Root Lignin Composition and Content in Oil Palm (Elaeis guineensis Jacq.) Genotypes with Different Defense Responses to Ganoderma boninense
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oil Palm Genotypes
2.2. Basal Stem Rot Disease Trial
2.2.1. Large-Scale Fungal Inoculum Preparation
2.2.2. Artificial Infection of Oil Palm Genotypes
2.3. Plant Functional Traits
2.4. Lignin Content and Composition
2.4.1. Preparation of Extractive-Free Root Samples
2.4.2. Thioglycolic Acid (TGA) Lignin Determination
2.4.3. Lignin Composition
2.5. Mineral Element Analysis
2.6. Statistical Analysis
3. Results
3.1. Oil Palm Genotypes and Basal Stem Rot Disease Incidence (%)
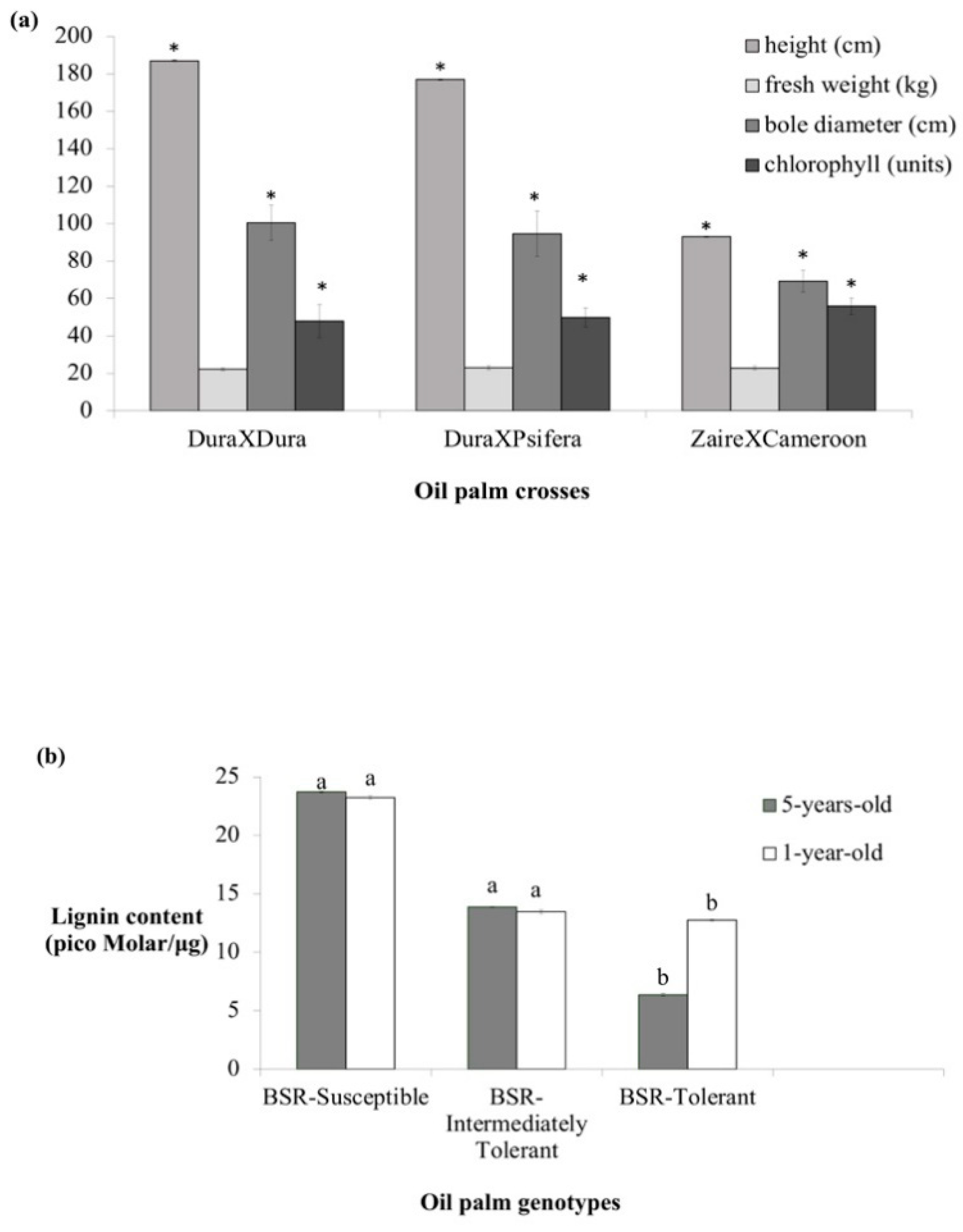
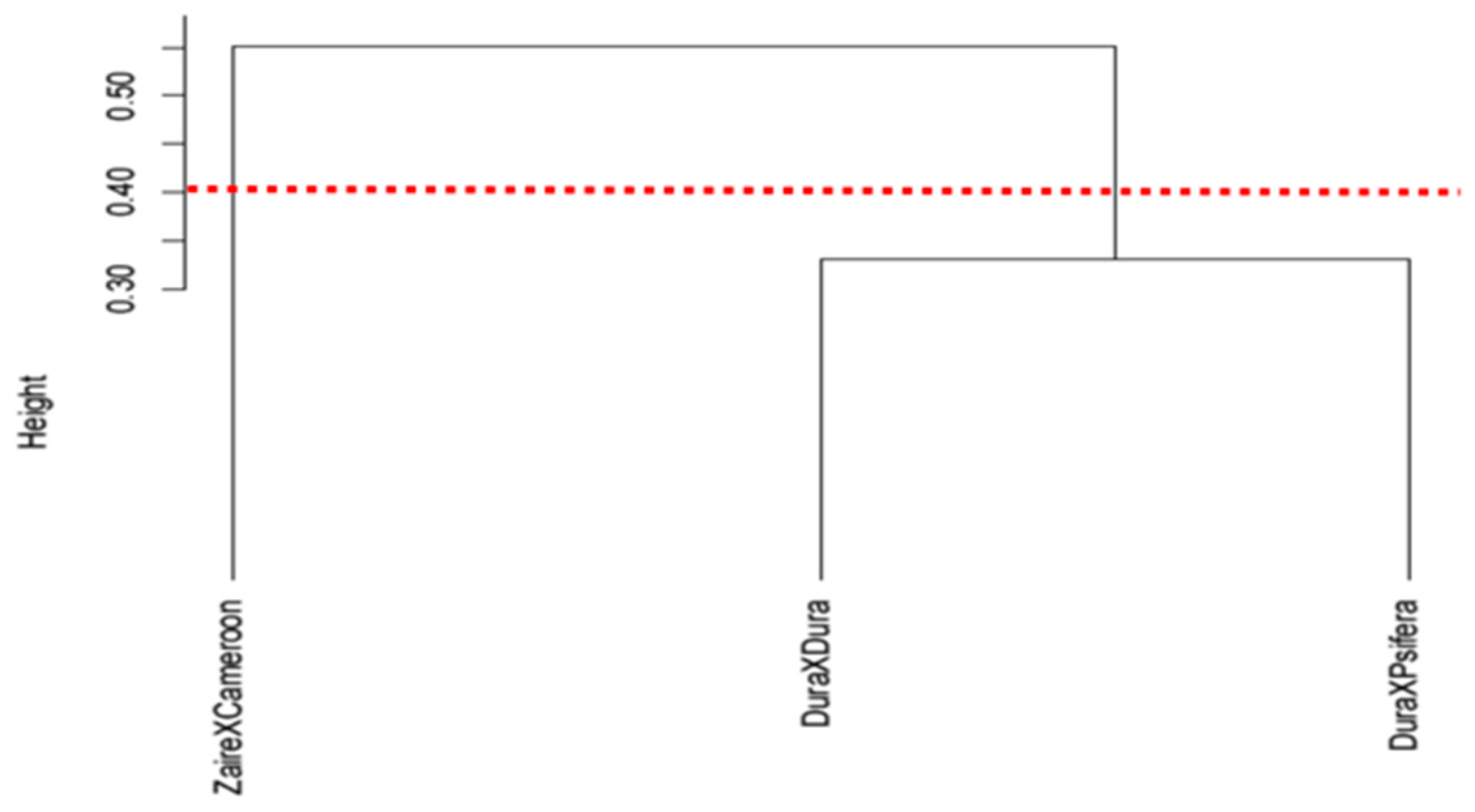
3.2. Variation in Plant Functional Traits Among the Oil Palm Genotypes
3.3. Lignin Content Distinguishes the Oil Palm Genotypes
3.4. Lignin Composition in Oil Palm Genotypes
3.5. Elemental Nutrient Content of Oil Palm Genotypes with Differing Defense Response to G. boninense
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wahid, B.; Siti, N.A.A.; Henson, I.E. Oil Palm-Achievements and Potentials. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Barcelos, E.; Rios, S.A.; Cunha, R.N.V.; Lopes, R.; Motoike, S.Y.; Babiychuk, E.; Skirycz, A.; Kushnir, S. Oil palm natural diversity and the potential for yield improvement. Front. Plant Sci. 2015, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Palm Oil Industry Worldwide-Statistics & Facts. Available online: www.statista.com/topics/6079/global-palm-oil-industry (accessed on 6 July 2020).
- Corley, R.H.V. How much palm oil do we need? Environ. Sci. Policy 2009, 12, 134–139. [Google Scholar] [CrossRef]
- Singh, G. Ganoderma—The scourge of oil palm in the coastal areas. Planter 1991, 786, 421–444. [Google Scholar]
- Singh, R.; Ong-Abdullah, M.; Low, E.L.; Manaf, M.A.A.; Rosli, R.; Nookiah, R.; Ooi, L.C.; Ooi, S.; Chan, K.; Halim, M.A.; et al. Oil palm genome sequence reveals divergence of infertile species in old and new worlds. Nature 2013, 500, 335–339. [Google Scholar] [CrossRef] [Green Version]
- Pilotti, C.A. Stem rots of oil palm caused by Ganoderma boninense: Pathogen biology and epidemiology. Mycopathologia 2005, 159, 129–137. [Google Scholar] [CrossRef]
- Flood, J.; Hasan, Y. Basal stem rot—Taxonomy, biology, epidemiology, economic status and control in South East Asia and Pacific Islands. In Proceedings of the Malaysian Palm Oil Board Conference, Kuala Lumpur, Malasia, 18–19 May 2004; MPOB: Kuala Lumpur, Malaysia, 2004; pp. 117–133. [Google Scholar]
- Rees, R.W.; Flood, J.; Hassan, Y.; Potterd, U.; Cooper, R.M. Basal stem rot of oil palm (Elaies guineensis): Mode of infection and lower stem invasion by Ganoderma boninense. Plant Pathol. 2009, 58, 982–989. [Google Scholar] [CrossRef]
- Govender, N.T.; Seman, I.A.; Mahmood, M.; Wong, M.Y. The phenylpropanoid pathway and lignin in defence against Ganoderma boninense colonized root tissues in oil palm (Elaeis guineensis Jacq.). Front. Plant Sci. 2017, 8, 1395. [Google Scholar] [CrossRef] [Green Version]
- Govender, N.T.; Wong, M.-Y. Detection of oil palm root penetration by Agrobacterium-mediated transformed Ganoderma boninense, expressing green fluorescent protein. Phytopathology 2017, 107, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Idris, A.S.; Ariffin, D. Basal Stem Rot-Biology, Detection and Control. In Proceedings of the International Conference on Pest and Disease of Importance to the Palm Industry, Kuala Lumpur, Malaysia, 18–19 May 2004. [Google Scholar]
- Idris, A.S.; Khusairi, D.; Ismail, S.; Ariffin, D. Selection for partial resistance in oil palm to Ganoderma basal stem rot. In Proceedings of the Seminar Recent Progress in the Management of Peat and Ganoderma, Bangi, Malaysia, 6–7 May 2002. [Google Scholar]
- Umezawa, T. The cinnamate/monolignol pathway. Phytochem. Rev. 2009, 9, 1–17. [Google Scholar] [CrossRef]
- Bose, S.K.; Francis, R.C.; Govender, M.; Bush, T.; Saprk, A. Lignin content versus syringyl to guaiacyl ratio amongst poplars. Bioresour. Technol. 2009, 100, 1628–1633. [Google Scholar] [CrossRef]
- Li, L.; Zhou, Y.; Cheng, X.; Sun, J.; Marita, J.M.; Ralph, J.; Chiang, V.L. Combinatorial modification of multiple lignin traits in trees through multigene co-transformation. Proc. Natl. Acad. Sci. USA 2003, 100, 4939–4944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skyba, O.; Douglas, C.J.; Mansfielda, S.D. Syringyl-rich lignin renders Poplars more resistant to degradation by wood decay fungi. Appl. Environ. Microbiol. 2013, 79, 2560–2571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallis, A.F.A.; Wearne, R.H.; Wright, P.J. Analytical characteristics of plantation eucalypt woods relating to kraft pulp yields. Appita J. 1996, 49, 427–432. [Google Scholar]
- Lapierre, C.; Pollet, B.; Petit-Conil, M.; Toval, G.; Romero, J.; Pilate, G.; Leple, J.C.; Boerjan, W.; Ferret, V.V.; De Nadai, V.; et al. Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid o-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol. 1999, 119, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Giraldo, L.; Posé, S.; Pattathil, S.; Peralta, A.; Hahn, M.G.; Ayre, B.G.; Sunuwar, J.; Hernandez, J.; Patel, M.A.; Shah, J.; et al. Elicitors and defence gene induction in plants with altered lignin compositions. New Phytol. 2018, 219, 1235–1251. [Google Scholar] [CrossRef] [Green Version]
- Agrios, N.G. Plant Pathology; Elsevier-Academic Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Vanholme, R.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin engineering. Curr. Opin. Plant Biol. 2008, 11, 278–285. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure: Updates on lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Dixon, R.A. Altering the cell wall and its impact on plant disease: From forage to bioenergy. Annu. Rev. Phytopathol. 2014, 52, 69–91. [Google Scholar] [CrossRef] [Green Version]
- Eynck, C.; Seguin-swartz, G.; Clarke, W.E.; Parkin, I.A.P. Monolignol biosynthesis is associated with resistance to Sclerotinia sclerotiorum in Camelia sativa. Mol. Plant Pathol. 2012, 13, 887–899. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Rodrigues, F.A.; Seebold, K.W. Silicon and plant disease. In Silicon in Agriculture; Datnoff, L.E., Elmer, W.H., Huber, D.M., Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Huber, D.M.; Graham, R.D. The role of nutrition in crop resistance and tolerance to disease. In Mineral Nutrition of Crops Fundamental Mechanisms and Implications; Rengel, Z., Ed.; Food Product Press: New York, NY, USA, 1999; pp. 205–226. [Google Scholar]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. Agro. Sustain. Develop. 2008, 28, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Bruce, R.J.; West, C.A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989, 91, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, K.; Blaschke, L.; Polle, A. Comparisons of different methods for lignin determination as a basis for calibration of near-infrared reflectance spectroscopy and implications of lignoproteins. J. Chem. Ecol. 2002, 28, 2483–2501. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1992; pp. 518–523. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 25 July 2020).
- Paterson, R.R.M. Ganoderma disease of oil palm-A white-rot perspective necessary for integrated control. Crop Prot. 2007, 26, 1369–1376. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.R.M.; Meon, S.; Lima, N. The feasibility of producing oil palm with altered lignin content to control Ganoderma disease. J. Phytopathol. 2009, 157, 649–656. [Google Scholar] [CrossRef]
- Ana, R.D.; Ian, A.D. Dubery Panama Disease: Cell wall reinforcement in banana roots in response to elicitors from Fusarium oxysporum f. s. cubense Race Four. Biochem. Cell Biol. 2000, 9, 1173–1180. [Google Scholar]
- Kostyn, K.; Czemplik, M.; Kulma, A.; Bortniczuk, M.; Skala, J.; Szopa, J. Genes of phenylpropanoid pathway are activated in early response to Fusarium attack in flax plants. Plant Sci. 2012, 190, 103–115. [Google Scholar] [CrossRef]
- Hancock, J.E.; Loya, W.M.; Giardina, C.P.; Chiang, V.L.; Pregitzer, K.S. Plant growth, biomass partitioning and soil carbon formation in response to latered lignin biosynthesis in Populus tremuloides. New Phytol. 2007, 173, 732–742. [Google Scholar] [CrossRef]
- Elissetche, J.P.; Valenzzuela, S.; Garcia, R.; Norambuena, M.; Iturra, C.; Rodriguez, J.; Mendonea, R.T.; Balocchi, C. Transcript abundance of enzymes involved in lignin biosynthesis of Eucalyptus globules genotypes with contrasting levels of pulp yield and wood density. Tree Genet. Genomes 2011, 7, 697–705. [Google Scholar] [CrossRef]
- Klash, A.; Ncube, E.; Toit, B.; Meincken, M. Determination of the cellulose and lignin content on wood fibre surfaces of Eucalypts as a function of genotype and site. Eur. J. For. Res. 2010, 129, 741–748. [Google Scholar] [CrossRef]
- Dowd, P.F.; Johnson, E.T. Differential resistance of switchgrass Panicum virgatum L. lines to fall armyworms Spodoptera frugiperda (J.E. Smith). Genet. Resour. Crop Evol. 2009, 56, 1077–1089. [Google Scholar] [CrossRef]
- Pedersen, J.F.; Vogel, K.P.; Funnell, D. Impact of reduced lignin on plant fitness. Crop Sci. 2005, 45, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Dowd, P.F.; Sarath, G.; Mitchell, R.B.; Saathoff, A.J.; Vogel, K.P. Insect resistance of a full sib family of tetraploid switchgrass Panicum virgatum L. with varying lignin levels. Genet. Resour. Crop Evol. 2013, 60, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.; Ishiga, T.; Decker, S.R.; Turner, G.B.; Craven, K.D. A novel delivery system for the root symbiotic fungus, Sebacina vermifera and consequent biomass enhancement of low lignin COMT Switchgras lines. Bioenergy Res. 2015, 8, 922–933. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Escamilla-Trevino, L.L.; Sathitsuksanoh, N.; Shen, Z.; Shen, H.; Zhang, Y.H.; Dixon, R.A.; Zhao, B. Silencing of 4-coumarate: Coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Sarkanen, K.V. Lignins: Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley-Science: New York, NY, USA, 1971. [Google Scholar]
- Blanchette, R.A. Delignification by wood-decay fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Highley, T.L. Influence of type and amount of lignin on decay by Coriolus versicolor. Can. J. For. Res. 1982, 12, 435–438. [Google Scholar] [CrossRef]
- Syafii, W.; Yoshimoto, T. Effect of lignin structure on decay resistance of some tropical woods. Indones. J. Trop. Agric. 1991, 3, 32–37. [Google Scholar]
- Stewart, J.J.; Akiyama, T.; Chapple, C.; Ralph, J.; Mansfield, S.D. The effects on lignin structure of overexpression of ferulate 5-hydroxylase in hybrid poplar. Plant Physiol. 2009, 150, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Stout, A.T.; Davis, A.A.; Domec, J.C.; Yang, C.; Shi, R.; King, J.S. Growth under field conditions affects lignin content and productivity in transgeneuc Populus trichocarpa with altered lignin biosynthesis. Biomass Bioenergy 2014, 68, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Voelker, S.; Lachenbruch, B.; Meinzer, F.; Jourdes, M.; Ki, C.; Patten, A. Antisense down-regulation of 4CL expression alters lignifications, tree growth, and saccharification potential of field-grown poplar. Plant Physiol. 2010, 154, 874–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stit, M. Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006, 46, 533–548. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological function of mineral micronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Marafon, A.C.; Endres, L. Silicon: Fertilization and nutrition in higher plants. Amazon. J. Agric. Environ. Sci. 2013, 56, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Datnoff, L.E.; Elmer, W.; Huber, D.M. Mineral Nutrition and Plant Disease; APS Press: St Paul, MN, USA, 2007. [Google Scholar]
- Liang, Y.C.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zheng, L.; He, F.; Zhao, F.J.; Shen, Z.; Zheng, L. Transcriptional and physiological analyses identify a regulatory role for hydrogen peroxide in the lignin biosynthesis of copper-stressed rice roots. Plant Soil 2014, 387, 323–336. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, H.; Zhang, Q.; Jin, G.M.; Jiang, S.J.; Jiang, D.; He, Q.Y.; Li, Z.P. Copper-induced oxidative stress and responses of the antioxidant system in roots of Medicago sativa. J. Agron. Crop Sci. 2011, 197, 418–429. [Google Scholar] [CrossRef]
- Colzi, I.; Arnetoli, M.; Gallo, A.; Doumett, S.; Del Bubba, M.; Pignattelli, S.; Gabbrielli, R.; Gonnelli, C. Copper tolerance strategies involving the root cell wall pectins in Silene paradoxa. Environ. Exp. Bot. 2015, 78, 91–98. [Google Scholar] [CrossRef]
- Colzi, I.; Doumett, S.; Del Bubba, M.; Fornaini, J.; Arnetoli, M.; Gabbrielli, R.; Gonnelli, C. On the role of the cell wall in the phenomenon of copper tolerance in Silene paradoxa L. Environ. Exp. Bot. 2012, 72, 77–83. [Google Scholar] [CrossRef]
- Tengoua, F.F.; Hanafi, M.M.; Idris, A.S.; Syed-Omar, S.R. Comparative study of lignin in roots of different oil palm progenies in relation to Ganoderma basal stem rot disease. J. Oil Palm Res. 2015, 27, 128–134. [Google Scholar]
- Tengoua, F.F.; Hanafi, M.M.; Idris, A.S.; Kadir, J.; Jamaludin, N.M.A.; Mohidin, H.; Syed-Rastan, S.O. Basal stem rot disease incidence and severity on oil palm (Elaeis guineensis jacq.) seedlings. Am. J. Appl. Sci. 2014, 11, 1841–1859. [Google Scholar] [CrossRef] [Green Version]

| Oil Palm Crosses | Disease Incidence (DI%) 1 |
|---|---|
| DuraXDura | 84.6 ± 12.4 |
| DeliXPsifera | 57.8 ± 12.7 |
| DuraXPsifera | 54.2 ± 18.52 |
| ZaireXCameroon | 23.6 ± 14.82 |
| DuraXCameroon | 20.4 ± 13.71 |
| Oil Palm Age (Years) | Oil Palm Genotypes | G Subunit (%) 1 | S Subunit (%) 1 | S/G Ratio |
|---|---|---|---|---|
| One | ZaireXCameroon | 72.3 ± 0.24 | 24.7 ± 0.38 | 0.34 |
| DuraXPsifera | 83.9 ± 0.3 | 16.1 ± 0.27 | 0.19 | |
| DuraXDura | 80.9 ± 0.25 | 19.1 ± 0.29 | 0.24 | |
| Five | DuraXCameroon | 67.6 ± 0.34 | 32.4 ± 0.33 | 0.48 |
| DeliXPsifera | 83.8 ± 0.33 | 16.2 ± 0.31 | 0.19 | |
| DuraXDura | 85.0 ± 0.28 | 15.0 ± 0.37 | 0.18 |
| Oil Palm Genotypes | |||
|---|---|---|---|
| Elements (%) | ZaireXCameroon | DuraXPsifera | DuraXDura |
| Potassium (K) | 59.92 ± 5.57 | 62.22 ± 3.3 * | 61.00 ± 6.49 |
| Calcium (Ca) | 11.17 ± 2.01 | 9.45 ± 1.49 | 9.09 ± 3.41 |
| Iron (Fe) | 9.99 ± 3.51 | 10.31 ± 3.23 | 13.21 ± 2.7 * |
| Silicon (Si) | 4.93 ± 1.78 | 3.96 ± 1.37 | 7.04 ± 2.17 * |
| Titanium (Ti) | 2.17 ± 0.55 | 2.03 ± 0.64 | 2.79 ± 0.41 * |
| Sulphur (S) | 1.73 ± 0.52 | 1.81 ± 0.44 | 2.26 ± 0.38 * |
| Phosphorus (P) | 1.56 ± 0.62 | 1.91 ± 0.48 | 1.61 ± 0.35 |
| Copper (Cu) | 0.11 ± 0.03 | 0.2 ± 0.08 | 0.19 ± 0.04 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govender, N.; Abu-Seman, I.; Mui-Yun, W. Root Lignin Composition and Content in Oil Palm (Elaeis guineensis Jacq.) Genotypes with Different Defense Responses to Ganoderma boninense. Agronomy 2020, 10, 1487. https://doi.org/10.3390/agronomy10101487
Govender N, Abu-Seman I, Mui-Yun W. Root Lignin Composition and Content in Oil Palm (Elaeis guineensis Jacq.) Genotypes with Different Defense Responses to Ganoderma boninense. Agronomy. 2020; 10(10):1487. https://doi.org/10.3390/agronomy10101487
Chicago/Turabian StyleGovender, Nisha, Idris Abu-Seman, and Wong Mui-Yun. 2020. "Root Lignin Composition and Content in Oil Palm (Elaeis guineensis Jacq.) Genotypes with Different Defense Responses to Ganoderma boninense" Agronomy 10, no. 10: 1487. https://doi.org/10.3390/agronomy10101487
APA StyleGovender, N., Abu-Seman, I., & Mui-Yun, W. (2020). Root Lignin Composition and Content in Oil Palm (Elaeis guineensis Jacq.) Genotypes with Different Defense Responses to Ganoderma boninense. Agronomy, 10(10), 1487. https://doi.org/10.3390/agronomy10101487





