Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects Origin and Rearing
2.2. Experimental Design
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT. Statistical Data; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- IOOC. World Olive Oil Figures. Available online: http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures (accessed on 17 January 2020).
- Tzanakakis, M. Seasonal development and dormancy of insects and mites feeding on olive: A review. Neth. J. Zool. 2003, 52, 87–224. [Google Scholar] [CrossRef] [Green Version]
- Haniotakis, G.E. Olive pest control: Present status and prospects. IOBC Wprs Bull. 2005, 28, 1. [Google Scholar]
- Raina, B.L. Olives. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4260–4267. [Google Scholar]
- Wiesman, Z. Desert Olive Oil Cultivation: Advanced Bio Technologies; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Villa, M.; Marrão, R.; Mexia, A.; Bento, A.; Pereira, J.A. Are wild flowers and insect honeydews potential food resources for adults of the olive moth, Prays oleae? J. Pest Sci. 2017, 90, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Delrio, G. Integrated Control in Olive Groves. In Proceedings of the Biological Control and Integrated Crop Protection: Towards Environmentally Dafer Agriculture, IOBC/WPRS, Veldhohen, The Netherlands, 8–13 September 1991; pp. 67–76. [Google Scholar]
- Rimoldi, F.; Schneider, M.I.; Ronco, A.E. Susceptibility of chrysoperla externa eggs (Neuroptera: Chrisopidae) to conventional and biorational insecticides. Environ. Entomol. 2008, 37, 1252–1257. [Google Scholar] [CrossRef]
- Arambourg, Y. La fauna entomológica del olivo. Olivae 1984, 1, 37–40. [Google Scholar]
- Gharbi, N.; Dibo, A.; Ksantini, M. Observation of arthropod populations during outbreak of olive psyllid Euphyllura olivina in Tunisian olive groves. Tunis. J. Plant. Prot. 2012, 7, 27–34. [Google Scholar]
- Pantaleoni, R.; Lentini, A.; Delrio, G. Lacewing in Sardinia olive groves. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: New York, NY, USA, 2001; pp. 435–446. [Google Scholar]
- Miller, G.L.; Oswald, J.D.; Miller, D.R. Lacewings and scale insects: A review of predator/prey associations between the Neuropterida and Coccoidea (Insecta: Neuroptera, Raphidioptera, Hemiptera). Ann. Entomol. Soc. Am. 2014, 97, 1103–1125. [Google Scholar] [CrossRef]
- Petanidou, T.; Potts, S.G. Mutual use of resources in Mediterranean plant–pollinator communities: How specialized are pollination webs. In Plant–Pollinator Interactions: From Specialization to Generalization; The University of Chicago Press: Chicago, IL, USA, 2006; pp. 220–244. [Google Scholar]
- Villa, M.; Santos, S.A.P.; Benhadi-Marín, J.; Mexia, A.; Bento, A.; Pereira, J.A. Life-history parameters of Chrysoperla carnea s.l. fed on spontaneous plant species and insect honeydews: Importance for conservation biological control. BioControl 2016, 61, 533–543. [Google Scholar] [CrossRef]
- McEwen, P.; Clow, S.; Jervis, M.; Kidd, N. Alteration in searching behaviour of adult female green lacewings Chrysoperla carnea (Neur.: Chrysopidae) following contact with honeydew of the black scale Saissetia oleae (Hom.: Coccidae) and solutions containing acidhydrolysed L-tryptophan. Entomophaga 1993, 38, 347–354. [Google Scholar] [CrossRef]
- Sengonca, C.; Al-Zyoud, F.; Blaeser, P. Prey consumption by larval and adult stages of the entomophagous ladybird Serangium parcesetosum Sicard (Col., Coccinellidae) of the cotton whitefly, Bemisia tabaci (Genn.)(Hom., Aleyrodidae), at two different temperatures. J. Pest Sci. 2005, 78, 179–186. [Google Scholar] [CrossRef]
- Holling, C.S. Some Characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Solomon, M. The natural control of animal populations. J. Anim. Ecol. 1949, 18, 1–35. [Google Scholar] [CrossRef]
- Beingolea, G. Estatus actual de la plaga de la quereza negra del olivo (Saissetia oleae Bern.) en los valles de Yauca E Ilo. Bol. Trimest. Exp. Agropecu. 1955, 4, 18–22. [Google Scholar]
- Holling, C.S. The functional response of predators to prey density and its role in mimicry and population regulation. Mem. Entomol. Soc. Can. 1965, 97, 5–60. [Google Scholar] [CrossRef] [Green Version]
- Schenk, D.; Bacher, S. Functional response of a generalist insect predator to one of its prey species in the field. J. Anim. Ecol. 2002, 71, 524–531. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Oaten, A. Predation and population stability. Adv. Ecol. Res. 1975, 9, 1–131. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; Smith, J.W., Jr. Attributes of natural enemies in ephemeral crop habitats. Biol. Control 1997, 10, 16–22. [Google Scholar] [CrossRef]
- Fathi, S.A.A.; Nouri-Ganbalani, G. Assessing the potential for biological control of potato field pests in Ardabil, Iran: Functional responses of Orius niger (Wolf.) and O. minutus (L.) (Hemiptera: Anthocoridae). J. Pest Sci. 2010, 83, 47–52. [Google Scholar] [CrossRef]
- Fathipour, Y.; Maleknia, B. Mite predators. In Ecofriendly Pest Management for Food Security; Elsevier: San Diego, CA, USA, 2016; pp. 329–366. [Google Scholar]
- Pritchard, D.W.; Paterson, R.A.; Bovy, H.C.; Barrios-O'Neill, D. Frair: An R package for fitting and comparing consumer functional responses. Methods Ecol. Evol. 2017, 8, 1528–1534. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Juliano, S.A. Non-linear curve fitting: Predation and functional response curve. In Design and Analysis of Ecological Experiment; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 159–182. [Google Scholar]
- Barrios-O'Neill, D.; Dick, J.T.; Emmerson, M.C.; Ricciardi, A.; MacIsaac, H.J.; Alexander, M.E.; Bovy, H.C. Fortune favours the bold: A higher predator reduces the impact of a native but not an invasive intermediate predator. J. Anim. Ecol. 2014, 83, 693–701. [Google Scholar] [CrossRef]
- Bolker, B.M. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Benhadi-Marín, J.; Pereira, J.A.; Barreales, D.; Sousa, J.P.; Santos, S.A. A simulation-based method to compare the pest suppression potential of predators: A case study with spiders. Biol. Control 2018, 123, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, R.N.; O'Neil, R.J. Laboratory measurement of the functional response of Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Environ. Entomol. 1991, 20, 610–614. [Google Scholar] [CrossRef]
- Atlıhan, R.; Kaydan, B.; Özgökçe, M. Feeding activity and life history characteristics of the generalist predator, Chrysoperla carnea (Neuroptera: Chrysopidae) at different prey densities. J. Pest Sci. 2004, 77, 17–21. [Google Scholar] [CrossRef]
- Hassanpour, M.; Nouri-Ganbalani, G.; Mohaghegh, J.; Enkegaard, A. Functional response of different larval instars of the green lacewing, Chrysoperla carnea (Neuroptera: Chrysopidae), to the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). J. Food Agric. Environ. 2009, 7, 424–428. [Google Scholar]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of prey and predator stages. Insect Sci. 2011, 18, 217–224. [Google Scholar] [CrossRef]
- Huang, N.; Enkegaard, A. Predation capacity and prey preference of Chrysoperla carnea on Pieris brassicae. BioControl 2010, 55, 379–385. [Google Scholar] [CrossRef]
- Klingen, I.; Johansen, N.; Hofsvang, T. The predation of Chrysoperla carnea (Neurop., Chrysopidae) on eggs and larvae of Mamestra brassicae (Lep., Noctuidae). J. Appl. Entomol. 1996, 120, 363–367. [Google Scholar] [CrossRef]
- Montoya-Alvarez, A.F.; Ito, K.; Nakahira, K.; Arakawa, R. Functional response of Chrysoperla nipponensis and C. carnea (Neuroptera: Chrysopidae) to the cotton aphid Aphis gossypii Glover (Homoptera: Aphididae) under laboratory conditions. Appl. Entomol. Zool. 2010, 45, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Rios-Velasco, C.; Nájera-Miramontes, D.; Jacobo-Cuellar, J.L.; Berlanga-Reyes, D.I.; Ruiz-Cisneros, M.F.; Zamudio-Flores, P.B.; Ornelas-Paz, J.J.; Acosta-Muñiz, C.H.; Romo-Chacón, A.; Salas Marina, M.A. Predation capability and functional response of Chrysoperla carnea to Choristoneura rosaceana under laboratory conditions. Southwest. Entomol. 2017, 42, 677–690. [Google Scholar] [CrossRef]
- Stark, S.; Whitford, F. Functional response of Chrysopa carnea [Neur: Chrysopidae] larvae feeding on Heliothis virescens [Lep.: Noctuidae] eggs on cotton in field cages. BioControl 1987, 32, 521–527. [Google Scholar] [CrossRef]
- Sultan, A.; Farhanullah Khan, M. Functional Response of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) to Sugarcane Whitefly Aleurolobus barodensis (Maskell) in Laboratory Conditions. J. Insect Behav. 2014, 27, 454–461. [Google Scholar] [CrossRef]
- Bento, A.; Lopes, J.; Torres, L.; Passos-Carvalho, P. Biological Control of Prays oleae (Bern) by Chrysopids in Trás-os-Montes Region (Northeastern Portugal). In Proceedings of the III International Symposium on Olive Growing, Chania, Greece, 22–26 September 1997; Volume 474, pp. 535–540. [Google Scholar]
- Ramos, P.; Campos, M.; Ramos, J.M. Estabilización del ataque de Prays oleae Bern. y de la actividad de los depredadores oófagos sobre el fruto del olivo. Bol. Sanid. Veg. Plagas. 1984, 10, 239–243. [Google Scholar]
- Campos, M. Lacewings in Andalusian olive orchards. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: New York, NY, USA, 2001; pp. 492–497. [Google Scholar]
- Szentkirályi, F. Lacewings in fruit and nut crops. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University Press: New York, NY, USA, 2001; pp. 172–238. [Google Scholar]
- Santos, S.A.; Pereira, J.A.; da Conceição Rodrigues, M.; Torres, L.M.; Pereira, A.M.N.; Nogueira, A.J. Identification of predator–prey relationships between coccinellids and Saissetia oleae (Hemiptera: Coccidae), in olive groves, using an enzyme-linked immunosorbent assay. J. Pest Sci. 2009, 82, 101–108. [Google Scholar] [CrossRef]
- Ehler, L.E. Competition between two natural enemies of Mediterranean black scale on olive. Environ. Entomol. 1978, 7, 521–523. [Google Scholar] [CrossRef]
- Scopes, N. The potential of Chrysopa carnea as a biological control agent of Myzus persicae on glasshouse chrysanthemums. Ann. Appl. Biol. 1969, 64, 433–439. [Google Scholar] [CrossRef]
- Yuksel, S. The effectiveness of Chrysoperla carnea (Stephens) (Neuroptera, Chrysopidae) as a predator on cotton aphid Aphis gossypii Glov.(Homoptera, Aphididae). In Proceedings of the Second Turkish National Entomological Congress, Adana, Turkey, 28–31 January 1992; pp. 209–216. [Google Scholar]
- Liu, T.X.; Chen, T.Y. Effects of a juvenile hormone analog, pyriproxyfen, on the apterous form of Lipaphis erysimi. Entomol. Exp. Appl. 2001, 98, 295–301. [Google Scholar] [CrossRef]
- Alcalá Herrera, R.; Campos, M.; González-Salvadó, M.; Ruano, F.J.I. Abundance and population decline factors of chrysopid juveniles in olive groves and adjacent trees. Insects 2019, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Borges, I.; Soares, A.O.; Magro, A.; Hemptinne, J.-L. Prey availability in time and space is a driving force in life history evolution of predatory insects. Evol. Ecol. 2011, 25, 1307–1319. [Google Scholar] [CrossRef]
- Pereira, J.A.C. Bioecologia da Cochonilha Negra, Saissetia Oleae (Olivier), na Oliveira, em Trás-os-Montes. Ph.D. Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 2004. [Google Scholar]
- Alrouechdi, K.; Pralavorio, P.; Canard, M.; Arambourg, Y. Coïncidence et relations prédatrices entre Chrysopa carnea (Stephens) (Neur., Chrysopidae) et quelques ravageurs de l'olivier dans le sud-est de la France. J. Appl. Entomol. 1981, 91, 411–417. [Google Scholar]
- Marrão, R.M. Effect of Parasitoid Competition, Ant Exclusion and Carbohydrate Sources on Biological Control of Saissetia oleae on Olive Trees. Ph.D. Thesis, Universidad de León, Leon, Spain, 2017. [Google Scholar]
- Pinheiro, L.A.; Torres, L.M.; Raimundo, J.; Santos, S.A. Effects of pollen, sugars and honeydew on lifespan and nutrient levels of Episyrphus balteatus. BioControl 2015, 60, 47–57. [Google Scholar] [CrossRef]
- Villa, M.; Santos, S.A.; Mexia, A.; Bento, A.; Pereira, J.A. Wild flower resources and insect honeydew are potential food items for Elasmus flabellatus. Agron. Sustain. Dev. 2017, 37, 15. [Google Scholar] [CrossRef] [Green Version]
- Guzman, L.M.; Srivastava, D.S. Prey body mass and richness underlie the persistence of a top predator. Proc. R. Soc. B. 2019, 286, 20190622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, T.; Kaartinen, R.; Jonsson, M.; Bommarco, R. Predictive power of food web models based on body size decreases with trophic complexity. Ecol. Lett. 2018, 21, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.J.; Dettmers, J.M.; Wahl, D.H.; Czesny, S.J. Effects of predator–prey interactions and benthic habitat complexity on selectivity of a foraging generalist. Trans. Am. Fish. Soc. 2010, 139, 1004–1013. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Pereira, J.A.; Sousa, J.P.; Santos, S.A. Functional responses of three guilds of spiders: Comparing single-and multiprey approaches. Ann. Appl. Biol. 2019, 175, 202–214. [Google Scholar] [CrossRef] [Green Version]

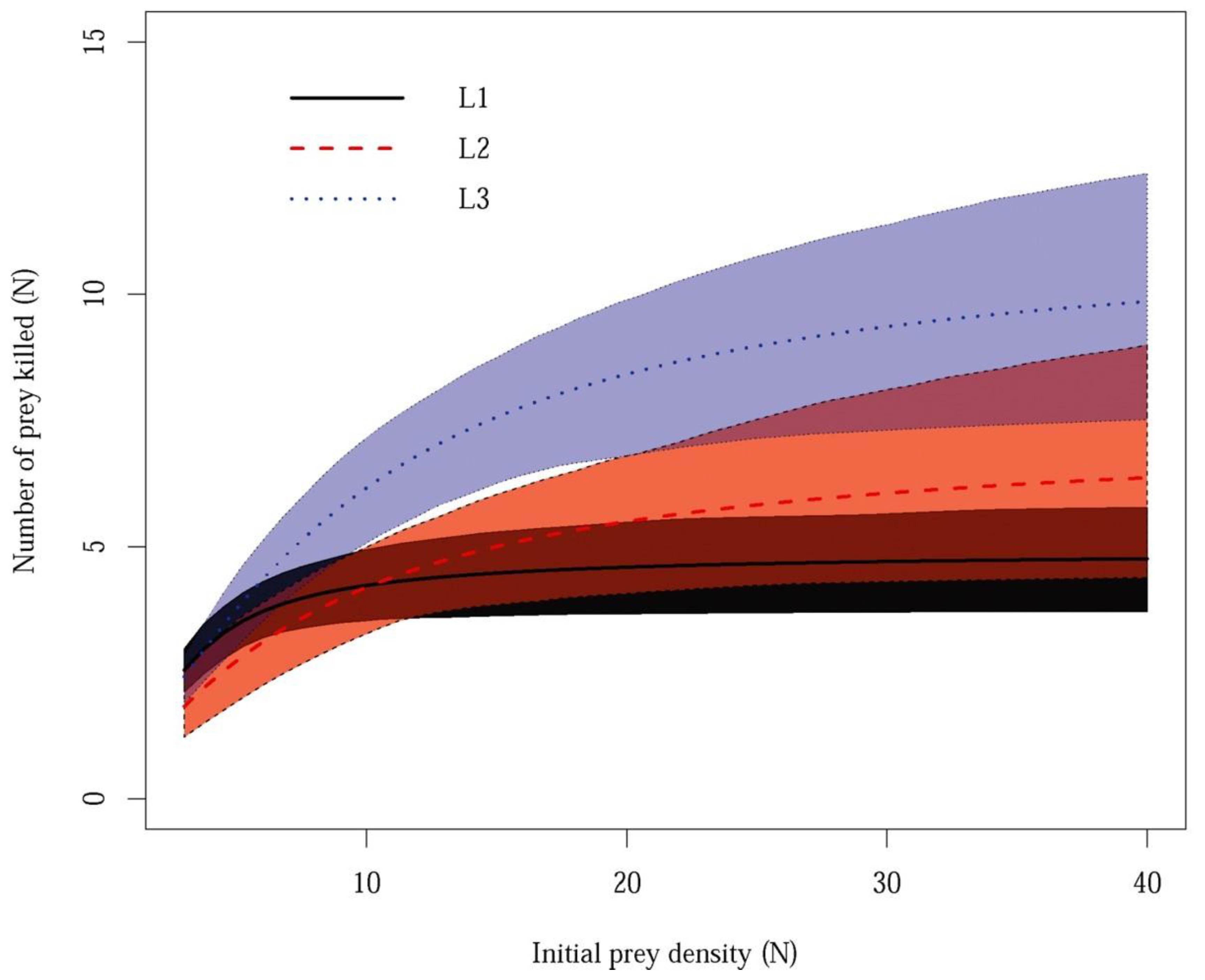
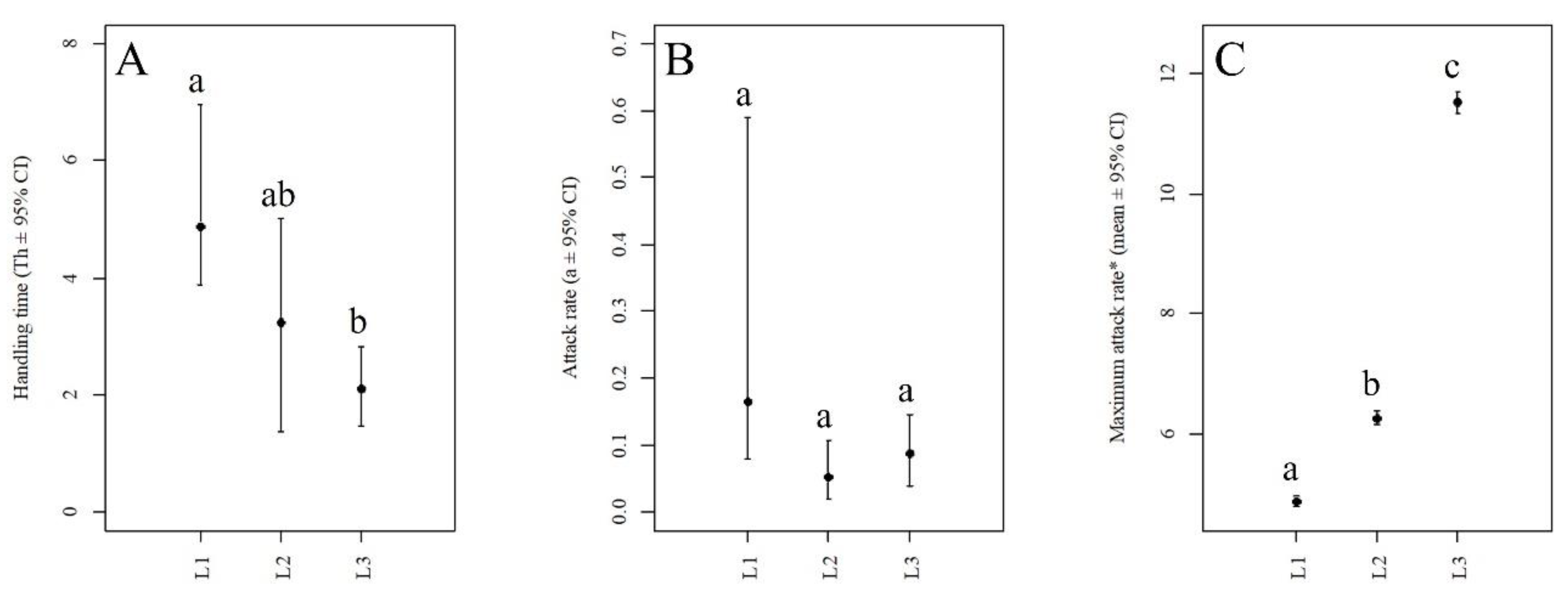
| Prey Density | n | Mean Number of Consumed Prey (Mean ± SE) | ||
|---|---|---|---|---|
| 1st instar | 2nd instar | 3rd instar | ||
| 3 | 25 | 2.7 ± 0.20 | 1.9 ± 0.39 | 2.4 ± 0.38 |
| 5 | 25 | 2.8 ± 0.51 | 3.2 ± 0.54 | 3.6 ± 0.60 |
| 10 | 24 | 5.6 ± 1.12 | 3.9 ± 0.90 | 6.4 ± 0.93 |
| 15 | 22 | 4.2 ± 0.90 | 2.9 ± 1.03 | 8.0 ± 1.67 |
| 25 | 23 | 4.3 ± 0.51 | 8.2± 1.12 | 8.3 ± 1.86 |
| 40 | 20 | 4.5 ± 0.59 | 5.25 ± 0.82 | 10.0 ± 1.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy 2020, 10, 1511. https://doi.org/10.3390/agronomy10101511
Mahzoum AM, Villa M, Benhadi-Marín J, Pereira JA. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy. 2020; 10(10):1511. https://doi.org/10.3390/agronomy10101511
Chicago/Turabian StyleMahzoum, Abdelkader Meni, María Villa, Jacinto Benhadi-Marín, and José Alberto Pereira. 2020. "Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control" Agronomy 10, no. 10: 1511. https://doi.org/10.3390/agronomy10101511
APA StyleMahzoum, A. M., Villa, M., Benhadi-Marín, J., & Pereira, J. A. (2020). Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) Larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for Biological Control. Agronomy, 10(10), 1511. https://doi.org/10.3390/agronomy10101511








