Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Soil Sampling
2.3. Measurement of Soil Properties and Soil Microbial Biomass
2.4. DNA Extraction and Amplicon Sequencing
2.5. Biological Information Analysis and Data Processing
2.6. Statistical Analysis
3. Results
3.1. Effects of Different Fertilizers on Soil Properties and Microbial Biomass
3.2. Effects of Different Fertilizers on Soil Bacteria Diversity Index
3.3. Effects of Different Fertilizers on Soil Bacterial Community Structure
3.4. Correlation between Bacterial Community and Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falkowski, P.G.; Tom, F.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2017, 624, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef]
- Phillips, R.P.; Bernhardt, E.S.; Schlesinger, W.H. Elevated CO2 increases root exudation from loblolly pine (Pinus taeda) seedlings as an N-mediated response. Tree Physiol. 2009, 29, 1513–1523. [Google Scholar] [CrossRef]
- Harsh Pal, B.; Sang-Wook, P.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar]
- Gabriele, B.; Kornelia, S. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2010, 68, 1–13. [Google Scholar]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Heikki, S.L.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Shang, Q.; Ling, N.; Feng, X.; Yang, X.; Wu, P.; Zou, J.; Shen, Q.; Guo, S. Soil fertility and its significance to crop productivity and sustainability in typical agroecosystem: A summary of long-term fertilizer experiments in China. Plant Soil 2014, 381, 13–23. [Google Scholar] [CrossRef]
- Yu, H.; Ling, N.; Wang, T.; Zhu, C.; Wang, Y.; Wang, S.; Gao, Q. Responses of soil biological traits and bacterial communities to nitrogen fertilization mediate maize yields across three soil types. Soil Tillage Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Berthrong, S.T.; Buckley, D.H.; Drinkwater, L.E. Agricultural Management and Labile Carbon Additions Affect Soil Microbial Community Structure and Interact with Carbon and Nitrogen Cycling. Microb. Ecol. 2013, 66, 158–170. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Zhang, J.; Huang, Q.; Deng, H.; Deng, Y.; Zhong, W. Long-term fertilization effects on active ammonia oxidizers in an acidic upland soil in China. Soil Biol. Biochem. 2015, 84, 28–37. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Min, L.; Qin, C.; Chen, Y.; Li, Y.; Huang, Q.; Wang, J.; Shen, Z.; Shen, Q. Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol. Fertil. Soils 2016, 52, 1–13. [Google Scholar] [CrossRef]
- Liu, L.; Li, T.; Wei, X.; Jiang, B.; Ping, F. Effects of a nutrient additive on the density of functional bacteria and the microbial community structure of bioorganic fertilizer. Bioresour. Technol. 2014, 172, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P.; Zeng, Z. Dynamics of Bacterial Communities in a 30-Year Fertilized Paddy Field under Different Organic–Inorganic Fertilization Strategies. Agronomy 2019, 9, 14. [Google Scholar] [CrossRef]
- Lazcano, C.; Gomez-Brandon, M.; Dominguez, J.; Revilla, P. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Hui, W.; Bai, E.; Lou, Y.; Zhang, A.; Zhuge, Y. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Gu, S.; Hu, Q.; Cheng, Y.; Bai, L.; Liu, Z.; Xiao, W.; Gong, Z.; Wu, Y.; Feng, K.; Deng, Y. Application of organic fertilizer improves microbial community diversity and alters microbial network structure in tea (Camellia sinensis) plantation soils. Soil Tillage Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Ling, N.; Wang, D.; Chen, Z.; Yang, S.; Yu, G.; Wei, R.; Huang, Q.; Guo, S.; Shen, Q. Response of the population size and community structure of Paenibacillus spp. to different fertilization regimes in a long-term experiment of red soil. Plant Soil 2014, 383, 87–98. [Google Scholar] [CrossRef]
- Murase, J.; Hida, A.; Ogawa, K.; Nonoyama, T.; Yoshikawa, N.; Imai, K. Impact of long-term fertilizer treatment on the microeukaryotic community structure of a rice field soil. Soil Biol. Biochem. 2015, 80, 237–243. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Wang, J.; Xue, C.; Song, Y.; Wang, L.; Huang, Q.; Shen, Q. Wheat and Rice Growth Stages and Fertilization Regimes Alter Soil Bacterial Community Structure, But Not Diversity. Front. Microbiol. 2016, 7, 1207. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tanja, M.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Christian, Q.; Elmar, P.; Pelin, Y.; Jan, G.; Timmy, S.; Pablo, Y.; Jorg, P.; Frank Oliver, G.C. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Pan, Y.; Cassman, N.; De Hollander, M.; Mendes, L.W.; Korevaar, H.; Geerts, R.H.E.M.; Van Veen, J.A.; Kuramae, E.E. Impact of long-term N, P, K, and NPK fertilization on the composition and potential functions of the bacterial community in grassland soil. FEMS Microbiol. Ecol. 2014, 90, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.C.; Swarup, A.; Wanjari, R.H.; Mishra, B.; Shahi, D.K. Long-term fertilization, manure and liming effects on soil organic matter and crop yields. Soil Tillage Res. 2007, 94, 397–409. [Google Scholar] [CrossRef]
- Sapp, M.; Harrison, M.; Hany, U.; Charlton, A.; Thwaites, R. Comparing the effect of digestate and chemical fertiliser on soil bacteria. Appl. Soil Ecol. 2015, 86, 1–9. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, C.; Farmer, J.; Sun, J. Effects of bio-organic fertilizer on pepper growth and Fusarium wilt biocontrol. Sci. Hortic. 2015, 193, 114–120. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Meidute, S.; Demoling, F.; Bååth, E. Antagonistic and synergistic effects of fungal and bacterial growth in soil after adding different carbon and nitrogen sources. Soil Biol. Biochem. 2008, 40, 2334–2343. [Google Scholar] [CrossRef]
- Girvan, M.S.; Campbell, C.D.; Killham, K.; Prosser, J.I.; Glover, L.A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef]
- Laurent, P.; Andersson, S.G.E.; Battin, T.J.; Prosser, J.I.; Schimel, J.P.; Whitman, W.B.; Sara, H. The ecological coherence of high bacterial taxonomic ranks. Nat. Rev. Microbiol. 2010, 8, 523–529. [Google Scholar]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Ping, H.; Wei, Z.; He, X. Reduced dependence of rhizosphere microbiome on plant-derived carbon in 32-year long-term inorganic and organic fertilized soils. Soil Biol. Biochem. 2015, 80, 70–78. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.M.; Shen, J.P.; Xu, Z.; He, J.Z. Soil type determines the abundance and community structure of ammonia-oxidizing bacteria and archaea in flooded paddy soils. J. Soils Sediments 2010, 10, 1510–1516. [Google Scholar] [CrossRef]
- Chu, H.; Noah, F.; Lauber, C.L.; Caporaso, J.G.; Rob, K.; Paul, G. Soil bacterial diversity in the Arctic is not fundamentally different from that found in other biomes. Environ. Microbiol. 2010, 12, 2998–3006. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Ling, N.; Chen, D.; Hui, G.; Wei, J.; Bai, Y.; Shen, Q.; Hu, S. Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 2017, 292, 25–33. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R. Identification of cellulose-responsive bacterial and fungal communities in geographically and edaphically different soils by using stable isotope probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef]
- Lopes, A.R.; Bello, D.; Prieto-Fernández, Á.; Trasar-Cepeda, C.; Manaia, C.M.; Nunes, O.C. Relationships among bulk soil physicochemical, biochemical, and microbiological parameters in an organic alfalfa-rice rotation system. Environ. Sci. Pollut. Res. 2015, 22, 11690–11699. [Google Scholar] [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 2016, 99, 137–149. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Ma, T.; Raza, W.; Li, J.; Howland, J.G.; Huang, Q.; Shen, Q. Impacts of inorganic and organic fertilization treatments on bacterial and fungal communities in a paddy soil. Appl. Soil Ecol. 2017, 112, 42–50. [Google Scholar] [CrossRef]
- Beauregard, M.S.; Hamel, C.; Atul-Nayyar; St-Arnaud, M. Long-Term Phosphorus Fertilization Impacts Soil Fungal and Bacterial Diversity but not AM Fungal Community in Alfalfa. Microb. Ecol. 2010, 59, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, D.; Yang, G.; Zhu, H.; Zhu, H.; Fei, Z.; Zhong, Z. Effects of tree species and soil properties on the composition and diversity of the soil bacterial community following afforestation. For. Ecol. Manag. 2018, 427, 342–349. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; Quadros, P.D.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; Mcgrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH Determines Microbial Diversity and Composition in the Park Grass Experiment. Microb. Ecol. 2015, 69, 395–406. [Google Scholar] [CrossRef] [PubMed]
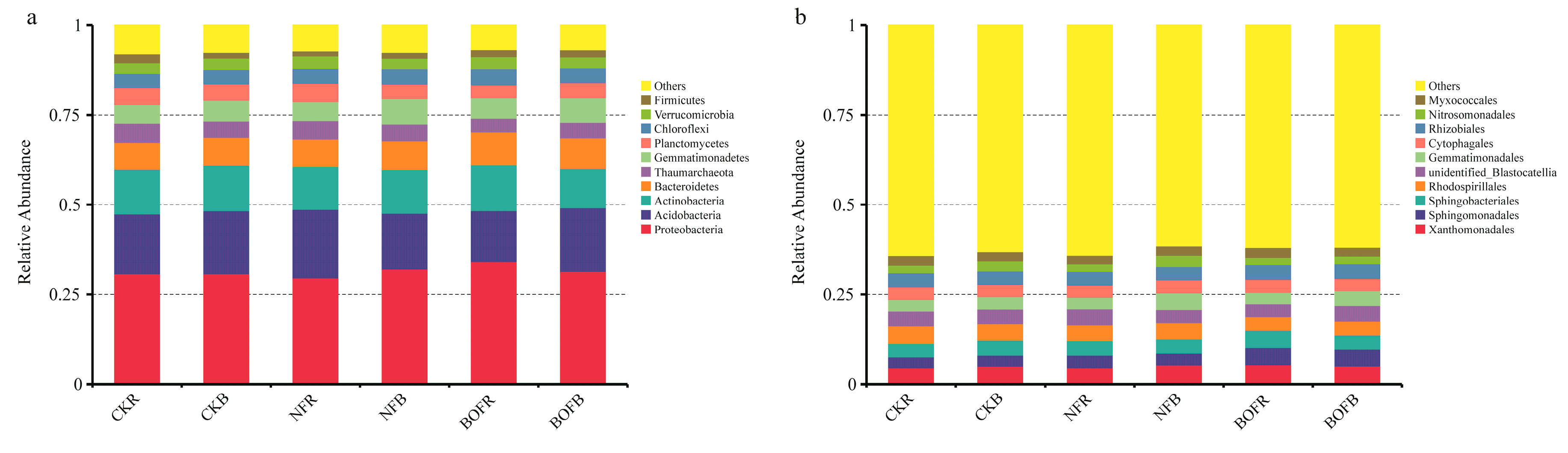
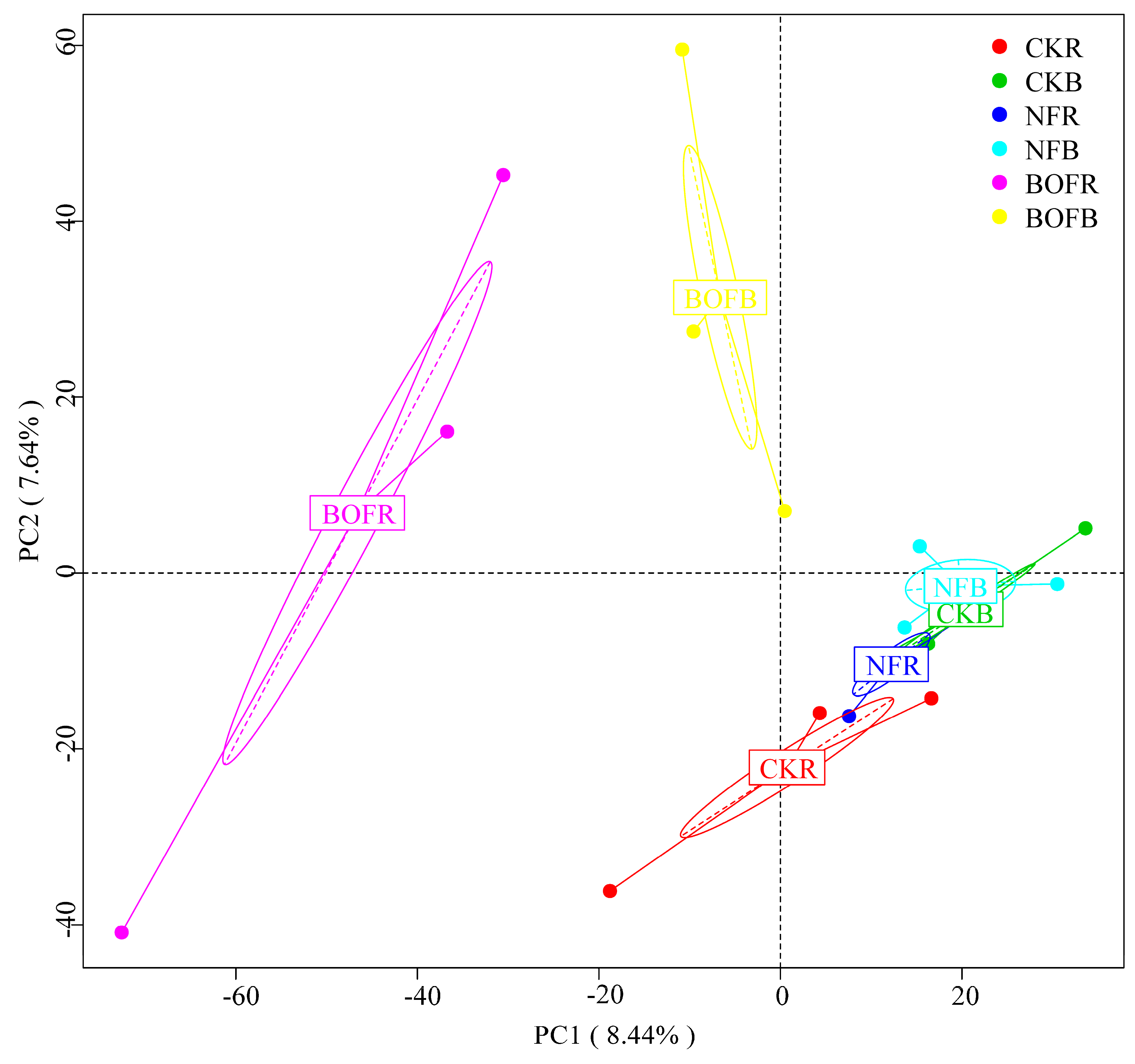
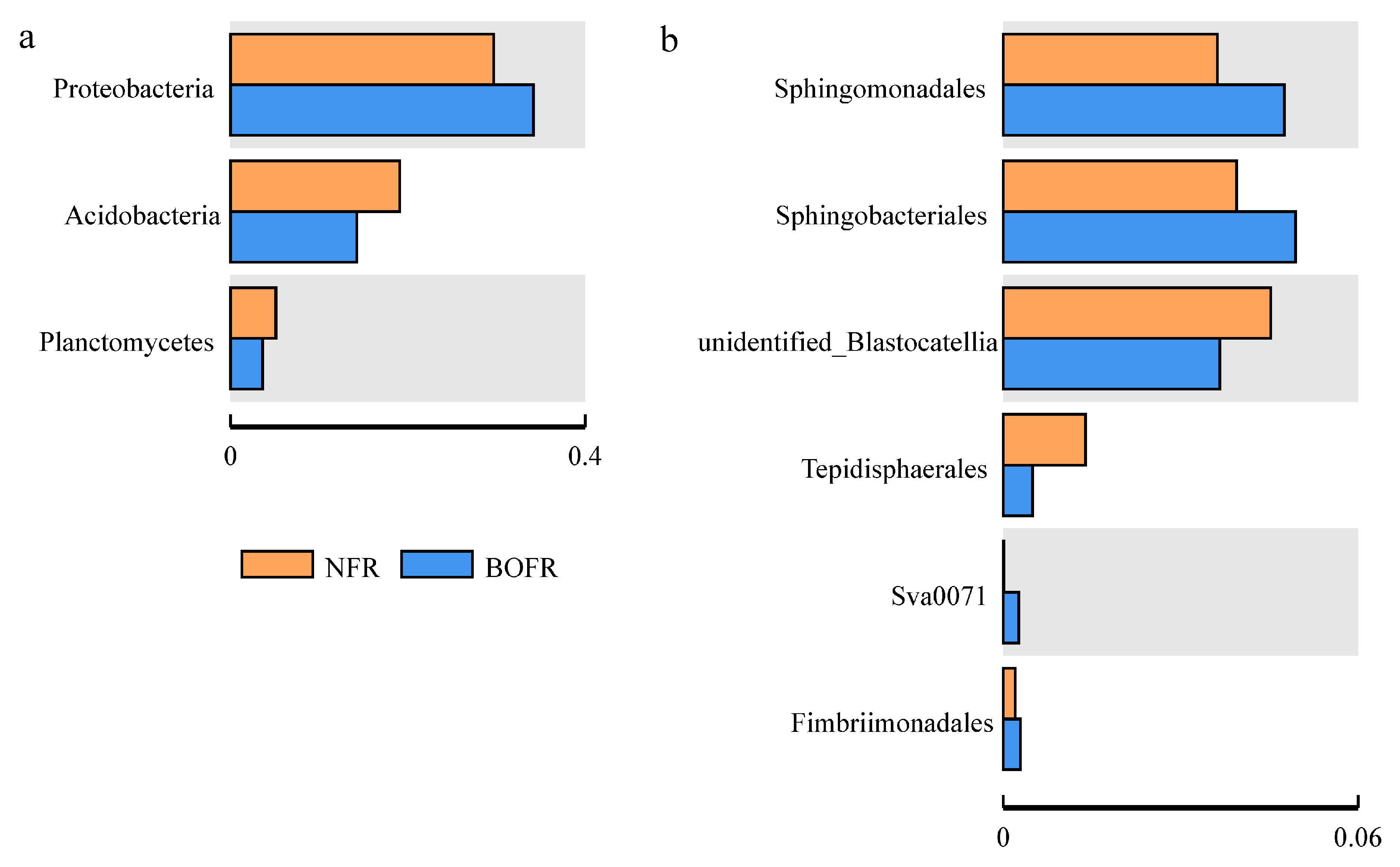
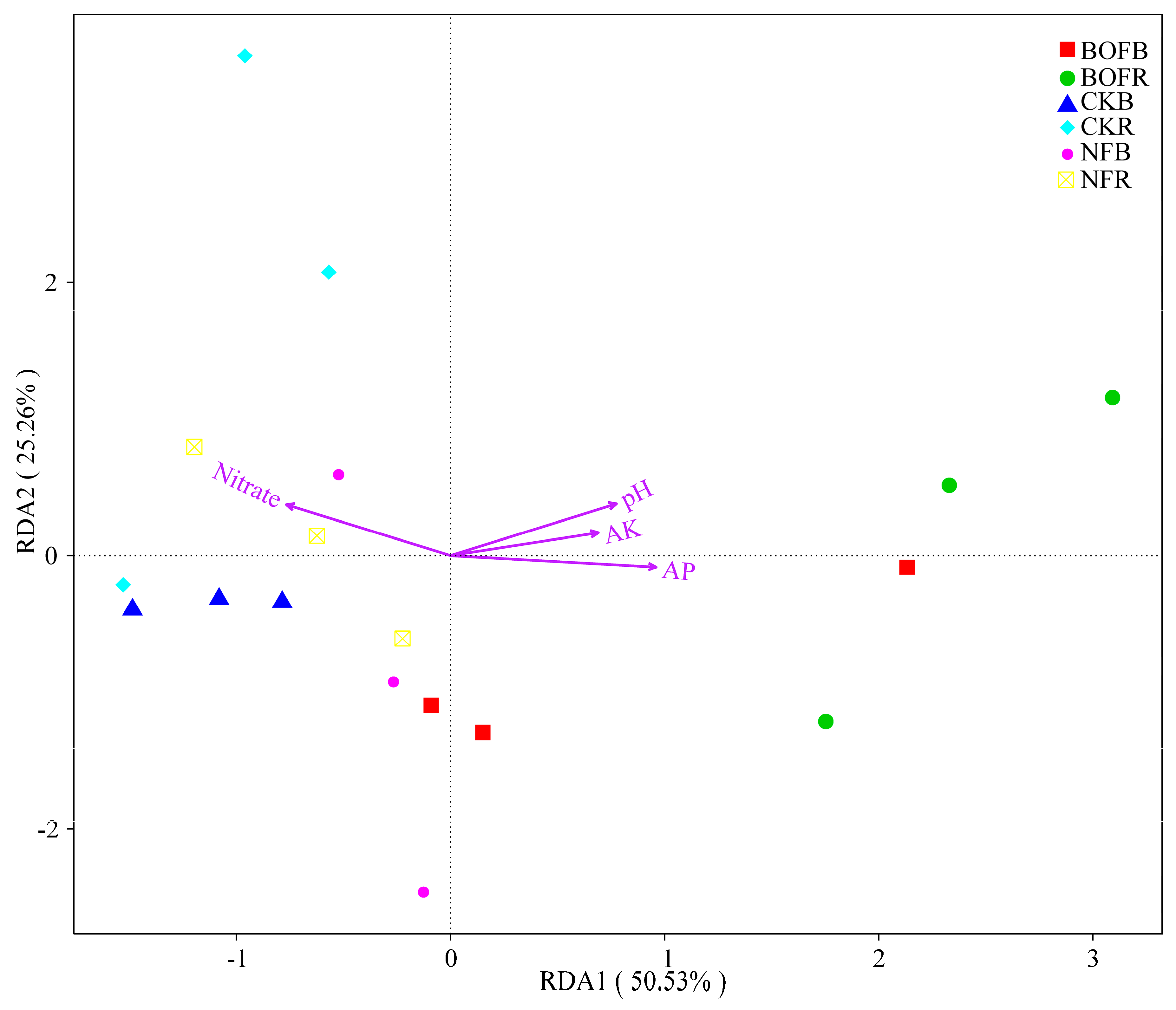
| Item | CKR | CKB | NFR | NFB | BOFR | BOFB |
|---|---|---|---|---|---|---|
| pH | 8.32 ± 0.02 b | 8.22 ± 0.03 c | 8.30 ± 0.06 b | 8.21 ± 0.03 c | 8.40 ± 0.05 a | 8.31 ± 0.06 b |
| SOM (g kg−1) | 16.74 ± 0.75 a | 16.43 ± 0.65 ab | 15.86 ± 0.97 ab | 15.14 ± 1.71 ab | 16.64 ± 0.23 ab | 14.84 ± 0.77 b |
| TN (g kg−1) | 0.94 ± 0.06 ab | 0.86 ± 0.02 b | 1.02 ± 0.02 a | 0.99 ± 0.06 a | 0.98 ± 0.08 ab | 0.97 ± 0.10 ab |
| Ammonium (mg kg−1) | 6.10 ± 0.12 a | 5.89 ± 0.25 a | 6.28 ± 0.45 a | 5.99 ± 0.40 a | 6.24 ± 0.14 a | 6.11 ± 0.16 a |
| Nitrate (mg kg−1) | 22.87 ± 4.62 ab | 23.24 ± 1.44 ab | 22.16 ± 2.89 ab | 24.17 ± 5.97 a | 12.65 ± 3.29 c | 16.49 ± 1.32 bc |
| AP (mg kg−1) | 10.16 ± 0.49 b | 10.08 ± 0.37 b | 10.16 ± 0.91 b | 10.48 ± 1.61 b | 16.88 ± 0.21 a | 12.88 ± 2.19 b |
| AK (mg kg−1) | 116.00 ± 2.90 b | 108.27 ± 1.67 c | 102.47 ± 4.42 cd | 98.60 ± 5.02 d | 130.50 ± 2.90 a | 115.03 ± 1.67 b |
| MBC (mg kg−1) | 423.27 ± 51.69 ab | 334.59 ± 36.63 c | 474.41 ± 23.76 a | 432.71 ± 26.99 ab | 432.47 ± 25.70 ab | 394.42 ± 25.27 b |
| MBN (mg kg−1) | 42.74 ± 5.03 a | 36.37 ± 3.25 abc | 40.06 ± 1.47 ab | 35.59 ± 3.83 bc | 39.16 ± 1.14 ab | 32.16 ± 3.763 c |
| Treatment | Observed Species | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| CKR | 3853 ± 47 a | 10.09 ± 0.07 ab | 0.998 ± 0.001 a | 5141.34 ± 245.51 a | 5270.75 ± 185.09 a |
| CKB | 3652 ± 191 a | 10.05 ± 0.03 ab | 0.998 ± 0.001 a | 4696.15 ± 650.13 a | 4712.37 ± 605.82 a |
| NFR | 3692 ± 94 a | 10.01 ± 0.07 b | 0.997 ± 0.001 a | 4838.85 ± 65.13 a | 4941.59 ± 10.71 a |
| NFB | 3562 ± 238 a | 10.06 ± 0.06 ab | 0.998 ± 0.001 a | 4302.26 ± 614.79 a | 4399.41 ± 588.79 a |
| BOFR | 3853 ± 130 a | 10.17 ± 0.09 a | 0.998 ± 0.001 a | 5032.43 ± 335.56 a | 5084.55 ± 337.89 a |
| BOFB | 3838 ± 244 a | 10.16 ± 0.10 a | 0.998 ± 0.000 a | 4803.29 ± 599.45 a | 4934.92 ± 602.96 a |
| Item | Observed Species | Shannon | Simpson | Chao1 | ACE |
|---|---|---|---|---|---|
| pH | 0.756 ** | 0.619 ** | 0.172 | 0.652 ** | 0.687 ** |
| SOM | −0.062 | −0.121 | −0.259 | 0.101 | 0.075 |
| TN | 0.004 | 0.152 | 0.010 | −0.027 | 0.018 |
| Ammonium | 0.094 | 0.220 | −0.029 | −0.023 | 0.034 |
| Nitrate | −0.560 * | −0.730 ** | −0.463 | −0.418 | −0.410 |
| AP | 0.354 | 0.616 ** | 0.180 | 0.239 | 0.218 |
| AK | 0.544 * | 0.601 ** | 0.169 | 0.440 | 0.426 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, R.; Hou, R.; Li, J.; Lyu, Y.; Hang, S.; Gong, H.; Ouyang, Z. Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain. Agronomy 2020, 10, 93. https://doi.org/10.3390/agronomy10010093
Liang R, Hou R, Li J, Lyu Y, Hang S, Gong H, Ouyang Z. Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain. Agronomy. 2020; 10(1):93. https://doi.org/10.3390/agronomy10010093
Chicago/Turabian StyleLiang, Rubiao, Ruixing Hou, Jing Li, Yun Lyu, Sheng Hang, Huarui Gong, and Zhu Ouyang. 2020. "Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain" Agronomy 10, no. 1: 93. https://doi.org/10.3390/agronomy10010093
APA StyleLiang, R., Hou, R., Li, J., Lyu, Y., Hang, S., Gong, H., & Ouyang, Z. (2020). Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain. Agronomy, 10(1), 93. https://doi.org/10.3390/agronomy10010093






