna, H. (2020). Raising Beet Tolerance to Salinity through Bioaugmentation with Halotolerant Endophytes. Agronomy, 10(10), 1571. https://doi.org/10.3390/agronomy10101571





