Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions and Crop Management
2.2. Soil Salinity
2.3. Growth and Yield Measurements
2.4. Physiological Parameters
2.5. Mineral Analysis
2.6. Statistical Analysis
3. Results
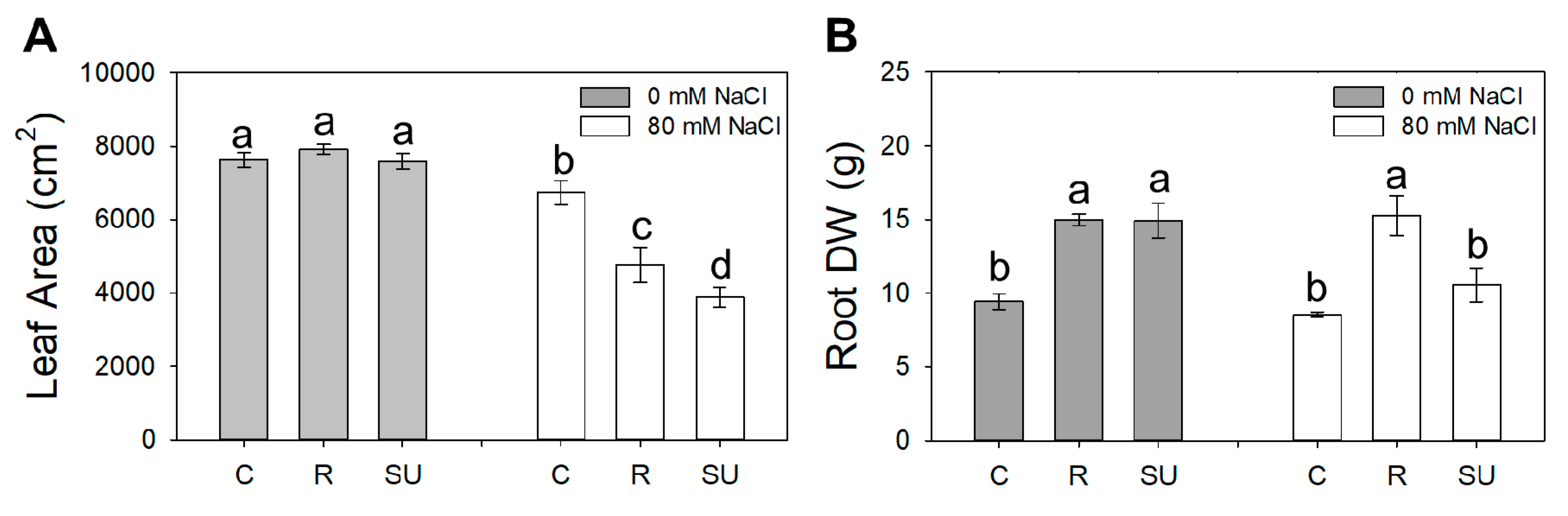
3.1. Biomass Components
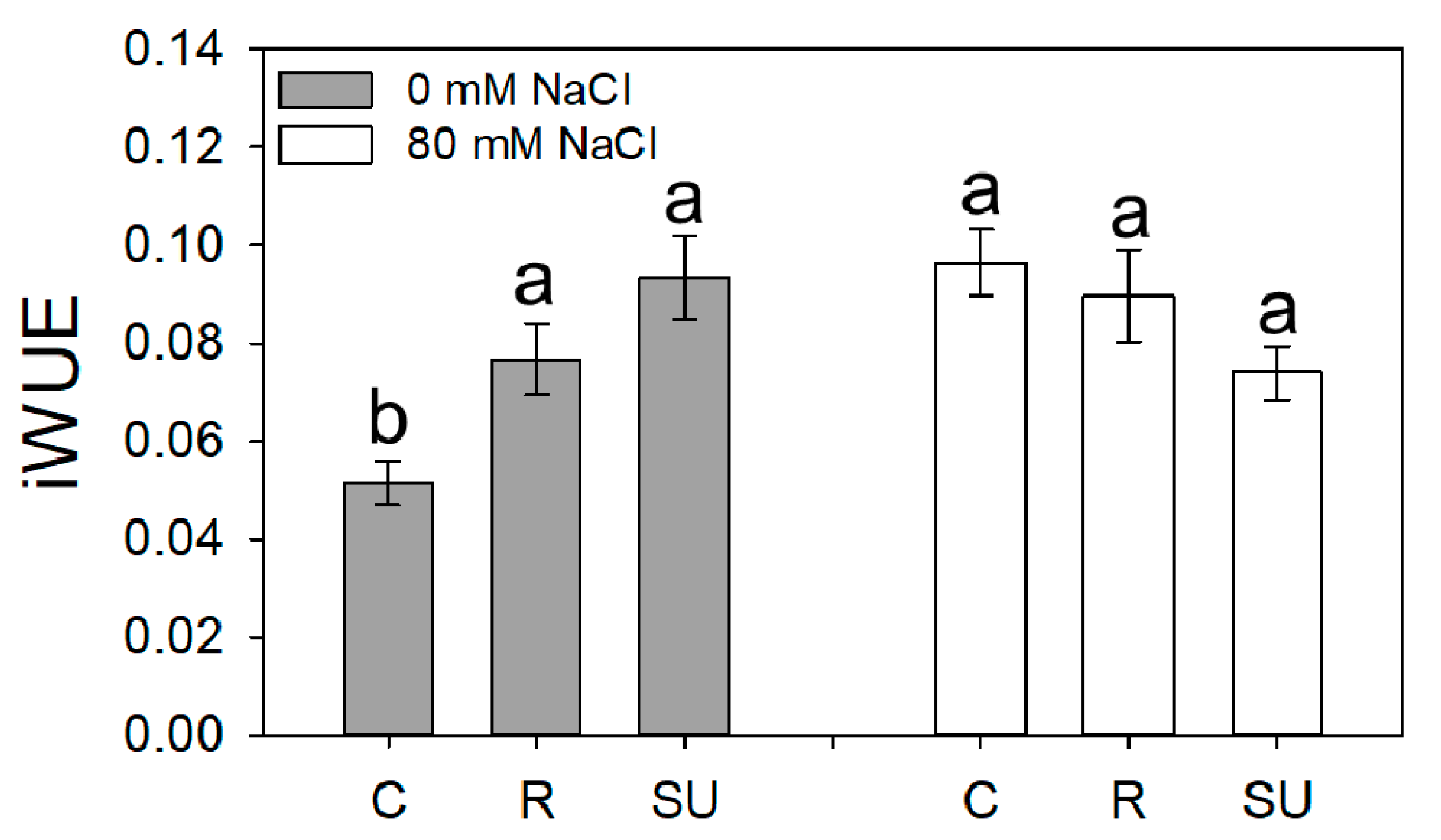
3.2. Leaf Gas Exchanges
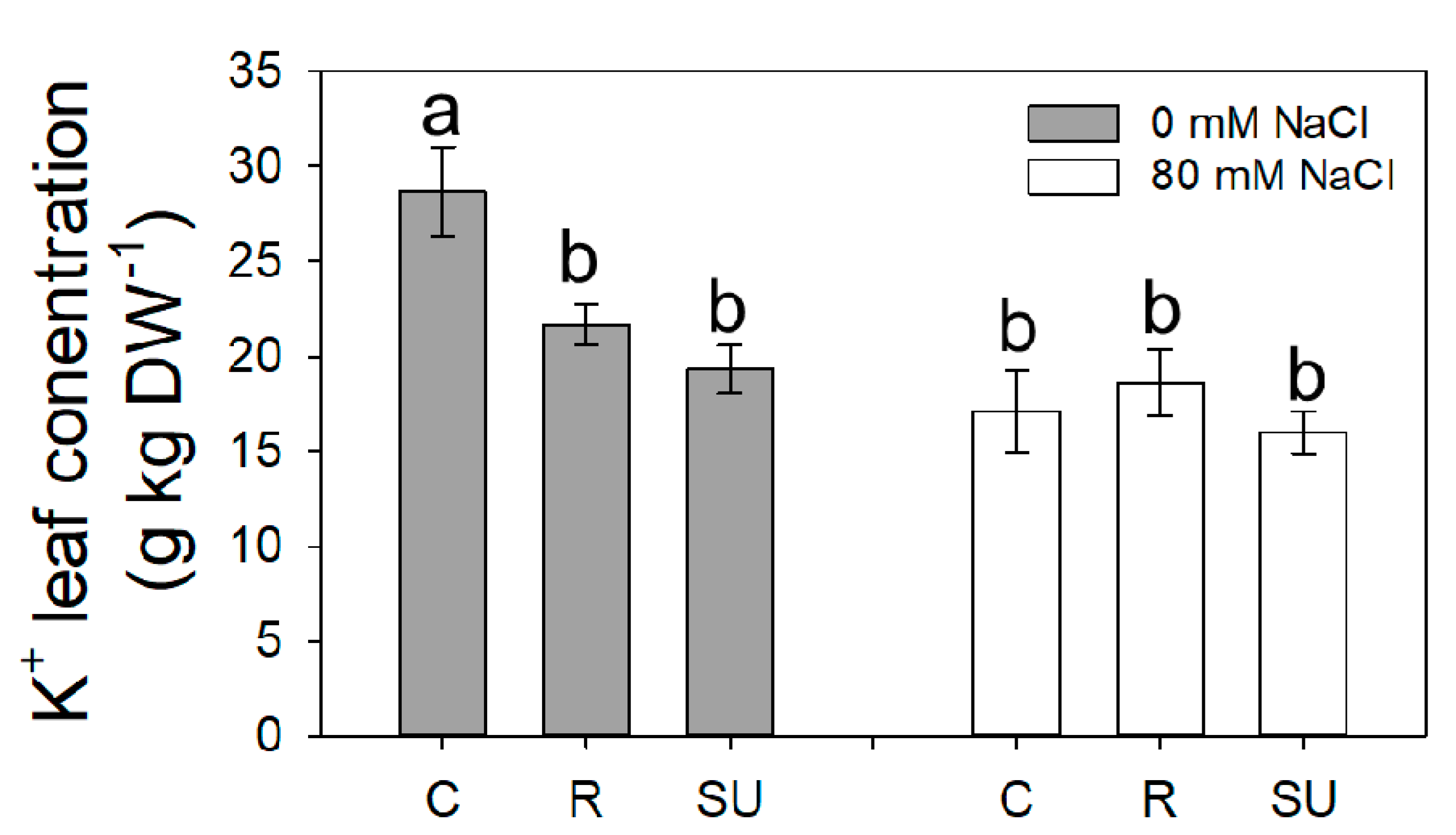
3.3. Leaf and Fruit Ions Content
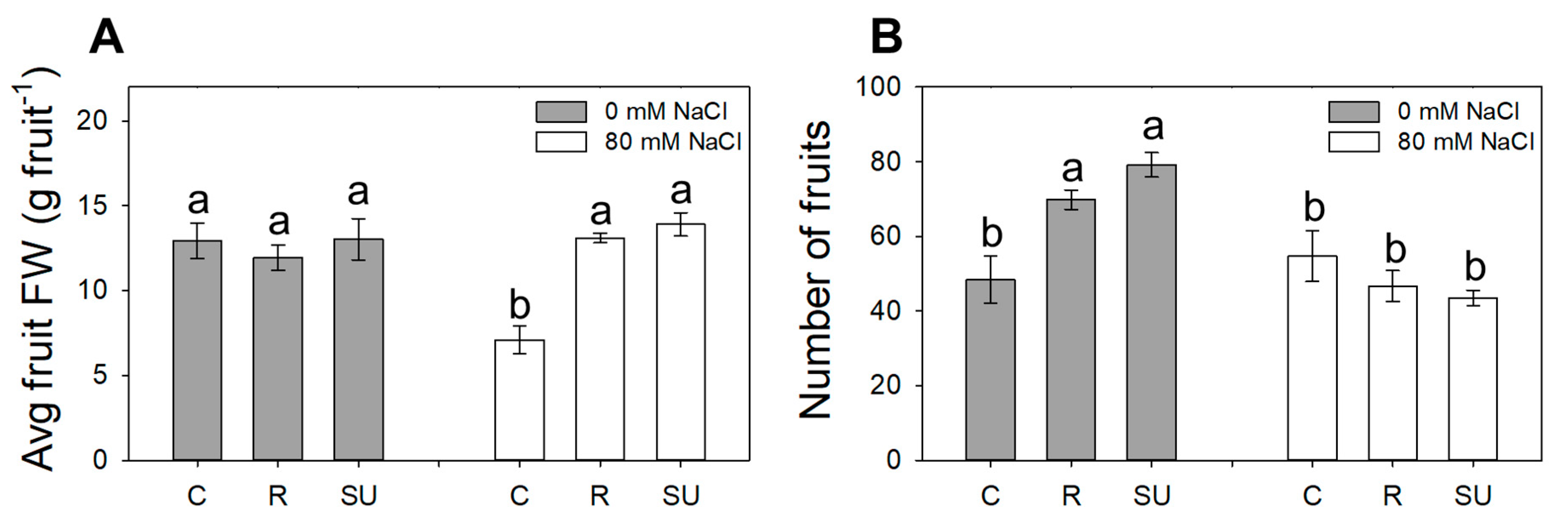
3.4. Yield Components
4. Discussion
4.1. Biostimulants Determine Stress Preadaptation
4.2. Biomass Redistribution Facilitates Stress Adaptation
4.3. Plant Biostimulants Mediate Nutrient Use Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libutti, A.; Monteleone, M. Irrigation management in Mediterranean salt affected agriculture: How leaching operates. Ital. J. Agron. 2012, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, P.; Bressan, R.; Zhu, J.; Bohnert, H. Plant cellular and molecular response to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, M.K.; Bhuiyan, S.I.; Franco, D.T. Soil salinity reduction and prediction of salt dynamics in the coastal ricelands of Bangladesh. Agric. Water Manag. 2001, 47, 9–23. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kavi Kishor, P.B. Engineering salinity tolerance in plants: Progress and prospects. Planta 2020, 251, 1–29. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Plaut, Z.; Edelstein, M.; Ben-Hur, M. Overcoming Salinity Barriers to Crop Production Using Traditional Methods. CRC Crit. Rev. Plant Sci. 2013, 32, 250–291. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- El Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattner, S.W.; Wite, D.; Riches, D.A.; Porter, I.J.; Arioli, T. The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol. Agric. Hortic. 2013, 29, 258–270. [Google Scholar] [CrossRef]
- Elansary, H.O.; Skalicka-Woźniak, K.; King, I.W. Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol. Biochem. 2016, 105, 310–320. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak®) and polyamines on nutrient-deprived (N, P and K) okra seedlings. Sci. Hortic. 2013, 151, 142–146. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Repiso, C.; Hennequart, F.; Nolasco, J.B.; Liu, F. Biostimulants enhance growth and drought tolerance in Arabidopsis thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Kawatra, M.; Kaur, K.; Kaur, G. Effect of osmo priming on sucrose metabolism in spring maize, during the period of grain filling, under limited irrigation conditions. Physiol. Mol. Biol. Plants 2019, 25, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Bourioug, M.; Ezzaza, K.; Bouabid, R.; Alaoui-Mhamdi, M.; Bungau, S.; Bourgeade, P.; Alaoui-Sossé, L.; Alaoui-Sossé, B.; Aleya, L. Influence of hydro- and osmo-priming on sunflower seeds to break dormancy and improve crop performance under water stress. Environ. Sci. Pollut. Res. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hakeem, A.; Liu, Y.; Zhang, L.; Si, T.; Jiang, D. Induction of osmotic stress resistance by seed osmo-priming in winter wheat (Triticum aestivum L.) during post-germinative stages. Seed Sci. Technol. 2017, 45, 485–498. [Google Scholar] [CrossRef]
- Lemmens, E.; Deleu, L.J.; De Brier, N.; De Man, W.L.; De Proft, M.; Prinsen, E.; Delcour, J.A. The Impact of Hydro-Priming and Osmo-Priming on Seedling Characteristics, Plant Hormone Concentrations, Activity of Selected Hydrolytic Enzymes, and Cell Wall and Phytate Hydrolysis in Sprouted Wheat (Triticum aestivum L.). ACS Omega 2019, 4, 22089–22100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabassum, T.; Farooq, M.; Ahmad, R.; Zohaib, A.; Wahid, A. Seed priming and transgenerational drought memory improves tolerance against salt stress in bread wheat. Plant Physiol. Biochem. 2017, 118, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.V.; Van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef] [PubMed]
- Mouradi, M.; Bouizgaren, A.; Farissi, M.; Latrach, L.; Qaddoury, A.; Ghoulam, C. Seed osmopriming improves plant growth, nodulation, chlorophyll fluorescence and nutrient uptake in alfalfa (Medicago sativa L.)–rhizobia symbiosis under drought stress. Sci. Hortic. 2016, 213, 232–242. [Google Scholar] [CrossRef]
- Pawar, V.A.; Laware, S.L. Seed Priming A Critical Review. Int. J. Sci. Res. Biol. Sci. 2018, 5, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Abebe, S.N. Effect of hydro and osmo priming on yield and yield components of Chickpea (Cicer arietinum L.). Afr. J. Agric. Res. 2016, 11, 3027–3036. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.S.S.; Selby, C.; Carmichael, E.; McRoberts, C.; Rao, J.R.; Ambrosino, P.; Chiurazzi, M.; Pucci, M.; Martin, T. Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chem. Biol. Technol. Agric. 2016, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Muthu-Pandian Chanthini, K.; Senthil-Nathan, S.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Sivanesh, H.; Sundar, N.S.; Palanikani, R.; Soranam, R. Chaetomorpha antennina (Bory) Kützing derived seaweed liquid fertilizers as prospective bio-stimulant for Lycopersicon esculentum (Mill). Biocatal. Agric. Biotechnol. 2019, 20, 101190. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2015. [Google Scholar]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouari, M.; Hassena, A.; Trabelsi, L.; Rouina, B.; Decou, R.; Labrousse, P. Exogenous Proline-Mediated Abiotic Stress Tolerance in Plants: Possible Mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Gholami Zali, A.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crops Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Haswell, E.S.; Verslues, P.E. The ongoing search for the molecular basis of plant osmosensing. J. Gen. Physiol. 2015, 145, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Roycewicz, P.; Malamy, J.E. Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Condon, A.G. Drying times: Plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 2020, 71, 2239–2252. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Guo, R.; Shi, D.; Liu, B.; Lin, X.; Yang, C. Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 2012, 12, 194. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Li, S.; Min, W.; Ye, J.; Hou, Z. Ionomic and transcriptomic analyses of two cotton cultivars (Gossypium hirsutum L.) provide insights into the ion balance mechanism of cotton under salt stress. PLoS ONE 2019, 14, e0226776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzen, B.; Brix, H.; Mendelssohn, I.A.; McKee, K.L.; Miao, S.L. Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquat. Bot. 2001, 70, 117–133. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Palta, J.; Whisson, K.; Peoples, M.B. Yield and nitrogen use efficiency of wheat increased with root length and biomass due to nitrogen, phosphorus, and potassium interactions. J. Plant Nutr. Soil Sci. 2018, 181, 364–373. [Google Scholar] [CrossRef]
- Chen, L.; Liao, H. Engineering crop nutrient efficiency for sustainable agriculture. J. Integr. Plant Biol. 2017, 59, 710–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Mao, Z.; Zhang, J.; Hemat, M.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Osmolyte accumulation plays important roles in the drought priming induced tolerance to post-anthesis drought stress in winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2019, 166, 103804. [Google Scholar] [CrossRef]
- Cirillo, V.; Masin, R.; Maggio, A.; Zanin, G. Crop-weed interactions in saline environments. Eur. J. Agron. 2018, 99, 51–61. [Google Scholar] [CrossRef]
- De Pascale, S.; Orsini, F.; Caputo, R.; Palermo, M.A.; Barbieri, G.; Maggio, A. Seasonal and multiannual effects of salinisation on tomato yield and fruit quality. Funct. Plant Biol. 2012, 39, 689–698. [Google Scholar] [CrossRef]

| Shoot FW | R/S | Leaf Area | Root DW | |
|---|---|---|---|---|
| g | cm2 | g | ||
| Biostimulant (B) | ||||
| Control | 877.1 a | 0.08 b | 6821 a | 8.99 b |
| Rygex | 718.7 b | 0.11 a | 6663 a | 15.1 a |
| Super Fifty | 693.2 b | 0.13 a | 5897 b | 12.7 a |
| Salinity (S) | ||||
| S0 | 879.6 a | 0.11 | 7918 a | 13.11 a |
| S80 | 646.4 b | 0.11 | 5002 b | 11.46 b |
| Significance | ||||
| B | *** | ** | * | *** |
| S | *** | ns | *** | * |
| BxS | ns | ns | * | * |
| Pn | gs | Ψleaf | iWUE | |
|---|---|---|---|---|
| µmol m−2 s−1 | mmol m−2 | MPa | ||
| Biostimulant (B) | ||||
| Control | 6.68 b | 109.7 | −1.32 | 0.071 |
| Rygex | 8.56 a | 108.4 | −1.51 | 0.081 |
| Super Fifty | 8.76 a | 104.7 | −1.53 | 0.084 |
| Salinity (S) | ||||
| S0 | 8.81 a | 124.8 a | −1.21 a | 0.074 |
| S80 | 7.17 b | 91.52 b | −1.69 b | 0.082 |
| Significance | ||||
| B | ** | ns | ns | ns |
| S | ** | * | *** | ns |
| BxS | ns | ns | * | * |
| Leaf | Fruit | |||||
|---|---|---|---|---|---|---|
| Na+ | NO3− | K+ | K+ | Ca2+ | Mg2+ | |
| g kg−1 DW | g kg−1 DW | g kg−1 DW | g kg−1 DW | g kg−1 DW | g kg−1 DW | |
| Biostimulant (B) | ||||||
| Control | 2.67 | 3.72 a | 22.9 a | 36.1 a | 0.46 a | 1.49 a |
| Rygex | 2.78 | 1.94 b | 20.1 a | 28.9 b | 0.30 b | 1.15 b |
| Super Fifty | 2.16 | 1.95 b | 17.6 b | 30.7 b | 0.33 b | 1.29 b |
| Salinity (S) | ||||||
| S0 | 0.43 b | 1.49 b | 23.20 a | 31.78 | 0.35 | 1.32 |
| S80 | 4.31 a | 3.59 a | 17.24 b | 32.02 | 0.38 | 1.29 |
| Significance | ||||||
| B | ns | * | * | * | * | * |
| S | *** | ** | *** | ns | ns | ns |
| BxS | ns | ns | * | ns | ns | ns |
| Fruits FW | Harvest Index | Firmness | Average Fruit FW | Number of Fruits | |
|---|---|---|---|---|---|
| (g) | (kg cm−1) | (g fruit−1) | (#) | ||
| Biostimulant (B) | |||||
| Control | 486.1 b | 0.25 b | 1.49 a | 10.01 b | 51.5 |
| Rygex | 722.8 a | 0.35 a | 0.91 b | 12.52 a | 58.2 |
| Super Fifty | 826.7 a | 0.37 a | 0.52 c | 13.47 a | 61.3 |
| Salinity (S) | |||||
| S0 | 828.9 a | 0.52 | - | 12.64 | 65.8 a |
| S80 | 528.3 b | 0.48 | - | 11.37 | 48.3 b |
| Significance | |||||
| B | *** | ** | *** | *** | ns |
| S | *** | ns | - | ns | *** |
| BxS | ns | ns | - | *** | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy 2020, 10, 1559. https://doi.org/10.3390/agronomy10101559
Di Stasio E, Cirillo V, Raimondi G, Giordano M, Esposito M, Maggio A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy. 2020; 10(10):1559. https://doi.org/10.3390/agronomy10101559
Chicago/Turabian StyleDi Stasio, Emilio, Valerio Cirillo, Giampaolo Raimondi, Maria Giordano, Marco Esposito, and Albino Maggio. 2020. "Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants" Agronomy 10, no. 10: 1559. https://doi.org/10.3390/agronomy10101559
APA StyleDi Stasio, E., Cirillo, V., Raimondi, G., Giordano, M., Esposito, M., & Maggio, A. (2020). Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy, 10(10), 1559. https://doi.org/10.3390/agronomy10101559






