Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Design
- (1)
- Control—plants were not treated with biostimulators or foliar fertilizers.
- (2)
- Titanit.
- (3)
- Optysil.
- (4)
- Metalosate potassium.
- (5)
- Rooter.
- (6)
- Bolero Mo.
- (7)
- Adob Zn IDHA.
- (8)
- Adob B.
- (9)
- Adob 2.0 Mo.
2.2. Weather Conditions
2.3. Soil Sampling for Biochemical Analysis
2.4. Soil Enzymatic Activity
- –
- Dehydrogenases (EC 1.1.1.)—with 1% TTC (triphenyltetrazolium chloride) as a substrate after 24-h incubation at 30 °C, at a wavelength of 485 nm, expressed as μmol triphenylformazan (TPF) 24 h−1 g−1dm of soil; colorimetry measurements [21];
- –
- Acid and alkaline phosphatase (EC 3.1.3.2)—with sodium p-nitrophenyl phosphate as a substrate after 1-h incubation at 37 °C, at a wavelength of 400 nm; expressed as μmol para-nitrophenol (PNP) h−1 g−1dm of soil (Novospac spectrophotometer); spectrophotometry measurements [22];
- –
- Catalase (EC 1.11.1.6)—with 0.3% hydrogen peroxide as a substrate after 20 min incubation at room temperature (about 20 °C); titrated with 0.02 M potassium permanganate until its colour was light pink; expressed as μmol H2O2 min−1 g−1dm of soil; manometrically measurements [23].
2.5. Biological Index of Fertility (BIF)
2.6. Statistical Analyses
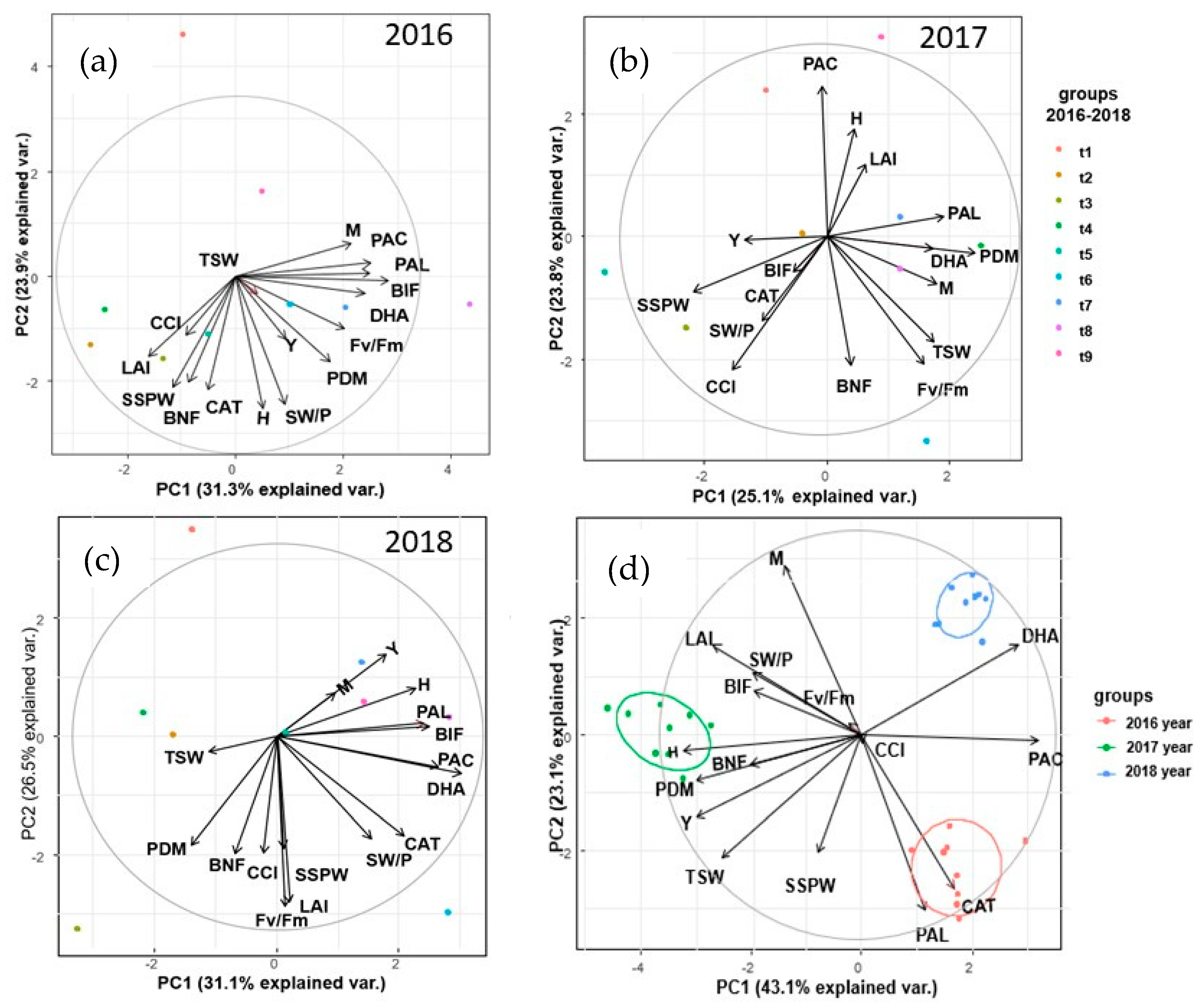
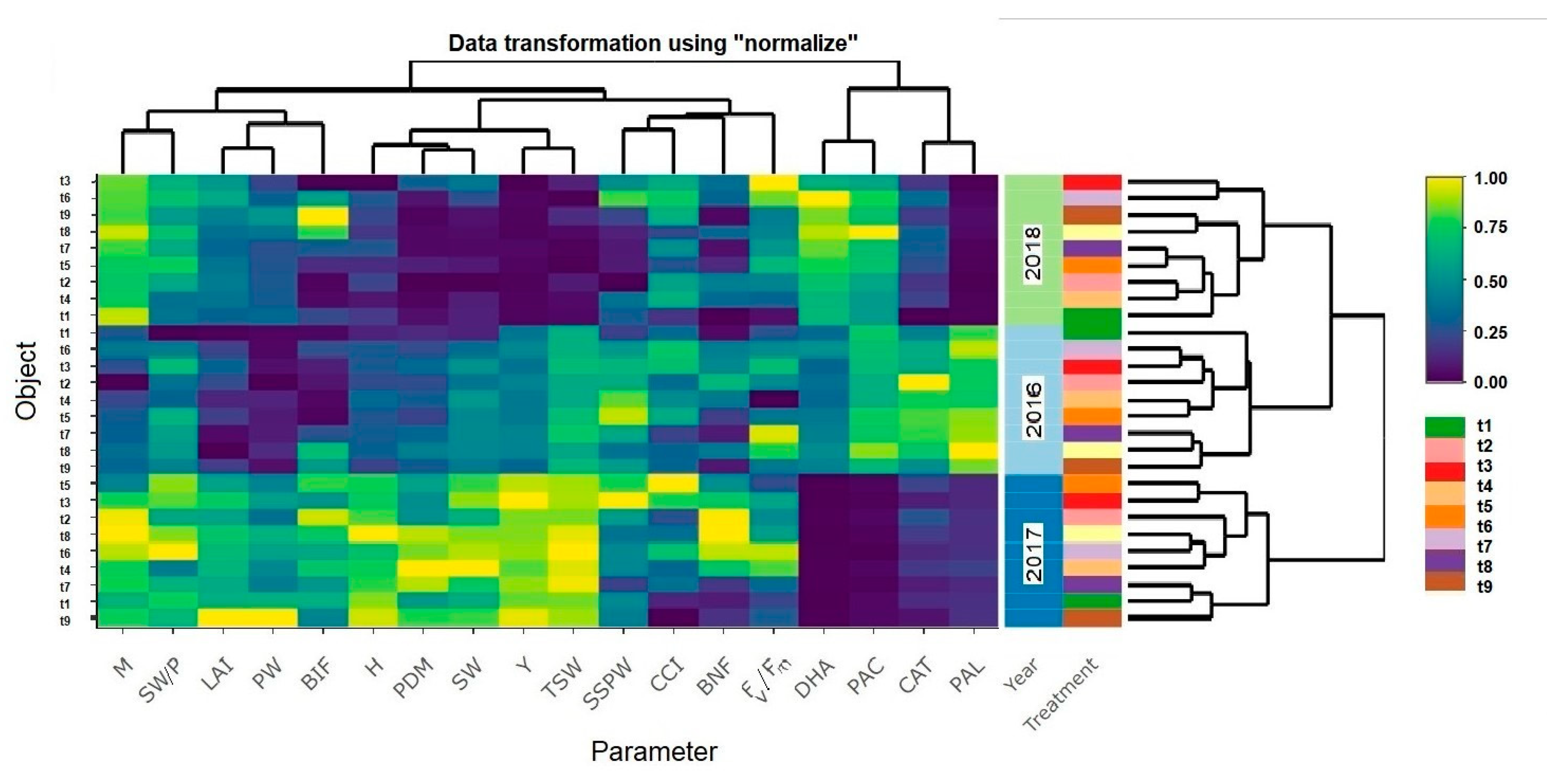
3. Results
3.1. Yield, Biometric and Physiological Traits of the Pea Plants
3.2. Biological Nitrogen Fixation
3.3. Soil Enzymatic Activity
4. Discussion
4.1. Yield, Biometric and Physiological Traits
4.2. Biological Nitrogen Fixation
4.3. Biochemical Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Daun, J.K. Effect of variety and crude protein content on nutrients and certain antinutrients in field peas (Pisum sativum). J. Sci. Food Agric. 2004, 84, 1021–1029. [Google Scholar] [CrossRef]
- N’Dayegamiye, A.; Whalen, J.K.; Tremblay, G.; Nyiraneza, J.; Grenier, M.; Drapeau, A.; Bipfubusa, M. The benefits of legume crops on corn and wheat yield, nitrogen nutrition, and soil properties improvement. Agron. J. 2015, 107, 1653–1665. [Google Scholar] [CrossRef]
- Michalk, K. The legal framework for short rotation coppice in Germany in the context of the ‘greening’of the EU’s common agricultural policy. In Bioenergy from Dendromass for the Sustainable Development of Rural Areas; Wiley-VCH: Hoboken, NJ, USA, 2015; pp. 367–374. [Google Scholar]
- Hillocks, R.J. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Vermeulen, S.J.; Aggarwal, P.K.; Ainslie, A.; Angelone, C.; Campbell, B.M.; Challinor, A.J.; Lau, C. Options for support to agriculture and food security under climate change. Environ. Sci. Policy 2012, 15, 136–144. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Flexas, J.; Medrano, H. Prospects for crop production under drought: Research priorities and future directions. Ann. App. Biol. 2005, 147, 211–226. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 26, 2049. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sekara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. Ann. 2010, 22, 9–13. [Google Scholar] [CrossRef]
- Ferreira, M.I.; Lourens, A.F. The efficacy of liquid seaweed extract on the field canola plants. S. Afr. J. Plant Soil 2002, 19, 159–161. [Google Scholar] [CrossRef]
- Sultana, V.; Ehteshamul-Haque, S.; Ara, J.; Athar, M. Comparative efficacy of Brown, Green and red Seaweeds in the control of Root infesting fungi and okra. Int. J. Environ. Sci. Technol. 2005, 21, 75–81. [Google Scholar]
- Piotrowska-Dlugosz, A.; Charzynski, P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the ekranic technosols of Toruń (Poland). J. Soil Sediment. 2015, 15, 47–59. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, S.M. Functional aspects of plant growth promoting Rhizobacteria: Recent Advancements. Insight Microbiol. 2011, 1, 39–54. [Google Scholar] [CrossRef]
- Sulewska, H.; Ratajczak, K.; Panasiewicz, K.; Kalaji, H.M. Can pyraclostrobin and epoxiconazole protect conventional and stay-green maize varieties grown under drought stress? PLoS ONE 2019, 14, e0221116. [Google Scholar] [CrossRef]
- Strażyński, P.; Mrówczyński, M. (Eds.) Metodyka Integrowanej Ochrony i Produkcji Grochu dla Doradców; Instytut Ochrony Roślin-Państwowy Instytut Badawczy: Poznań, Poland, 2016. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007. [Google Scholar]
- Molga, M. Agrometeorology; PWRiL: Warsaw, Poland, 1986. (In Polish) [Google Scholar]
- Sawicka, A. The ecological aspects of dinitrogen fixation. In Annals of the University of Agriculture in Poznan; Dissertations Scientific: Poznań, Poland, 1983; Volume 134. [Google Scholar]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenase aktivität im Boden mittels triphenytetrazoliumchlorid (TTC). Landwirtsch Forsch 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil 1. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Stefanic, F.; Ellade, G.; Chirnageanu, J. Researches concerning a biological index of soil fertility. In Proceedings of the 5th Symposium of Soil Biology, Bucharest, Romania, 28 June–3 July 1981; Nemes, M.P., Kiss, S., Papacostea, P., Stefanic, C., Rusan, M., Eds.; Romanian National Society of Soil Science: Bucharest, Romania, 1984; pp. 35–45. [Google Scholar]
- COBORU. Available online: http://www.coboru.pl/PlikiWynikow/14_2016_WPDO_12_GRS.pdf (accessed on 27 May 2020).
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Growth components and zinc recovery efficiency of upland rice genotypes. Pesq. Agropec. Bras. 2005, 40, 1211–1215. [Google Scholar] [CrossRef][Green Version]
- Duffy, B. Zinc and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D.M., Eds.; APS Press: St. Paul, MN, USA, 2007; pp. 155–175. [Google Scholar]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Waraczewska, Z.; Budka, A. The Influence of Bio-Stimulants and Foliar Fertilizers on Yield, Plant Features, and the Level of Soil Biochemical Activity in White Lupine (Lupinus albus L.) Cultivation. Agronomy 2020, 10, 150. [Google Scholar] [CrossRef]
- Raj, A.B.; Raj, S.K. Zinc and boron nutrition in pulses: A review. J. App. Nat. Sci. 2019, 11, 673–679. [Google Scholar] [CrossRef]
- Sulewska, H.; Ratajczak, K. Chemical composition of selected preparations supporting plant development and evaluation of their activity in soybean cultivation. Chem. Indus. 2017, 96, 1352–1355. [Google Scholar]
- Kuniya, N.; Chaudhary, N.; Patel, S. Effect of sulphur and zinc application on growth, yield attributes, yield and quality of summer cluster bean [Cyamopsis tetragonoloba (L.)] in light textured soil. IJCS 2018, 6, 1529–1532. [Google Scholar]
- Hassanein, M.S.; Shalaby, M.A.F.; Rashad, E.M. Improving growth and yield of some faba bean cultivars by using some plant growth promoters in newly cultivated land. Ann. Agric. Sci. Moshtohor. 2000, 38, 2141–2155. [Google Scholar]
- Tobbal, Y.F.M. Physiological Studies on the Effect of Some Nutrients and Growth Regulators on Plant Growth and Metabolism. Ph.D. Thesis, Faculty of Science Al-Azhar University, Cairo, Egypt, 2006. [Google Scholar]
- El-Sallami, I.H.; Gad, M.M. Growth and flowering responses of New York aster (Aster novibelgii L.) to a slow release fertilizer and foliar applied zinc. Assuit J. Agric. Sci. 2005, 36, 121–136. [Google Scholar]
- Mostafavi, K. Grain yield and yield components of soybean upon application of different micronutrient foliar fertilizers at different growth stages. Int. J. Agric. Res. Rev. 2012, 2, 389–394. [Google Scholar]
- Gomaa, A.O. Studies on the response of Matthiola incana plants to some growth conditions. 2-In Field: Effect of foliar spray with zinc sulphate, calcium and paclobutrazol on growth and flowering. Egypt. J. Appl. Sci. 2003, 18, 291–318. [Google Scholar]
- Hansch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant. Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Brkić, S.; Milaković, Z.; Kristek, A.; Antunović, M. Pea yield and its quality depending on inoculation, nitrogen and molybdenum fertilization. Plant Soil Environ. 2004, 50, 39–45. [Google Scholar] [CrossRef]
- Omer, F.A.; Abbas, D.N.; Khalaf, A.S. Effect of molybdenum and potassium application on nodulation, growth and yield of lentil (Lens culinaris MEDIC). Pak. J. Bot. 2016, 48, 2255–2259. [Google Scholar]
- Madny, A.E.M. Response of Some Field Crops Grown under Newly Reclaimed Soil Conditions to Boron Fertilization. Ph.D. Thesis, Faculty of Science Al-Azhar University, Cairo, Egypt, 2004. [Google Scholar]
- Shaaban, M.M.; Abdalla, F.E.; Abou El-Nour, E.A.A.; El-Saady, A.M. Boron nitrogen interaction effect on growth and yield of faba bean plants grown under sandy soil conditions. Int. J. Agric. Res. 2006, 1, 322–330. [Google Scholar]
- Sulewska, H.; Ratajczak, K.; Niewiadomska, A.; Koziara, W.; Panasiewicz, K.; Faligowska, A. Preparaty zawierające tytan, krzem, bor, cynk i molibden w uprawie łubinu białego i grochu siewnego. Przem chem. 2018, 97, 1182–1185. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.S.; Pandey, A.C. Effect of seed soaking in nitrogen, phosphorus, potassium and boron on growth yield of garden pea (Pisum sativum L.). Ecol. Environ. Conserv. 2006, 14, 383–386. [Google Scholar]
- Moghazy, A.M.; Saed, S.M.E.; Awad, E.M.S. The influence of boron foliar spraying with compost and mineral fertilizers on growth, green pods and seed yield of pea. Nat. Sci. 2014, 12, 50–57. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Zobel, R.W. Yield, nutrient uptake and soil chemical properties as influenced by liming and boron application in common bean in a No–Tillage system. Commun. Soil Sci. Plan. 2007, 38, 1637–1653. [Google Scholar] [CrossRef]
- Subasinghe, S.; Dayatilake, G.A.; Senaratne, R. Effect of B, Co and Mo on nodulation, growth and yield of cowpea (Vigna unguiculata). Trop. Agric. Res. Ext. 2003, 6, 108–112. [Google Scholar]
- Bonilla, I.; Perez, H.; Cassab, G.; Lara, M.; Sanchez, F. The effect of boron deficiency on development in determinate nodules: Changes in cell wall pectin contents and nodule polypeptide expression. In Boron in Soils and Plants. Developments in Plant and Soil Sciences; Bell, R.W., Rerkasem, B., Eds.; Springer: Dordrecht, The Netherlands, 1997; Volume 76. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar]
- Ross, J.R.; Slaton, N.A.; Brye, K.R.; Delong, R.E. Boron fertilization influences on soybean yield and leaf and seed born concentration. Agron. J. 2006, 98, 198–205. [Google Scholar] [CrossRef]
- Malinowska, E.; Kalembasa, S. The yield and content of Ti, Fe, Mn, Cu in celery leaves (Apium graveolens L. var. dulce mill. pers.) as a result of Tytanit application. Acta Sci. Pol. Hortorum Cultus 2012, 11, 69–80. [Google Scholar]
- Grenda, A. Tytanit—An activator of metabolic processes. Chem. Sustain. Agric. 2003, 4, 263–269. [Google Scholar]
- Sacała, E. Role of silicon in plant resistance to water stress. J. Elementol. 2009, 14, 619–630. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kuchcińska, K. The effect of silicon foliar fertilization in sugar beet–Beta vulgaris (L.) ssp. vulgaris conv. crassa (Alef.) prov. altissima (Döll). Turk. J. Field Crops 2015, 20, 115–119. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Römheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, K.; Zielony, T. Effect of Goteo treatment on yield and fruit quality of tomato grown on rock wool. In Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops; Dąbrowski, Z.T., Ed.; Editorial House Wieś Jutra: Warsaw, Poland, 2008; pp. 21–26. [Google Scholar]
- Gajc-Wolska, J. The Influence of Grafting and Biostimulators on the Yield and Fruit Quality of Greenhouse Tomato CV. (Lycopersicon esculentum Mill.) Grown in the Field. Veg. Crops Res. Bull. 2010, 72, 63–70. [Google Scholar] [CrossRef]
- Gajewski, M.; Gos, K.; Bobruk, J. The influence of Goëmar Goteo biostimulator on yield and quality of two Chinese cabbage cultivars. In Monographs Series: Biostimulators in Modern Agriculture: Vegetable Crops; Dąbrowski, Z.T., Ed.; Editorial House Wieś Jutra: Warsaw, Poland, 2008; pp. 21–27. [Google Scholar]
- Zboińska, M. W jaki sposób rośliny pobierają i asymilują azot? Eduk. Biol. Środowiskowa 2018, 2, 19–31. [Google Scholar]
- Weisany, W.; Raei, Y.; Allahverdipoor, K.H. Role of some of mineral nutrients in biological nitrogen fixation. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 77–84. [Google Scholar]
- Sládková, A.; Száková, J.; Havelcová, M.; Najmanová, J.; Tlustoš, P. The contents of selected risk elements and organic pollutants in soil and vegetation within a former military area. Soil Sediment. Contam. Int. J. 2015, 24, 325–342. [Google Scholar] [CrossRef]
- Kováčik, P.; Havrlentová, M.; Šimanský, V. Growth and Yield Stimulation of Winter Oilseed Rape (Brasssica Napus, L.) by Mg-Titanit Fertiliser. Agriculture 2014, 1, 132–141. [Google Scholar]
- Rousk, K.; Sorensen, P.L.; Michelsen, A. What drives biological nitrogen fixation in high arctic tundra: Moisture or temperature? Ecosphere 2018, 9, e02117. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Majchrzak, L.; Borowiak, K.; Wolna-Maruwka, A.; Waraczewska, Z.; Budka, A.; Gaj, R. The Influence of Tillage and Cover Cropping on Soil Microbial Parameters and Spring Wheat Physiology. Agronomy 2020, 10, 200. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; Intech: Rijeka, Croatia, 2020; Chapter 8; pp. 183–210. [Google Scholar]
- Januszek, K.; Błońska, E.; Długa, J.; Socha, J. Dehydrogenase activity of forest soils depends on the assay used. Int. Agrophys. 2014, 29, 47–59. [Google Scholar] [CrossRef]
- Brzezińska, M.; Włodarczyk, T.; Stępniewski, W.; Przywara, G. Soil oxygen status and catalase activity. Acta Agrophys. 2005, 5, 555–565. [Google Scholar]
- Bastida, F.; Kandeler, E.; Moreno, J.L.; Ros, M.; García, C.; Hernández, T. Application of Fresh and Composted Organic Wastes Modifies Structure, Size and Activity of Soil Microbial Community under Semiarid Climate. Appl. Soil Ecol. 2008, 40, 318–329. [Google Scholar] [CrossRef]
- Salazar, S.; Sanchez, L.; Alvarez, J.; Valverde, A.; Galindo, P.; Igual, J.; Peix, A.; Santa-Regina, I. Correlation Among Soil Enzyme Activities Under Different Forest System Management Practices. Ecol. Eng. 2011, 37, 1123–1131. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Perzyński, A.; Gałązka, R.; Księżak, J. Changes in Enzymatic Activities and Microbial Communities in Soil under Long-Term Maize Monoculture and Crop Rotation. Pol. J. Environ. Stud. 2017, 26, 39–46. [Google Scholar] [CrossRef]
- Siwik-Ziomek, A.; Szczepanek, M. Soil Extracellular Enzyme Activities and Uptake of N by Oilseed Rape Depending on Fertilization and Seaweed Biostimulant Application. Agronomy 2019, 9, 480. [Google Scholar] [CrossRef]
- Prashantha, G.M.; Prakash, S.S.; Umesha, S.; Chikkaramappa, T.; Subbarayappa, C.T.; Ramamurthy, V. Direct and residual effect of zinc and boron on soil enzyme activities at harvest in finger millet-groundnut cropping system. J. Pharm. Phytochem. 2019, 8, 2447–2451. [Google Scholar]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Schneider, K.; Turrion, M.B.; Grierson, P.F.; Gallardo, J.F. Phosphatase activity, microbial phosphorus, and fine root growth in forest soils in the Sierra de Gata, western Spain. Biol. Fert. Soils 2001, 34, 151–155. [Google Scholar] [CrossRef]
- Bielińska, E.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Aon, M.A.; Colaneri, A.C. Temporal and spatial evolution of enzymatic activities and physical-chemical properties in an agricultural soil. Appl. Soil Ecol. 2001, 18, 155–270. [Google Scholar]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 2, 1337. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, A.; Sulewska, H.; Wolna-Maruwka, A.; Ratajczak, K.; Głuchowska, K.; Waraczewska, Z.; Budka, A. An Assessment of the Influence of Co-Inoculation with Endophytic Bacteria and Rhizobia, and the Influence of PRP SOL and PRP EBV Fertilisers on the Microbial Parameters of Soil and Nitrogenase Activity in Yellow Lupine (Lupinus luteus L.) Cultivation. Pol. J. Environ. Stud. 2018, 6, 2687–2702. [Google Scholar] [CrossRef]
- Fukuda, T.; Osaki, M.; Shinano, T.; Wasaki, J. Cloning and Characterization of Two Secreted Acid Phosphatases from Rice Call, Plant Nutrition: Food Security and Sustainability of Agro-Ecosystems through Basic and Applied Research; Kluwer Academic Publisher: New York, NY, USA, 2001; pp. 34–35. [Google Scholar]
- Adetunji, A.T.; Lewu, F.B.; Mulidzi, R.; Ncube, B. The biological activities of β-glucosidase, phosphatase and urease as soil quality indicators: A review. J. Soil Sci. Plant. Nutr. 2017, 17, 794–807. [Google Scholar] [CrossRef]
- Chelikani, P.; Ramana, T.; Radhakrishnan, T.M. Catalase: A repertoire of unusual features. Indian J. Clin. Biochem. 2005, 20, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, Z.; Wolińska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef]
- Hupe, A.; Schulz, H.; Bruns, C.; Haase, T.; Heß, J.; Joergensen, R.G.; Wichern, F. Even flow? Changes of carbon and nitrogen release from pea roots over time. Plant Soil 2018, 431, 143–157. [Google Scholar] [CrossRef]

| Biostimulator and Foliar Fertilizer | Term and Dose of Biostimulators | Biostimulator and Fertilizer Characteristics | |
|---|---|---|---|
| Biostimulators | Tytanit | I: BBCH 13–14 II: BBCH 31–32 0.3 0.3 dm3 ha−1 | Liquid, mineral stimulant containing titanium (Ti). It increases the yield, volume and development of plants, improves yield quality parameters and increases plants’ natural resistance to stress. Composition: 8.5 g Ti (dm3)−1 |
| Rooter | BBCH 13–14 1 dm3 ha−1 | Biostimulator—it stimulates the growth of the root system, accelerates regeneration and improves the uptake of soil minerals. Composition: P2O5 13.0%; K2O 5.0% | |
| Foliar fertilizers | Optysil | I: BBCH 16–18 II: BBCH 52–55 III: BBCH 71–73 0.5 dm3 ha−1 | Liquid, silicon antistressor stimulating the growth and development of plants, activating their natural immune system, and increasing tolerance to unfavourable cultivation conditions. Composition: 200 g SiO2 (dm3)−1 |
| Metalosate potassium | I: BBCH 11–13 II: BBCH 18–20 III: BBCH 31–32 3 dm3 ha−1 | Liquid foliar fertilizer containing an easily absorbable form of potassium, which supplements potassium deficiency in plants with amino acids. Composition: K2O 24% | |
| Bolero Mo | BBCH 58 1.5 dm3 ha−1 | Liquid foliar fertilizer containing boron and molybdenum to supplement deficiency in plants. Composition: B 8.2%; Mo 0.8% | |
| Adob Zn IDHA | BBCH 58 1 dm3 ha−1 | Foliar fertilizer containing zinc (Zn) fully chelated by biodegradable chelating agent IDHA. Composition: Zn 100 g kg−1 (weight percentage content 10, chelated by IDHA) | |
| Adob B | BBCH 55–58 2 dm3 ha −1 | Liquid, highly concentrated foliar fertilizer containing boron that regulates auxin activity and participates in cell division. Composition: N 78 g kg−1; B 150 g kg−1 | |
| Adob 2.0 Mo | BBCH 11–13 0.15 dm3 ha −1 | Liquid, single-component fertilizer which increases the rate and efficiency of use of nitrogen by plants and improves interaction with iron. Composition: Mo 20% | |
| Treatment | 2016 | 2017 | 2018 |
|---|---|---|---|
| Tilling set | 04.04 | 03.04 | 05.04 |
| Sowing date | 04.04 | 04.04 | 07.04 |
| Herbicide spraying | 05.04 Afalon Dyspersyjny 1.1 L/ha | 04.04 Stomp Aqua 455 CS 2.6 L/ha | 09.04 Stom Aqua 455 CS 2.6 L/ha |
| Herbicide spraying | 13.05 Basagran 480 SL 2.6 L/ha | 26.05 Panthera 040 EC 1.75 L/ha | 16.05—Fusilade forte 150 EC 1.7 L/ha |
| Insecticide spraying | _ | 17.05 and 29.05 Dursban 480 EC 1.25 L/ha | 15.05 and 13.06 Superkill 500 EC 0.06 L/ha |
| Fungicide spraying | 30.05 Gwarant 500 SL 2.0 L/ha | 05.06. and 24.06 Azoksystrobina 250 SC 1.0 L/ha | _ |
| Fungicide spraying | 30.06 Korazzo 250 SC 1.0 L/ha | 12.07 Signum 33 WG 0.8 kg/ha + Piorun 200 SC 0.2 L/ha | 22.05 Korazzo 050 SC 1.2 L/ha |
| Harvest date | 19.07 | 31.07 | 29.06 |
| Mineral Component | mg·kg−1 | Soil Abundance |
|---|---|---|
| Manganese | 164.1 | average |
| Zinc | 15.4 | high |
| Copper | 2.6 | average |
| Iron | 728.0 | average |
| Boron | 10.2 | high |
| Molybdenum | below testing limits | very low |
| Phosphorus | 8.64 mg P/100 g soil | high |
| Potassium | 12.28 mg K/100 g soil | average |
| Magnesium | 5.3 mg mg/100 g soil | high |
| Humus content | 0.8% | poor |
| C-org % | 0.48 | |
| pH in 1 M KCl | 6.5 | slightly acid |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulator or foliar fertilizers applied to the plants | 3.07 c | 5.21 cd | 1.33 ab |
| Tytanit | 3.34 ab | 5.31 cd | 1.26 ab |
| Optysil | 3.00 cd | 6.01 a | 1.21 b |
| Metalosate potassium | 3.12 c | 5.15 d | 1.30 ab |
| Rooter | 3.13 bc | 5.60 b | 1.34 ab |
| Bolero Mo | 3.43 a | 5.42 bc | 1.34 ab |
| Adob Zn IDHA | 3.35 a | 5.40 bc | 1.39 a |
| Adob B | 3.33 ab | 5.33 cd | 1.29 ab |
| Adob 2.0 Mo | 2.79 d | 5.83 a | 1.30 ab |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 12.6 ab | 14.0 ab | 15.0 a |
| Tytanit | 11.6 b | 15.2 a | 14.3 a |
| Optysil | 12.5 ab | 14.5 ab | 14.7 a |
| Metalosate potassium | 12.4 ab | 14.4 ab | 14.3 a |
| Rooter | 12.6 ab | 13.2 b | 14.3 a |
| Bolero Mo | 13.0 a | 15.0 a | 14.6 a |
| Adob Zn IDHA | 12.7 ab | 14.5 ab | 14.6 a |
| Adob B | 12.9 a | 15.3 a | 15.0 a |
| Adob 2.0 Mo | 12.8 a | 14.4 ab | 14.6 a |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 258.9 bcd | 284.7 b | 189.6 a |
| Tytanit | 255.3 cd | 285.2 b | 195.3 a |
| Optysil | 264.7 abc | 290.5 ab | 197.1 a |
| Metalosate potassium | 255.6 cd | 296.9 ab | 190.7 a |
| Rooter | 265.4 ab | 290.0 ab | 185.6 a |
| Bolero Mo | 259.7 bcd | 302.8 a | 185.3 a |
| Adob Zn IDHA | 270.4 a | 299.8 ab | 186.6 a |
| Adob B | 254.4 d | 298.1 ab | 189.9 a |
| Adob 2.0 Mo | 261.7 abcd | 289.0 ab | 201.4 a |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 1.56 b | 4.33 a | 1.55 c |
| Tytanit | 3.08 ab | 4.40 a | 0.98 c |
| Optysil | 3.78 a | 5.01 a | 3.16 a |
| Metalosate potassium | 3.89 a | 5.39 a | 1.51 c |
| Rooter | 3.54 a | 5.42 a | 1.44 c |
| Bolero Mo | 2.90 ab | 5.71 a | 2.31 b |
| Adob Zn IDHA | 3.56 ab | 5.79 a | 1.20 c |
| Adob B | 3.51 ab | 5.96 a | 1.24 c |
| Adob 2.0 Mo | 3.20 ab | 6.49 a | 1.32 c |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 37.9 a | 48.6 ab | 41.4 b |
| Tytanit | 53.8 a | 51.4 ab | 30.4 b |
| Optysil | 55.2 a | 68.9 a | 49.2 ab |
| Metalosate potassium | 62.3 a | 48.0 ab | 44.9 ab |
| Rooter | 66.0 a | 60.5 ab | 33.8 b |
| Bolero Mo | 50.9 a | 48.4 ab | 61.6 a |
| Adob Zn IDHA | 53.1 a | 38.0 b | 33.6 b |
| Adob B | 45.0 a | 44.8 ab | 34.2 b |
| Adob 2.0 Mo | 51.5 a | 46.6 ab | 38.8 b |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 49.8 b | 92.3 ab | 54.0 abc |
| Tytanit | 56.9 ab | 89.1 ab | 55.7 ab |
| Optysil | 59.3 ab | 85.6 ab | 43.8 d |
| Metalosate potassium | 63.3 a | 86.5 ab | 47.7 cd |
| Rooter | 58.9 ab | 87.3 ab | 51.5 bcd |
| Bolero Mo | 59.4 ab | 83.0 b | 59.3 a |
| Adob Zn IDHA | 60.1 a | 88.6 ab | 59.1 ab |
| Adob B | 60.6 a | 100.0 a | 53.4 abc |
| Adob 2.0 Mo | 55.2 ab | 96.3 ab | 56.1 ab |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 4.57 b | 8.93 bc | 3.78 b |
| Tytanit | 5.71 ab | 8.47 c | 3.19 b |
| Optysil | 6.85 ab | 8.45 c | 6.43 a |
| Metalosate potassium | 6.24 ab | 13.89 a | 3.43 b |
| Rooter | 5.36 ab | 9.29 bc | 4.25 b |
| Bolero Mo | 5.70 ab | 12.25 abc | 3.90 b |
| Adob Zn IDHA | 6.70 ab | 12.94 ab | 3.59 b |
| Adob B | 7.78 a | 12.98 ab | 3.59 b |
| Adob 2.0 Mo | 6.18 ab | 11.66 abc | 3.39 b |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 16.6 cd | 12.8 cd | 13.5 c |
| Tytanit | 16.6 cd | 15.1 cd | 20.3 b |
| Optysil | 22.4 a | 23.8 ab | 20.9 ab |
| Metalosate potassium | 18.8 bc | 16.3 c | 21.4 ab |
| Rooter | 20.9 ab | 27.4 a | 15.2 c |
| Bolero Mo | 22.8 a | 22.5 b | 23.1 a |
| Adob Zn IDHA | 14.3 d | 17.1 c | 19.2 b |
| Adob B | 16.0 cd | 16.9 c | 14.5c |
| Adob 2.0 Mo | 16.2 cd | 11.0 d | 21.4 ab |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 0.743 cd | 0.738 c | 0.723 c |
| Tytanit | 0.763 a-d | 0.763 abc | 0.760 abc |
| Optysil | 0.783 abc | 0.773 abc | 0.813 a |
| Metalosate potassium | 0.718 d | 0.795 ab | 0.748 bc |
| Rooter | 0.755 bcd | 0.740 bc | 0.780 ab |
| Bolero Mo | 0.763 a-d | 0.805 a | 0.798 ab |
| Adob Zn IDHA | 0.808 a | 0.750 abc | 0.768 abc |
| Adob B | 0.793 ab | 0.770 abc | 0.760 abc |
| Adob 2.0 Mo | 0.755 bcd | 0.745 bc | 0.758 abc |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 1.78 e | 3.44 cd | 2.61 e |
| Tytanit | 2.40 ab | 3.33 d | 2.86 cd |
| Optysil | 2.60 a | 3.78 b | 3.19 b |
| Metalosate potassium | 2.05 cd | 3.52 bcd | 2.79 cde |
| Rooter | 2.28 bc | 3.33 d | 2.80 cde |
| Bolero Mo | 2.30 b | 3.58 bcd | 3.39 a |
| Adob Zn IDHA | 1.88 de | 3.39 d | 2.63 e |
| Adob B | 1.75 e | 3.71 bc | 2.73 de |
| Adob 2.0 Mo | 2.28 bc | 4.48 a | 2.93 c |
| Experimental Combination | Year of Analysis | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Control—no biostimulators or foliar fertilizers applied to the plants | 46.5 d | 62.3 f | 34.0 d |
| Tytanit | 244.0 a | 351.0 a | 169.0 a |
| Optysil | 183.5 b | 268.5 c | 147.5 b |
| Metalosate potassium | 161.5 b | 263.8 c | 143.0 b |
| Rooter | 95.8 c | 192.3 d | 74.5 c |
| Bolero Mo | 168.8 b | 328.0 b | 136.3 b |
| Adob Zn IDHA | 61.0 cd | 86.8 e | 51.5 d |
| Adob B | 164.3 b | 353.4 a | 141.0 b |
| Adob 2.0 Mo | 57.3 cd | 82.4 ef | 43.9 d |
| Experimental Combination | Term of Analysis/BBCH | |||
|---|---|---|---|---|
| I/BBCH5-10 | II/BBCH35-40 | III/BBCH51-59 | IV/After Harvest | |
| 2016 | ||||
| 1 | 0.0021 no | 0.0046 mn | 0.0195 c | 0.0071 i–m |
| 2 | 0.0021 no | 0.0073 h–m | 0.0195 c | 0.0060 j–m |
| 3 | 0.0018 no | 0.0056 lm | 0.0153 de | 0.0088 h–k |
| 4 | 0.0020 no | 0.0066 i–m | 0.0183 cd | 0.0059 k–m |
| 5 | 0.0015 o | 0.0089 h–k | 0.0200 bc | 0.0088 h–k |
| 6 | 0.0018 no | 0.0095 f–i | 0.0295 a | 0.0091 h–j |
| 7 | 0.0015 no | 0.0091 g–i | 0.0189 c | 0.0124 ef |
| 8 | 0.0016 no | 0.0078 h–l | 0.0267 a | 0.0103 f–h |
| 9 | 0.0018 no | 0.0056 lm | 0.0230 b | 0.0122 fg |
| 2017 | ||||
| 1 | 0.0054 a–c | 0.0005 c | 0.0068 a–c | 0.0022 bc |
| 2 | 0.0062 a–c | 0.0037 a–c | 0.0095 a–c | 0.0003 c |
| 3 | 0.0031 a–c | 0.0010 c | 0.0109 ab | 0.0014 bc |
| 4 | 0.0062 a–c | 0.0014 bc | 0.0099 a–c | 0.0003 c |
| 5 | 0.0021 bc | 0.0018 bc | 0.0024 bc | 0.0007 c |
| 6 | 0.0035 a–c | 0.0019 bc | 0.0111 ab | 0.0123 a |
| 7 | 0.0023 bc | 0.0057 a–c | 0.0054 a–c | 0.0081 a–c |
| 8 | 0.0017 bc | 0.0024 bc | 0.0014 bc | 0.0054 a–c |
| 9 | 0.0076 a–c | 0.0086 a–c | 0.0008 c | 0.0080 a–c |
| 2018 | ||||
| 1 | 0.0041 no | 0.0090 mn | 0.0379 d | 0.0141 i–m |
| 2 | 0.0042 no | 0.0144 h–m | 0.0381 d | 0.0119 j–m |
| 3 | 0.0036 no | 0.0109 lm | 0.0288 e | 0.0173 h–k |
| 4 | 0.0039 no | 0.0130 i–m | 0.0352 d | 0.0116 k–m |
| 5 | 0.0029 o | 0.0175 h–k | 0.0393 cd | 0.0173 h–k |
| 6 | 0.0034 no | 0.0188 f–i | 0.0578 a | 0.0179 g–j |
| 7 | 0.0030 no | 0.0181 g–i | 0.0358 d | 0.0247 ef |
| 8 | 0.0032 no | 0.0154 h–l | 0.0506 b | 0.0204 f–h |
| 9 | 0.0035 no | 0.0109 lm | 0.0446 bc | 0.0240 e–g |
| Experimental Combination | Term of Analysis/BBCH | |||
|---|---|---|---|---|
| I/BBCH 5–10 | II/BBCH 35–40 | III/BBCH 51–59 | IV/After Harvest | |
| 2016 | ||||
| 1 | 0.424 k | 1.059 g–j | 3.476 b | 0.954 ij |
| 2 | 0.353 k | 1.198 f–j | 2.654 e | 0.968 h–j |
| 3 | 0.384 k | 1.049 g–j | 3.170 b–d | 0.974 h–j |
| 4 | 0.372 k | 1.217 f–j | 2.793 de | 0.868 j |
| 5 | 0.355 k | 1.398 fg | 3.198 bc | 1.122 f–j |
| 6 | 0.476 k | 1.340 f–h | 2.541 e | 1.268 f–l |
| 7 | 0.379 k | 1.463 e | 2.901 c–e | 1.182 f–j |
| 8 | 0.329 k | 1.233 f–j | 3.929 a | 1.378 fg |
| 9 | 0.290 k | 0.940 ij | 2.850 c–e | 1.399 fg |
| 2017 | ||||
| 1 | 0.195 h–k | 0.398 ab | 0.353 a–d | 0.216 g–k |
| 2 | 0.191 i–k | 0.384 a–c | 0.283 c–j | 0.281 c–j |
| 3 | 0.151 k | 0.310 a–g | 0.279 c–j | 0.233 e–k |
| 4 | 0.166 k | 0.403 a | 0.279 c–j | 0.277 d–j |
| 5 | 0.203 g–k | 0.396 ab | 0.246 d–k | 0.236 e–k |
| 6 | 0.202 g–k | 0.289 b–j | 0.234 e–k | 0.199 h–k |
| 7 | 0.166 k | 0.333 a–e | 0.331 a–f | 0.184 jk |
| 8 | 0.221 f–k | 0.303 a–h | 0.298 a–i | 0.216 g–k |
| 9 | 0.311 a–g | 0.303 a–h | 0.338 a–e | 0.223 f–k |
| 2018 | ||||
| 1 | 0.181 l | 0.904 kl | 2.454 b | 1.119 i–k |
| 2 | 0.181 l | 1.440 e–k | 1.905 b–g | 1.407 f–k |
| 3 | 0.244 l | 1.092 jk | 2.125 b–f | 1.464 e–k |
| 4 | 0.196 l | 1.298 g–k | 1.965 b–g | 1.132 h–k |
| 5 | 0.190 l | 1.753 b–j | 2.233 b–d | 1.581 c–k |
| 6 | 0.197 l | 1.882 b–h | 2.337 bc | 1.892 b–h |
| 7 | 0.186 l | 1.806 b–j | 1.877 b–i | 1.817 b–j |
| 8 | 0.230 l | 1.537 d–k | 3.983 a | 2.015 b–g |
| 9 | 0.224 l | 1.087 jk | 2.183 b–e | 2.236 b–d |
| Experimental Combination | Term of Analysis | |||
|---|---|---|---|---|
| I/BBCH5–10 | II/BBCH35–40 | III/BBCH51–59 | IV/After Harvest | |
| 2016 | ||||
| 1 | 0.316 m | 0.760 kl | 2.711 b | 0.834 i–l |
| 2 | 0.283 m | 0.964 g–k | 2.302 d | 0.786 j–l |
| 3 | 0.282 m | 0.803 i–l | 2.352 d | 0.925 h–l |
| 4 | 0.286 m | 0.939 g–l | 2.311 d | 0.729 l |
| 5 | 0.254 m | 1.144 e–g | 2.601 bc | 1.002 f–j |
| 6 | 0.326 m | 1.145 e–g | 2.743 b | 1.088 f–h |
| 7 | 0.267 m | 1.189 ef | 2.393 cd | 1.213 ef |
| 8 | 0.244 m | 1.007 f–i | 3.299 a | 1.205 ef |
| 9 | 0.234 m | 0.749 kl | 2.574 bc | 1.308 e |
| 2017 | ||||
| 1 | 0.173 i–k | 0.319 b–d | 0.250 c–i | 0.238 d–i |
| 2 | 0.169 i–k | 0.345 b | 0.262 b–h | 0.205 f–k |
| 3 | 0.178 h–k | 0.201 g–k | 0.234 d–j | 0.237 d–i |
| 4 | 0.222 e–j | 0.485 a | 0.294 b–g | 0.269 b–h |
| 5 | 0.124 k | 0.310 b–e | 0.238 d–i | 0.174 i–k |
| 6 | 0.227 d–j | 0.343 bc | 0.202 g–k | 0.210 f–k |
| 7 | 0.142 jk | 0.246 d–i | 0.208 f–k | 0.274 b–g |
| 8 | 0.141 jk | 0.296 b–f | 0.244 d–i | 0.276 b–g |
| 9 | 0.168 i–k | 0.297 b–f | 0.244 d–i | 0.280 b–g |
| 2018 | ||||
| 1 | 0.013 h | 0.017 h | 0.163 b–d | 0.043 f–h |
| 2 | 0.013 h | 0.007 h | 0.124 cd | 0.023 h |
| 3 | 0.011 h | 0.022 h | 0.119 b–e | 0.025 h |
| 4 | 0.016 h | 0.017 h | 0.151 cd | 0.035 gh |
| 5 | 0.017 h | 0.027 h | 0.119 b–e | 0.048 e–h |
| 6 | 0.005 h | 0.028 gh | 0.190 bc | 0.099 d–g |
| 7 | 0.005 h | 0.023 h | 0.113 d–f | 0.109 d–f |
| 8 | 0.003 h | 0.021 h | 0.225 ab | 0.133 cd |
| 9 | 0.010 h | 0.018 h | 0.139 cd | 0.270 a |
| Experimental Combination | Term of Analysis/BBCH | |||
|---|---|---|---|---|
| I/BBCH5–10 | II/BBCH35–40 | III/BBCH51–59 | IV/After Harvest | |
| 2016 | ||||
| 1 | 22.692 gh | 28.276 gh | 44.846 ef | 19.767 gh |
| 2 | 20.580 gh | 42.620 f | 72.124 a | 20.974 gh |
| 3 | 25.691 gh | 23.572 gh | 55.345 c–f | 33.000 gh |
| 4 | 21.507 gh | 35.703 gh | 72.429 a | 23.249 gh |
| 5 | 27.440 gh | 39.026 gh | 67.390 a–c | 33.525 gh |
| 6 | 22.283 gh | 26.695 gh | 57.982 b–d | 30.047 gh |
| 7 | 27.466 gh | 34.392 gh | 68.860 ab | 32.881 gh |
| 8 | 23.013 gh | 34.076 gh | 59.076 b–d | 41.569 g |
| 9 | 14.933 h | 21.821 gh | 54.870 de | 25.644 gh |
| 2017 | ||||
| 1 | 22.726 z | 21.723 z | 25.257 y | 33.962 k |
| 2 | 22.666 z | 32.811 l | 28.155 p | 44.407 a |
| 3 | 14.366 z | 17.502 z | 26.240 w | 36.803 f |
| 4 | 26.387 u | 16.629 z | 29.289 o | 38.730 e |
| 5 | 14.196 z | 27.569 r | 39.211 c | 36.601 h |
| 6 | 17.732 z | 16.554 z | 27.184 s | 39.308 b |
| 7 | 10.839 z | 9.009 z | 39.033 d | 36.630 g |
| 8 | 18.267 z | 26.443 t | 32.289 m | 35.145 i |
| 9 | 10.784 z | 13.189 z | 31.404 n | 34.014 j |
| 2018 | ||||
| 1 | 13.477 t | 21.286 p | 31.711 k | 10.259 w |
| 2 | 14.370 t | 37.776 hi | 38.910 g | 19.011 r |
| 3 | 23.299 o | 26.861 m | 37.363 i | 22.729 o |
| 4 | 8.146 y | 38.546 gh | 52.811 d | 16.344 s |
| 5 | 16.411 s | 36.202 j | 47.716 e | 24.500 n |
| 6 | 14.388 t | 40.710 f | 61.607 b | 27.577 m |
| 7 | 14.346 t | 41.326 f | 52.351 d | 24.559 n |
| 8 | 12.352 u | 30.921 kl | 64.465 a | 27.635 m |
| 9 | 4.7124 z | 30.413 l | 54.256 c | 22.517 o |
| Experimental Combination | Term of Analysis | |||
|---|---|---|---|---|
| I/BBCH5–10 | II/BBCH35–40 | III/BBCH51–59 | IV/After Harvest | |
| 2016 | ||||
| 1 | 2.288 hi | 4.080 g–i | 14.337 c–e | 7.223 f–i |
| 2 | 1.940 i | 4.112 g–i | 15.117 cd | 8.370 f–i |
| 3 | 1.343 i | 4.810 g–i | 17.358 bc | 5.401 g–i |
| 4 | 2.688 hi | 4.310 g–i | 16.451 cd | 2.561 hi |
| 5 | 3.338 g–i | 5.935 f–i | 11.409 de | 9.616 f–i |
| 6 | 2.518 i | 5.029 g–i | 17.223 bc | 13.776 c–e |
| 7 | 1.038 i | 5.299 g–i | 17.445 bc | 14.522 c–e |
| 8 | 0.758 i | 4.982 g–i | 28.795 b | 18.148 bc |
| 9 | 1.773 i | 5.730 g–i | 15.690 cd | 33.907 a |
| 2017 | ||||
| 1 | 17.047 z | 16.292 z | 18.946 y | 25.472 k |
| 2 | 17.002 z | 24.610 l | 21.121 p | 33.306 a |
| 3 | 10.776 z | 13.127 z | 19.686 w | 27.603 f |
| 4 | 19.794 u | 12.472 z | 21.972 o | 29.047 e |
| 5 | 10.648 z | 20.678 r | 29.409 c | 27.451 h |
| 6 | 13.300 z | 12.416 z | 20.393 s | 29.487 b |
| 7 | 8.130 z | 6.759 z | 29.277 d | 27.477 g |
| 8 | 13.701 z | 19.833 t | 24.217 m | 26.361 i |
| 9 | 8.092 z | 9.896 z | 23.553 n | 25.514 j |
| 2018 | ||||
| 1 | 2.899 h | 3.881 h | 37.256 bc | 9.892 f–h |
| 2 | 2.877 h | 1.609 h | 28.433 cd | 8.802 gh |
| 3 | 2.451 h | 4.944 h | 27.258 d | 5.918 h |
| 4 | 3.708 h | 3.903 h | 34.924 cd | 10.260 e–h |
| 5 | 3.785 h | 6.069 h | 27.597 d | 11.215 e–h |
| 6 | 1.223 h | 6.479 h | 43.747 ab | 22.826 d–g |
| 7 | 1.115 h | 5.272 h | 26.276 de | 24.911 d–f |
| 8 | 0.799 h | 4.694 h | 51.813 a | 30.487 cd |
| 9 | 2.290 h | 4.205 h | 32.277 cd | 31.161 cd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulewska, H.; Niewiadomska, A.; Ratajczak, K.; Budka, A.; Panasiewicz, K.; Faligowska, A.; Wolna-Maruwka, A.; Dryjański, L. Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators. Agronomy 2020, 10, 1558. https://doi.org/10.3390/agronomy10101558
Sulewska H, Niewiadomska A, Ratajczak K, Budka A, Panasiewicz K, Faligowska A, Wolna-Maruwka A, Dryjański L. Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators. Agronomy. 2020; 10(10):1558. https://doi.org/10.3390/agronomy10101558
Chicago/Turabian StyleSulewska, Hanna, Alicja Niewiadomska, Karolina Ratajczak, Anna Budka, Katarzyna Panasiewicz, Agnieszka Faligowska, Agnieszka Wolna-Maruwka, and Leszek Dryjański. 2020. "Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators" Agronomy 10, no. 10: 1558. https://doi.org/10.3390/agronomy10101558
APA StyleSulewska, H., Niewiadomska, A., Ratajczak, K., Budka, A., Panasiewicz, K., Faligowska, A., Wolna-Maruwka, A., & Dryjański, L. (2020). Changes in Pisum sativum L. Plants and in Soil as a Result of Application of Selected Foliar Fertilizers and Biostimulators. Agronomy, 10(10), 1558. https://doi.org/10.3390/agronomy10101558









