Agronomic and Environmental Implications of Substituting Pig Slurry for Synthetic Nitrogen in Mediterranean Wheat Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
2.2. Measurements and Determinations
2.2.1. Soil Sampling
2.2.2. Crop Sampling
2.2.3. Nitrous Oxide Emissions
2.3. Data and Statistical Analysis
3. Results
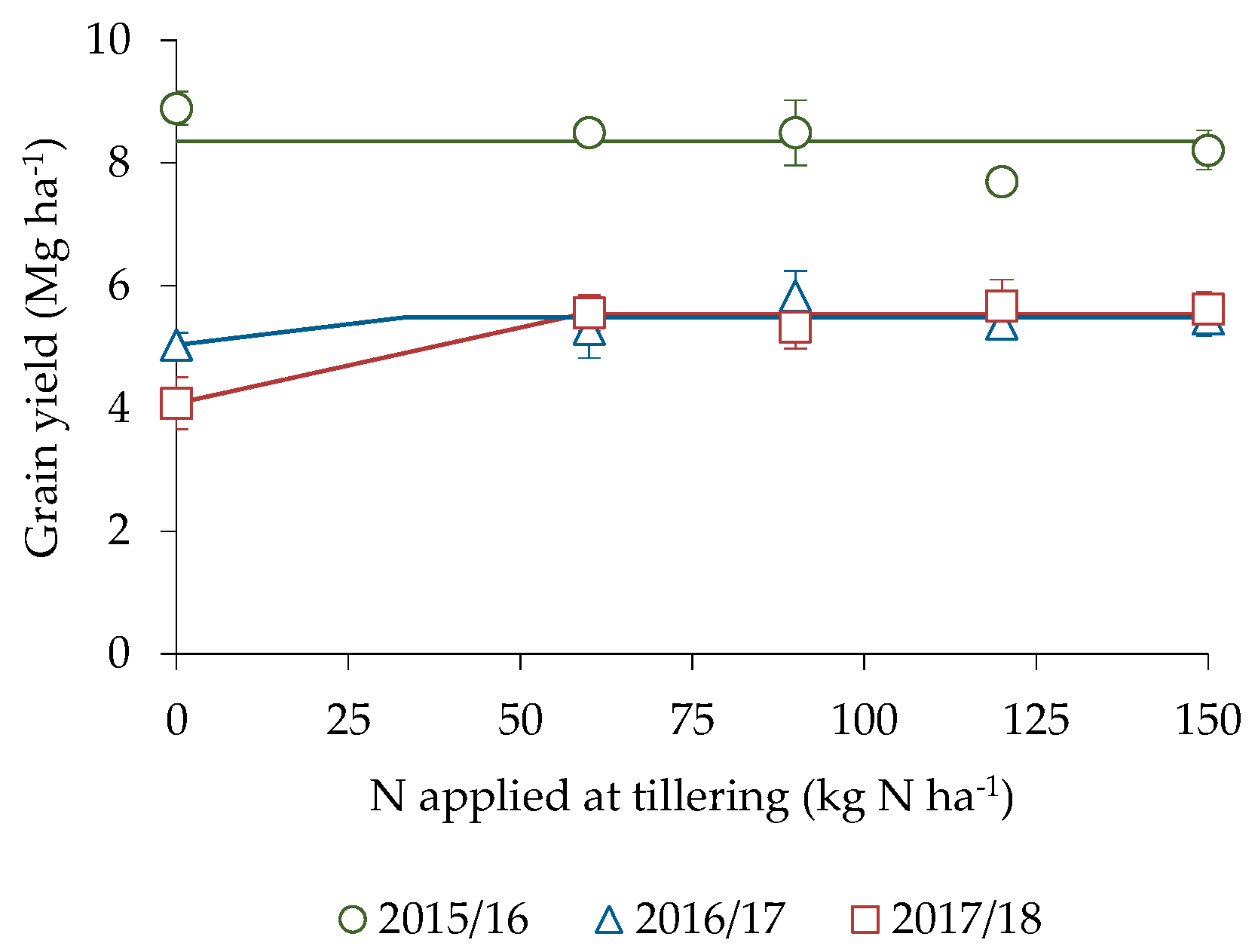
3.1. Productive Parameters
3.2. Nitrous Oxide Emissions
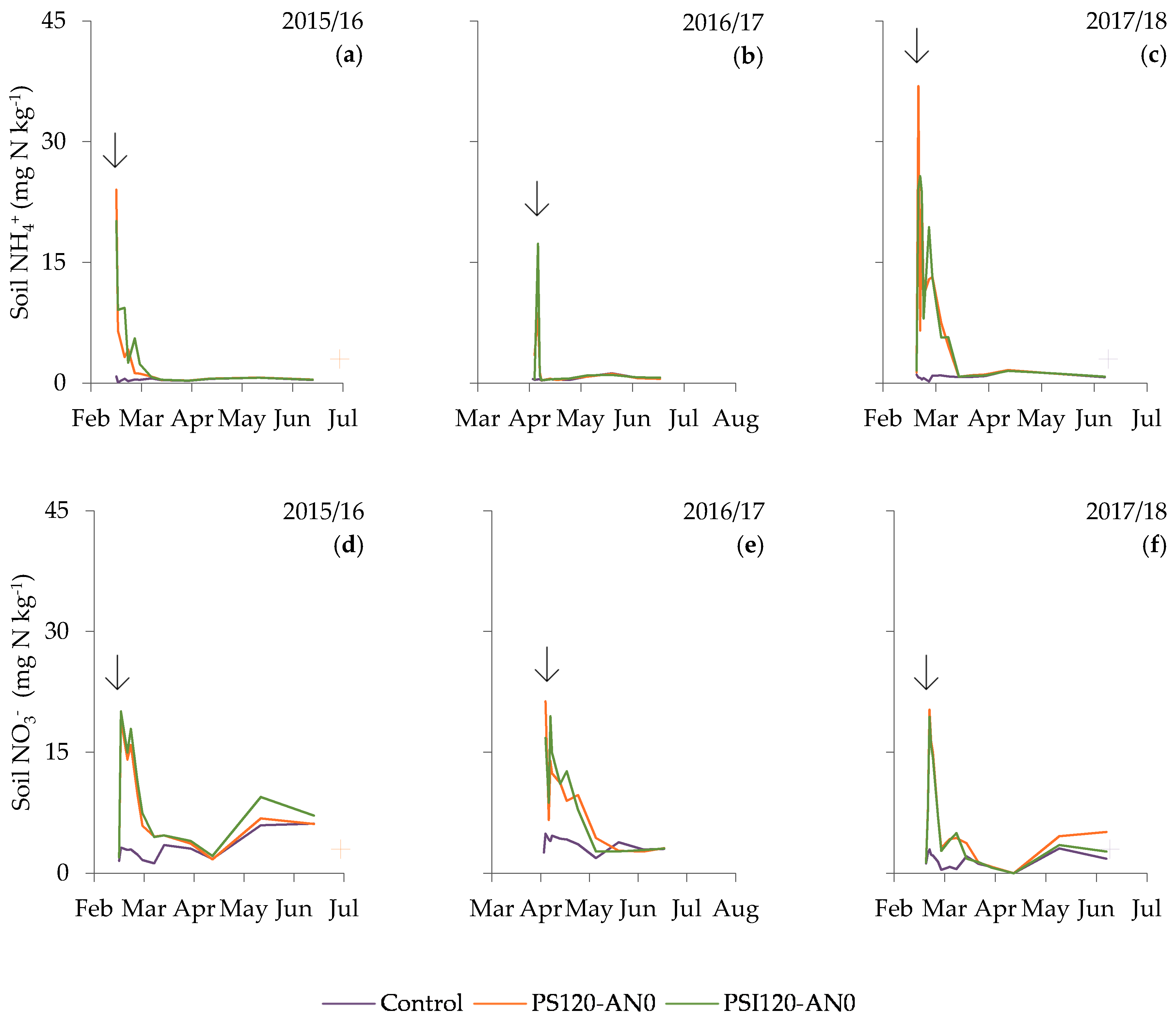
3.3. Dynamic of Soil Mineral Nitrogen
3.4. Nitrogen Balance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT—Wheat Production in 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 7 May 2020).
- Ministerio de Agricultura. Pesca y Alimentación Superficies y Producciones Anuales de Cultivos. Datos Avances de Cereales del Año 2018. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/superficies-producciones-anuales-cultivos/ (accessed on 7 May 2020).
- Borghi, B.; Corbellini, M.; Minoia, C.; Palumbo, M.; Di Fonzo, N.; Perenzin, M. Effects of Mediterranean climate on wheat bread-making quality. Eur. J. Agron. 1997, 6, 145–154. [Google Scholar] [CrossRef]
- European Commission. Pig Population—Annual Data (2018). Available online: https://ec.europa.eu/eurostat/data/database (accessed on 7 May 2020).
- Daudén, A.; Quílez, D. Pig slurry versus mineral fertilization on corn yield and nitrate leaching in a Mediterranean irrigated environment. Eur. J. Agron. 2004, 21, 7–19. [Google Scholar] [CrossRef]
- Gómez-Garrido, M.; Faz-Cano, Á.; Martínez-Martínez, S.; Carmona-Garcés, D.M.; Büyükkılıç-Yanardag, A. Nitrogen dynamic and leaching in calcareous soils amended with pig slurry. In Soil Management and Climate Change; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–187. [Google Scholar]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Cobena, A.; Misselbrook, T.H.; Hernáiz, P.; Vallejo, A. Impact of rainfall to the effectiveness of pig slurry shallow injection method for NH3 mitigation in a Mediterranean soil. Atmos. Environ. 2019, 216, 116913. [Google Scholar] [CrossRef]
- Diez, J.A.; de la Torre, A.I.; Cartagena, M.C.; Carballo, M.; Vallejo, A.; Muñoz, M.J. Evaluation of the Application of Pig Slurry to an Experimental Crop Using Agronomic and Ecotoxicological Approaches. J. Environ. Qual. 2001, 30, 2165–2172. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Valdez, A.S.; Bosch-Serra, À.D.; Ortiz, C.; Castellví, F. A short-term study to compare field strategies for ammonia emission mitigation. J. Environ. Qual. 2019, 48, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissions from soils: How well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Freney, J.R. Emission of nitrous oxide from soils used for agriculture. Nutr. Cycl. Agroecosyst. 1997, 49, 1–6. [Google Scholar] [CrossRef]
- Coyne, M.S. Biological Denitrification. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; Agronomy Monographs; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2008; Chapter 7; Volume 49, pp. 201–253. [Google Scholar]
- Prosser, J.I. Nitrogen in soil. Nitrification. Encycl. Soils Environ. 2005, 31–39. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and adaptive management of nitrification in agricultural soils. Front. Microbiol. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Debaeke, P.; Aussenac, T.; Fabre, J.L.; Hilaire, A.; Pujol, B.; Thuries, L. Grain nitrogen content of winter bread wheat (Triticum aestivum L.) as related to crop management and to the previous crop. Eur. J. Agron. 1996, 5, 273–286. [Google Scholar] [CrossRef]
- Goss, M.J.; Tubeileh, A.; Goorahoo, D. A Review of the Use of Organic Amendments and the Risk to Human Health. In Advances in Agronomy; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 120, pp. 275–379. [Google Scholar]
- Bosch-Serra, À.D.; Ortiz, C.; Yagüe, M.R.; Boixadera, J. Strategies to optimize nitrogen efficiency when fertilizing with pig slurries in dryland agricultural systems. Eur. J. Agron. 2015, 67, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-de-Santiago, D.E.; Yagüe, M.R.; Bosch-Serra, À.D. Soil water repellency after slurry fertilization in a dryland agricultural system. Catena 2019, 174, 536–545. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Lassaletta, L.; Aguilera, E.; del Prado, A.; Garnier, J.; Billen, G.; Iglesias, A.; Sánchez, B.; Guardia, G.; Abalos, D.; et al. Strategies for greenhouse gas emissions mitigation in Mediterranean agriculture: A review. Agric. Ecosyst. Environ. 2017, 238, 5–24. [Google Scholar] [CrossRef] [Green Version]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manage. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements. FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Yagüe, M.R.; Quílez, D. On-farm measurement of electrical conductivity for the estimation of ammonium nitrogen concentration in pig slurry. J. Environ. Qual. 2012, 41, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, E.A.; Robertson, G.P.; Greenberg, J.; Groffman, P.M.; Boone, R.D.; Gosz, J.R. Soil CO2, N2O, and CH4 exchange. In Standard Soil Methods for Long-Term Ecological Research; Robertson, G.P., Bledsoe, C.S., Coleman, D.C., Sollins, P., Eds.; Oxford University Press: New York, NY, USA, 1999; pp. 185–201. [Google Scholar]
- Alves, B.J.R.; Smith, K.A.; Flores, R.A.; Cardoso, A.S.; Oliveira, W.R.D.; Jantalia, C.P.; Urquiaga, S.; Boddey, R.M. Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol. Biochem. 2012, 46, 129–135. [Google Scholar] [CrossRef]
- Mateo-Marín, N.; Quílez, D.; Guillén, M.; Isla, R. Feasibility of stabilised nitrogen fertilisers decreasing greenhouse gas emissions under optimal management in sprinkler irrigated conditions. Agric. Ecosyst. Environ. 2020, 290, 106725. [Google Scholar] [CrossRef]
- MacKenzie, A.F.; Fan, M.X.; Cadrin, F. Nitrous Oxide Emission in Three Years as Affected by Tillage, Corn-Soybean-Alfalfa Rotations, and Nitrogen Fertilization. J. Environ. Qual. 1998, 27, 698. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Grossman, R.B.; Reinsch, T.G. 2.1 Bulk Density and Linear Extensibility. In Methods of Soil Analysis: Part 4 Physical Methods; Dane, J.H., Topp, C.G., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2002; pp. 201–228. [Google Scholar]
- Cerrato, M.E.; Blackmer, A.M. Comparison of models for describing corn yield response to nitrogen fertilizer. Agron. J. 1990, 82, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Wrigley, C.W.; Batey, I.L. Assessing grain quality. In Breadmaking; Elsevier: Amsterdam, The Netherlands, 2012; pp. 149–187. [Google Scholar]
- Meisinger, J.J.; Randall, G.W. Estimating Nitrogen Budgets for Soil-Crop Systems. In Managing Nitrogen for Groundwater Quality and Farm Profitability; Follett, R.F., Keeney, D.R., Cruse, R.N., Eds.; Soil Science Society of America: Madison, WI, USA, 1991; pp. 85–124. [Google Scholar]
- Schwede, D.B.; Simpson, D.; Tan, J.; Fu, J.S.; Dentener, F.; Du, E.; deVries, W. Spatial variation of modelled total, dry and wet nitrogen deposition to forests at global scale. Environ. Pollut. 2018, 243, 1287–1301. [Google Scholar] [CrossRef] [PubMed]
- Recio, J.; Alvarez, J.M.; Rodriguez-Quijano, M.; Vallejo, A. Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environ. Pollut. 2019, 245, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the Dinitrogen/Nitrous Oxide Ratio as Affected by Soil Water, Available Carbon, and Nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Richards, L.A.; Weaver, L.R. Moisture retention by some irrigated soils as related to soil-moisture tension. J. Agric. Res. 1944, 69, 215–235. [Google Scholar]
- Hernández, D.; Polo, A.; Plaza, C. Long-term effects of pig slurry on barley yield and N use efficiency under semiarid Mediterranean conditions. Eur. J. Agron. 2013, 44, 78–86. [Google Scholar] [CrossRef]
- Moreno-García, B.; Guillén, M.; Quílez, D. Response of paddy rice to fertilisation with pig slurry in northeast Spain: Strategies to optimise nitrogen use efficiency. F. Crop. Res. 2017, 208, 44–54. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Cantero-Martínez, C.; Bareche, J.; Arrúe, J.L.; Lampurlanés, J.; Álvaro-Fuentes, J. Do no-till and pig slurry application improve barley yield and water and nitrogen use efficiencies in rainfed Mediterranean conditions? F. Crop. Res. 2017, 203, 74–85. [Google Scholar] [CrossRef] [Green Version]
- López-Bellido, L.; López-Bellido, R.J.; Redondo, R. Nitrogen efficiency in wheat under rainfed Mediterranean conditions as affected by split nitrogen application. F. Crop. Res. 2005, 94, 86–97. [Google Scholar] [CrossRef]
- Lloveras, J.; Lopez, A.; Ferran, J.; Espachs, S.; Solsona, J. Bread-making wheat and soil nitrate as affected by nitrogen fertilization in irrigated Mediterranean conditions. Agron. J. 2001, 93, 1183–1190. [Google Scholar] [CrossRef]
- Mateo-Marín, N.; Isla, R.; Guillén, M.; Quílez, D. Efectos de dos aditivos sobre la volatilización de amoniaco en la aplicación agrícola de purín porcino. In Proceedings of the VI Workshop REMEDIA “Mitigación y Adaptación Con-ciencia”, Granada, Spain, 11–12 April 2018. [Google Scholar]
- EU Nitrogen Expert Panel. Nitrogen Use Efficiency (NUE)—An Indicator for the Utilization of Nitrogen in Agriculture and Food Systems; EU Nitrogen Expert Panel: Wageningen, The Netherlands, 2015. [Google Scholar]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 87, pp. 85–156. [Google Scholar]
- Dobermann, A. Nutrient use efficiency—Measurement and management. In Fertilizer Best Management Practies. General Principles, Strategy for Their Adoption and Voluntary Initiatives vs. Regulations; International Fertilizer Industry Association: Paris, France, 2007; pp. 1–28. [Google Scholar]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Aust. J. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Liu, X.J.; Mosier, A.R.; Halvorson, A.D.; Zhang, F.S. The impact of nitrogen placement and tillage on NO, N2O, CH4 and CO2 fluxes from a clay loam soil. Plant Soil 2006, 280, 177–188. [Google Scholar] [CrossRef]
- Liu, Z.; Powers, W. Meta-analysis of greenhouse gas emissions from swine manure land application. In Proceedings of the 2012 ASABE Annual International Meeting, Dallas, TX, USA, 29 July–1 August 2012; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012. [Google Scholar]
- Intergovernmental Panel on Climate Change Chapter 11: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. In 2006 IPCC Guidelines for National Greenhouse Gas Inventories Volume 4: Agriculture, Forestry and Other Land Use; Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) Institute for Global Enviromental Strategies: Kanagawa, Japan, 2006; p. 54. [Google Scholar]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryals, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Intergovernmental Panel on Climate Change Chapter 11: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Volume 4: Agriculture, Forestry and Other Land Use; Calvo-Buendia, E.; Tanabe, K.; Kranjc, A.; Baasansuren, J.; Fukuda, M.S.N.; Osako, A.; Pyrozhenko, Y.; Shermanau, P.; Federici, S. (Eds.) Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; p. 48. [Google Scholar]



| Soil Property | 0–30 cm | 30–60 cm | 60–90 cm |
|---|---|---|---|
| Soil Texture | Silty-Loam | Silty-Loam | Loam |
| Sand (%) | 32.5 | 31.1 | 38.2 |
| Silt (%) | 50.5 | 51.9 | 49.5 |
| Clay (%) | 17.0 | 17.0 | 12.3 |
| Stoniness (%vol.) | 1 | 1 | 1 |
| Equivalent Calcium Carbonate (g kg−1) | 40 | 41 | 39 |
| Total Nitrogen (Kjeldahl) (mg kg−1) | 1350 | 940 | 620 |
| Phosphorous (Olsen) (mg kg−1) | 43 | 12.1 | < 5.0 |
| Potassium (NH4Ac) (mg kg−1) | 408 | 231 | 101 |
| Organic Matter (%) | 1.84 | 0.92 | 0.50 |
| pH (1:2.5H2O) | 8.36 | 8.36 | 8.28 |
| Electrical Conductivity (1:5H2O) (dS m−1) | 0.265 | 0.261 | 0.307 |
| Crop Management Characteristic | 2015/16 | 2016/17 | 2017/18 |
|---|---|---|---|
| Sowing Date | 26/11/2015 | 30/12/2016 | 16/11/2017 |
| Harvest Date | 07/07/2016 | 04/07/2017 | 06/07/2018 |
| Seed Rate (kg ha−1) | 170 | 200 | 175 |
| Date N Side-Dress at tillering | 24/02/2016 | 21/03/2017 | 00/03/2018 |
| Date N Side-Dress at Stem Elongation | 05/04/2016 | 08/04/2017 | 24/04/2018 |
| Irrigation + Rain (mm) 1 | 380 | 435 | 428 |
| Crop ET (mm) | 416 | 429 | 383 |
| Pig Slurry Characteristic | 2015/16 | 2016/17 | 2017/18 | |
|---|---|---|---|---|
| Density (kg m−3) | 1018 ± 2 | 1020 ± 1 | 1012 ± 0 | |
| pH | - | 7.6 ± 0.0 | 8.4 ± 0.0 | |
| Dry Matter (kg DM m−3) | 24.1 ± 1.4 | 36.4 ± 0.9 | 12.7 ± 0.2 | |
| Organic Matter (kg OM m−3) | 10.3 ± 0.7 | 21.6 ± 0.9 | 6.1 ± 0.2 | |
| Ammonium Nitrogen (kg N m−3) | 2.8 ± 0.1 | 3.1 ± 0.1 | 2.2 ± 0.1 | |
| Organic Nitrogen (kg N m−3) | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.2 | |
| Phosphorous (kg P2O5 m−3) | 0.4 ± 0.1 | 0.8 ± 0.0 | 0.9 ± 0.5 | |
| Potassium (kg K2O m−3) | 2.8 ± 0.0 | 3.7 ± 0.1 | 3.4 ± 0.1 | |
| PS Rate 1 | (m3 ha−1) | 27.2 | 37.1 | 56.6 |
| PS Rate | (kg NH4+-N ha−1) | 74 | 114 | 118 |
| PSI Rate 2 | (m3 ha−1) | 28.6 | 34.5 | 57.5 |
| PSI Rate | (kg NH4+-N ha−1) | 85 | 110 | 129 |
| U120-AN0 | PS120-AN0 | PSI120-AN0 | p-Value | |
|---|---|---|---|---|
| NUEb | ||||
| 2015/16 | 2.79 ± 0.29 | 4.97 ± 0.94 | 4.56 ± 0.61 | 0.105 |
| 2016/17 | 1.85 ± 0.04 | 1.71 ± 0.06 | 1.91 ± 0.14 | 0.345 |
| 2017/18 | 1.48 ± 0.17 | 1.38 ± 0.13 | 1.35 ± 0.12 | 0.737 |
| NUEg | ||||
| 2015/16 | 1.29 ± 0.04 | 2.69 ± 0.38 | 2.23 ± 0.30 | 0.132 |
| 2016/17 | 0.96 ± 0.03 | 0.93 ± 0.04 | 1.02 ± 0.08 | 0.160 |
| 2017/18 | 0.90 ± 0.09 | 0.96 ± 0.11 | 0.89 ± 0.05 | 0.688 |
| REN | ||||
| 2015/16 | −0.05 ± 0.29 | 0.12 ± 0.59 | 0.31 ± 0.10 | 0.836 |
| 2016/17 | 0.48 ± 0.04 | 0.26 ± 0.04 | 0.39 ± 0.13 | 0.367 |
| 2017/18 | 0.65 ± 0.17 | 0.52 ± 0.09 | 0.56 ± 0.09 | 0.657 |
| Control | U120-AN30 | PS120-AN30 | PSI120-AN30 | p-Value | |
|---|---|---|---|---|---|
| N2O (g N ha−1) | |||||
| 2015/16 | 233 ± 86 b | 1624 ± 650 a | 1314 ± 330 a | 1428 ± 587 a | <0.001 |
| 2016/17 | 576 ± 167 b | 2101 ± 321 ab | 2427 ± 447 a | 2638 ± 715 a | 0.009 |
| 2017/18 | 519 ± 127 b | 2129 ± 400 ab | 3094 ± 817 a | 2538 ± 770 a | 0.008 |
| 2015/18 | 1532 ± 387 b | 6140 ± 1441 a | 7262 ± 1390 a | 7086 ± 2235 a | 0.007 |
| U120-AN30 | PS120-AN30 | PSI120-AN30 | p-Value | |
|---|---|---|---|---|
| EF (%) | ||||
| 2015/16 | 0.93 ± 0.38 | 0.91 ± 0.25 | 0.92 ± 0.49 | 0.999 |
| 2016/17 | 1.02 ± 0.21 | 1.06 ± 0.23 | 1.26 ± 0.39 | 0.929 |
| 2017/18 | 1.07 ± 0.20 | 1.42 ± 0.56 | 1.01 ± 0.37 | 0.510 |
| 2015/18 | 1.02 ± 0.25 | 1.18 ± 0.26 | 1.12 ± 0.44 | 0.469 |
| YSN2O (g N2O-N kg−1 grain) | ||||
| 2015/16 | 0.19 ± 0.08 | 0.15 ± 0.03 | 0.15 ± 0.06 | 0.919 |
| 2016/17 | 0.41 ± 0.05 | 0.49 ± 0.11 | 0.49 ± 0.12 | 0.964 |
| 2017/18 | 0.38 ± 0.05 | 0.48 ± 0.12 | 0.41 ± 0.11 | 0.763 |
| 2015/18 | 0.31 ± 0.06 | 0.36 ± 0.06 | 0.33 ± 0.10 | 0.893 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo-Marín, N.; Isla, R.; Guillén, M.; Quílez, D. Agronomic and Environmental Implications of Substituting Pig Slurry for Synthetic Nitrogen in Mediterranean Wheat Systems. Agronomy 2020, 10, 1498. https://doi.org/10.3390/agronomy10101498
Mateo-Marín N, Isla R, Guillén M, Quílez D. Agronomic and Environmental Implications of Substituting Pig Slurry for Synthetic Nitrogen in Mediterranean Wheat Systems. Agronomy. 2020; 10(10):1498. https://doi.org/10.3390/agronomy10101498
Chicago/Turabian StyleMateo-Marín, Noemí, Ramón Isla, Mónica Guillén, and Dolores Quílez. 2020. "Agronomic and Environmental Implications of Substituting Pig Slurry for Synthetic Nitrogen in Mediterranean Wheat Systems" Agronomy 10, no. 10: 1498. https://doi.org/10.3390/agronomy10101498
APA StyleMateo-Marín, N., Isla, R., Guillén, M., & Quílez, D. (2020). Agronomic and Environmental Implications of Substituting Pig Slurry for Synthetic Nitrogen in Mediterranean Wheat Systems. Agronomy, 10(10), 1498. https://doi.org/10.3390/agronomy10101498





