Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Manure Treatment Methods and Derived Products
2.3. Experimental Design for Fertilizer Tests
2.4. Economic Analysis of Different Fertilizer Treatment Systems
2.5. Data Collection
2.6. Data Analysis
3. Results and Discussions
3.1. Characteristics of Soil, Manure, and Manure-Derived Products
3.2. Maize Plant Height and Number of Leaves as Influenced by Treatments
3.3. Cob and Grain Yields
3.4. Economic Performance of Different Fertilizer Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bationo, A.; Hartemink, A.; Lungu, O.; Naimi, M.; Okoth, P.; Smaling, E.; Thiombiano, L.; Waswa, B. Knowing the African soils to improve fertilizer recommendations. In Improving Soil Fertility Recommendations in Africa Using the Decision Support System for Agrotechnology Transfer (DSSAT); Al, K.E., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 19–42. [Google Scholar] [CrossRef]
- Epule, E.T.; Bryant, C.R.; Akkari, C.; Daouda, O. Can organic fertilizers set the pace for a greener arable agricultural revolution in Africa? Analysis, synthesis and way forward. Land Use Policy 2015, 47, 179–187. [Google Scholar] [CrossRef]
- McArthur, J.W.; McCord, G.C. Fertilizing growth: Agricultural inputs and their effects in economic development. J. Dev. Econ. 2017, 127, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Sekumade, A.B. Economic Effect of Organic and Inorganic Fertilizers on the Yield of Maize in Oyo State, Nigeria. Int. J. Agric. Econ. 2017, 2, 63–68. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. In FAOSTAT Statistical Database; FAO: Rome, Italy, 2017. [Google Scholar]
- MAAIF. Fertilizer Consumption and Fertilizer Use by Crop in Uganda. Available online: https://africafertilizer.org/wp-content/uploads/.../FUBC-Uganda-final-report-2015.pdf (accessed on 12 May 2018).
- Masso, C.; Baijukya, F.; Ebanyat, P.; Bouaziz, S.; Wendt, J.; Bekunda, M.; Vanlauwe, B. Dilemma of nitrogen management for future food security in sub-Saharan Africa—A review. Soil Res. 2017, 55, 425–434. [Google Scholar] [CrossRef]
- Ciceri, D.; Allanore, A. Local fertilizers to achieve food self-sufficiency in Africa. Sci. Total Environ. 2019, 648, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Abayomi, A.O.; Adebayo, O.J. Effect of fertilizer types on the growth and yield of Amaranthus caudatus in Ilorin, Southern Guinea, Savanna Zone of Nigeria. Adv. Agric. 2014, 2014, 947062. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility—A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Komakech, A.J.; Sundberg, C.; Jönsson, H.; Vinnerås, B. Life cycle assessment of biodegradable waste treatment systems for sub-Saharan African cities. Resour. Conserv. Recycl. 2015, 99, 100–110. [Google Scholar] [CrossRef]
- Savci, S. Investigation of effect of chemical fertilizers on environment. Apcbee Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Komakech, A.J.; Banadda, N.E.; Gebresenbet, G.; Vinnerås, B. Maps of animal urban agriculture in Kampala City. Agron. Sustain. Dev. 2014, 34, 493–500. [Google Scholar] [CrossRef]
- Teenstra, E.; Vellinga, T.V.; Aktasaeng, N.; Amatayaku, W.; Ndambi, A.; Pelster, D.; Germer, L.; Jenet, A.; Opio, C.; Andeweg, K. Global Asessment of Manure Management Policies and Practices; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2014. [Google Scholar]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Thomas, C.L.; Acquah, G.E.; Whitmore, A.P.; McGrath, S.P.; Haefele, S.M. The Effect of Different Organic Fertilizers on Yield and Soil and Crop Nutrient Concentrations. Agronomy 2019, 9, 776. [Google Scholar] [CrossRef]
- Komakech, A.J.; Zurbrügg, C.; Semakula, D.; Kiggundu, N.; Vinnerås, B. Evaluation of the performance of different organic fertilizers on maize yield: A case study of Kampala, Uganda. J. Agric. Sci. 2015, 7, 28–38. [Google Scholar] [CrossRef]
- Komakech, A.; Zurbrügg, C.; Miito, G.; Wanyama, J.; Vinnerås, B. Environmental impact from vermicomposting of organic waste in Kampala, Uganda. J. Environ. Manag. 2016, 181, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kaizzi, K.C.; Nansamba, A.; Kabuye, M.F. Optimizing Fertilizer Use within the context of Integrated Soil Fertility in Uganda. In Fertilizer Use Optimization in Sub-Saharan Africa; Wortmann, C.S., Sones, K., Eds.; CABI: Nairobi, Kenya, 2017; pp. 193–209. [Google Scholar]
- Karungi, J.; Kyamanywa, S.; Ekbom, B. Comparison of the effect of market crop wastes and chemical soil fertility amendments on insect pests, natural enemies and yield of Brassica oleracea. Ann. Appl. Biol. 2006, 149, 103–109. [Google Scholar] [CrossRef]
- Okalebo, J.; Gathua, K.; Woomer, P. Laboratory methods of plant and soil analysis: A working manual. TSBF-UNESCO Nairobi Kenya 2002, 2, 29–68. [Google Scholar]
- Aziiba, E.A.; Qiang, C.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- Kaizzi, K.C.; Byalebeka, J.; Semalulu, O.; Alou, I.; Zimwanguyizza, W.; Nansamba, A.; Musinguzi, P.; Ebanyat, P.; Hyuha, T.; Wortmann, C.S. Maize response to fertilizer and nitrogen use efficiency in Uganda. Agron. J. 2012, 104, 73–82. [Google Scholar] [CrossRef]
- Jjagwe, J.; Komakech, A.J.; Karungi, J.; Amann, A.; Wanyama, J.; Lederer, J. Assessment of a Cattle Manure Vermicomposting System Using Material Flow Analysis: A Case Study from Uganda. Sustainability 2019, 11, 5173. [Google Scholar] [CrossRef]
- Katuromunda, S.; Sabiiti, E.; Bekunda, A.M. Effect of method of storing cattle faeces on the physical and chemical characteristics of the resultant composted cattle manure. Uganda J. Agric. Sci. 2012, 13, 95–106. [Google Scholar]
- Yadav, A.; Gupta, R.; Garg, V.K. Organic manure production from cow dung and biogas plant slurry by vermicomposting under field conditions. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–7. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of total solids in biomass and total dissolved solids in liquid process samples. Natl. Renew. Energy Lab. 2008, 1617, 1–6. [Google Scholar]
- De Groote, H.; Gunaratna, N.S.; Ergano, K.; Friesen, D. Extension and adoption of biofortified crops: Quality protein maize in East Africa. In Proceedings of the African Agricultural Economics Association Meetings, AAAE Third Conference/AEASA 48th Conference, Cape Town, South Africa, 19–23 September 2010. [Google Scholar]
- Pasqual, J.; Padilla, E.; Jadotte, E. Equivalence of different profitability criteria with the net present value. Int. J. Prod. Econ. 2013, 142, 205–210. [Google Scholar] [CrossRef]
- Schwab, B.; Lusztig, P. A comparative analysis of the Net Present Value and the benefit-cost ratio as measures of the economic desirability of investments. J. Financ. 1969, 24, 507–516. [Google Scholar] [CrossRef]
- Žižlavský, O. Net present value approach: Method for economic assessment of innovation projects. Procedia-Social Behav. Sci. 2014, 156, 506–512. [Google Scholar] [CrossRef]
- Keča, L.; Keča, N.; Pantić, D. Net Present Value and Internal Rate of Return as indicators for assessment of cost-efficiency of poplar plantations: A Serbian case study. Int. For. Rev. 2012, 14, 145–156. [Google Scholar] [CrossRef]
- Arshad, A. Net present value is better than internal rate of return. Interdiscip. J. Contemp. Res. Bus. 2012, 4, 211–219. [Google Scholar]
- De Reyck, B.; Degraeve, Z.; Vandenborre, R. Project options valuation with net present value and decision tree analysis. Eur. J. Oper. Res. 2008, 184, 341–355. [Google Scholar] [CrossRef]
- Jenkins, G.P.; Miklyaev, M.; Afra, S.; Kuo, C.Y. National Parameters and Commodity Specific Conversion Factors for Project Evaluation in Uganda. JDI Exec. Programs 2018. Available online: https://cri-world.com/publications/qed_dp_403.pdf (accessed on 12 May 2018).
- Kariko-Buhwezi, B.; Mwesigye, A.; Arinaitwe, J.; Colonna, G. Challenges to the sustainability of small scale biogas technologies in Uganda. In Proceedings of the Second International Conference on Advances in Engineering and Technology, Entebbe, Uganda, 31 January–2 February 2011; pp. 499–504. [Google Scholar]
- Jiang, Y.; Zhao, G.; Li, S. An economic analysis methodology for project evaluation and programming. Jt. Transp. Res. Program 2013, 1–44. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, P.; Li, X.; Li, C. Determination of the critical soil mineral nitrogen concentration for maximizing maize grain yield. Plant Soil 2013, 372, 41–51. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef]
- Elvira, C.; Sampedro, L.; Benitez, E.; Nogales, R. Vermicomposting of sludges from paper mill and dairy industries with Eisenia andrei: A pilot-scale study. Bioresour. Technol. 1998, 63, 205–211. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, J.; Vig, A.P. Potential utilization of bagasse as feed material for earthworm Eisenia fetida and production of vermicompost. Springerplus 2015, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Tatàno, F.; Pagliaro, G.; Di Giovanni, P.; Floriani, E.; Mangani, F. Biowaste home composting: Experimental process monitoring and quality control. Waste Manag. 2015, 38, 72–85. [Google Scholar] [CrossRef]
- Sonowal, P.; Khwairakpam, M.; Kalamdhad, A.S. Vermicomposting of solid pulp and paper mill sludge (SPPMS) using Eudrilus Eugeniae Earthworm. Int. J. Environ. Sci. 2014, 5, 502–514. [Google Scholar] [CrossRef]
- Wani, K.; Rao, R. Bioconversion of garden waste, kitchen waste and cow dung into value-added products using earthworm Eisenia fetida. Saudi J. Boil. Sci. 2013, 20, 149–154. [Google Scholar] [CrossRef]
- Soobhany, N.; Mohee, R.; Garg, V.K. Recovery of nutrient from Municipal Solid Waste by composting and vermicomposting using earthworm Eudrilus eugeniae. J. Environ. Chem. Eng. 2015, 3, 2931–2942. [Google Scholar] [CrossRef]
- Tumuhairwe, J.B.; Tenywa, J.S.; Otabbong, E.; Ledin, S. Comparison of four low-technology composting methods for market crop wastes. Waste Manag. 2009, 29, 2274–2281. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparative study. Bioresour. Technol. 2006, 97, 391–395. [Google Scholar] [CrossRef]
- Shah, G.M.; Groot, J.; Oenema, O.; Lantinga, E. Covered storage reduces losses and improves crop utilisation of nitrogen from solid cattle manure. Nutr. Cycl. Agroecosystems 2012, 94, 299–312. [Google Scholar] [CrossRef]
- Pattey, E.; Trzcinski, M.; Desjardins, R. Quantifying the reduction of greenhouse gas emissions as a resultof composting dairy and beef cattle manure. Nutr. Cycl. Agroecosystems 2005, 72, 173–187. [Google Scholar] [CrossRef]
- Shisanya, C.A.; Mucheru, M.W.; Mugendi, D.N.; Kung’u, J.B. Effect of organic and inorganic nutrient sources on soil mineral nitrogen and maize yields in central highlands of Kenya. Soil Tillage Res. 2009, 103, 239–246. [Google Scholar] [CrossRef]
- Okoroafor, I.; Okelola, E.; Edeh, O.; Nemehute, V.; Onu, C.; Nwaneri, T.; Chinaka, G. Effect of organic manure on the growth and yield performance of Maize in Ishiagu, Ebonyi State, Nigeria. IOSR J. Agric. Vet. Sci. (IOSR-JAVS) 2013, 5, 28–31. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Bayite-kasule, S. Inorganic Fertilizer in Uganda—Knowledge Gaps, Profitability, Subsidy, and Implications of a National Policy. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.225.7493&rep=rep1&type=pdf (accessed on 23 April 2018).
- Doan, T.T.; Henry-des-Tureaux, T.; Rumpel, C.; Janeau, J.L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Celestina, C.; Hunt, J.R.; Sale, P.W.; Franks, A.E. Attribution of crop yield responses to application of organic amendments: A critical review. Soil Tillage Res. 2019, 186, 135–145. [Google Scholar] [CrossRef]
- Chen, G.; Cao, H.; Liang, J.; Ma, W.; Guo, L.; Zhang, S.; Jiang, R.; Zhang, H.; Goulding, K.; Zhang, F. Factors affecting nitrogen use efficiency and grain yield of summer maize on smallholder farms in the North China Plain. Sustainability 2018, 10, 363. [Google Scholar] [CrossRef]
- Hijbeek, R.; van Ittersum, M.K.; ten Berge, H.F.; Gort, G.; Spiegel, H.; Whitmore, A.P. Do organic inputs matter–a meta-analysis of additional yield effects for arable crops in Europe. Plant Soil 2017, 411, 293–303. [Google Scholar] [CrossRef]
- Mahmood, F.; Khan, I.; Ashraf, U.; Shahzad, T.; Hussain, S.; Shahid, M.; Abid, M.; Ullah, S. Effects of organic and inorganic manures on maize and their residual impact on soil physico-chemical properties. J. Soil Sci. Plant Nutr. 2017, 17, 22–32. [Google Scholar] [CrossRef]
- Mukta, S.; Rahman, M.; Mortuza, M. Yield and Nutrient Content of Tomato as influenced by the Application of Vermicompost and Chemical Fertilizers. J. Environ. Sci. Nat. Res. 2015, 8, 115–122. [Google Scholar] [CrossRef]
- Atiyeh, R.; Subler, S.; Edwards, C.; Bachman, G.; Metzger, J.; Shuster, W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. [Google Scholar] [CrossRef]
- Mohanty, M.; Reddy, K.S.; Probert, M.; Dalal, R.C.; Rao, A.S.; Menzies, N. Modelling N mineralization from green manure and farmyard manure from a laboratory incubation study. Ecol. Model. 2011, 222, 719–726. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Guo, L.; Wu, G.; Li, C.; Liu, W.; Yu, X.; Cheng, D.; Jiang, G. Vermicomposting with maize increases agricultural benefits by 304%. Agron. Sustain. Dev. 2015, 35, 1149–1155. [Google Scholar] [CrossRef]
- Choudhary, V.; Suresh, K. Maize production, economics and soil productivity under different organic source of nutrients in eastern himalayan region, India. Int. J. Plant Prod. 2013, 7, 167–186. [Google Scholar]

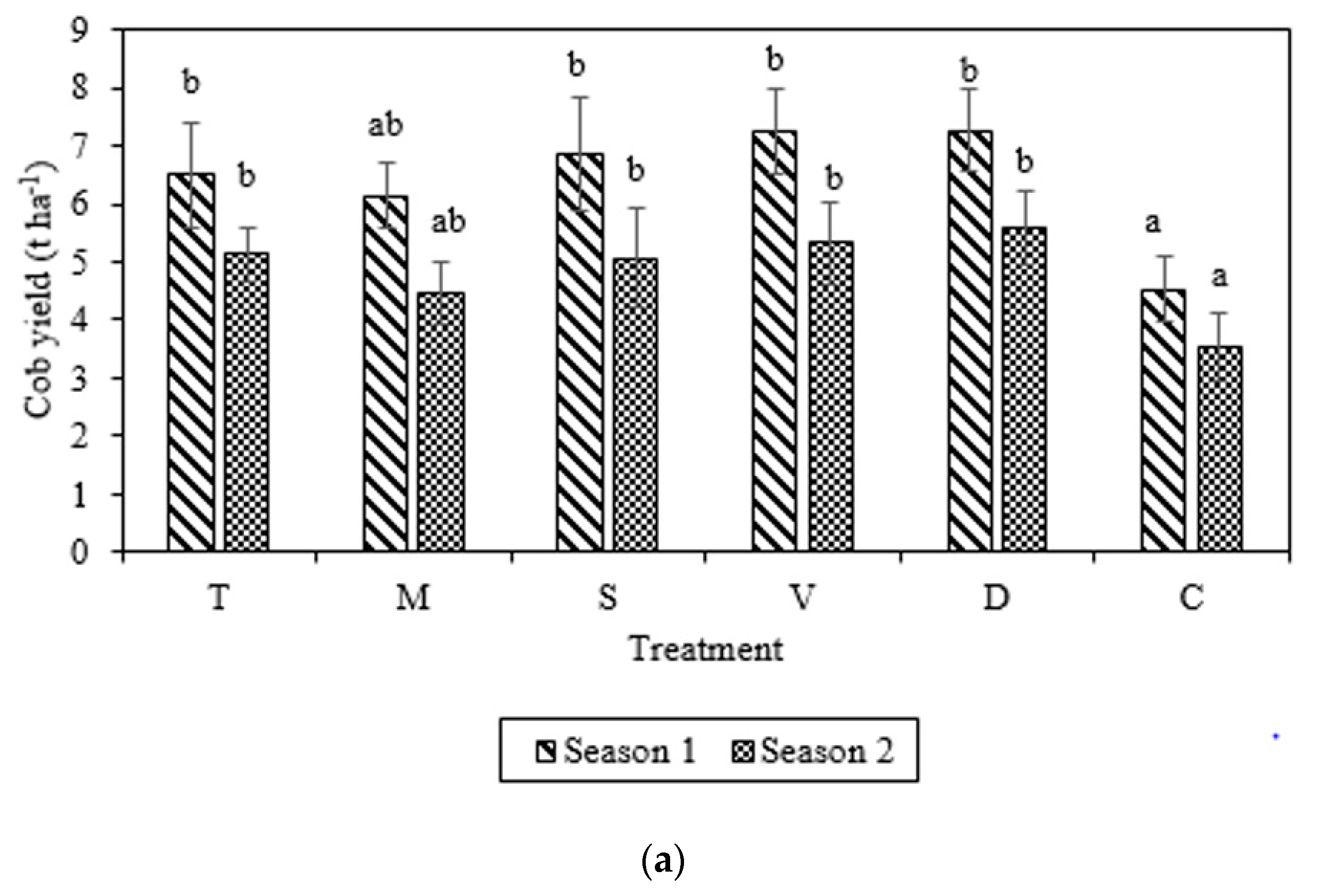
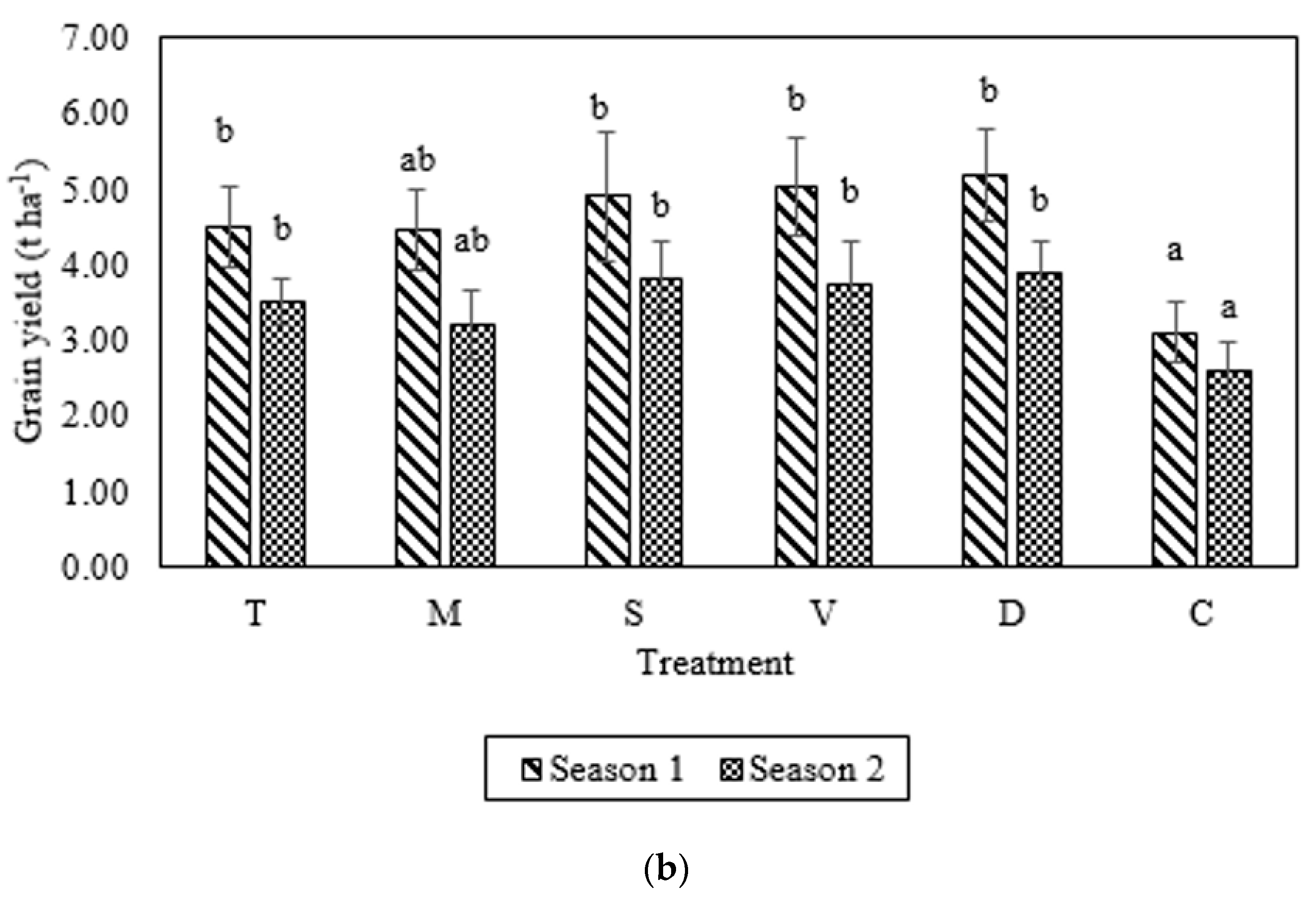
| Parameter | Manure Specification | ||||
|---|---|---|---|---|---|
| FCM | T | M | S | V | |
| MC (%) | 78.48 ± 1.23 | 38.24 ± 0.92 | 48.60 ± 1.34 | 74.32 ± 0.82 | 69.74 ± 0.37 |
| pH | 8.05 ± 0.14 | 7.91 ± 0.12 | 7.86 ± 0.06 | 7.82 ± 0.24 | 6.43 ± 0.11 |
| TKN (%ww) | 0.31 ± 0.04 | 1.02 ± 0.07 | 0.71 ± 0.06 | 0.48 ± 0.02 | 0.64 ± 0.06 |
| TP (%ww) | 0.11 ± 0.05 | 0.26 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.03 | 0.33 ± 0.03 |
| TK (%ww) | 0.24 ± 0.08 | 0.43 ± 0.07 | 0.44 ± 0.03 | 0.19 ± 0.02 | 0.50 ± 0.06 |
| TOC (%ww) | 7.46 ± 0.21 | 11.23 ± 0.31 | 10.11 ± 0.09 | 4.73 ± 0.06 | 6.13 ± 0.65 |
| C/N ratio | 24.06 ± 0.36 | 11.01 ±0.05 | 14.24 ± 0.42 | 9.85 ± 1.02 | 9.58 ± 0.53 |
| Treatment | Maize Height (cm) | |||
|---|---|---|---|---|
| Season 1 (2018 A) | ||||
| 2WAP | 4WAP | 6WAP | 8WAP | |
| T | 21.2 ± 5.1b | 42.9 ± 3.7b | 63.1 ± 4.6b | 108.1 ± 24.5ab |
| M | 25.1 ± 4.5bc | 44.2 ± 3.6b | 64.8 ± 4.4b | 107.0 ± 19.3ab |
| S | 24.9 ± 4.5bc | 44.2 ± 6.2b | 66.9 ± 6.2b | 108.3 ± 24.5ab |
| V | 25.3 ± 4.0bc | 48.2 ± 3.9b | 69.9 ± 7.5b | 112.9 ± 19.7ab |
| D | 26.8 ± 5.4c | 58.9 ± 11.6c | 81.0 ± 8.7c | 123.5 ± 34.3b |
| C | 15.6 ± 4.4a | 33.0 ± 7.1a | 54.0 ± 8.5a | 86.8 ± 8.4a |
| Grand Mean | 23.1 | 45.3 | 66.6 | 110.8 |
| s.e.d | 1.65 | 2.34 | 2.42 | 9.34 |
| Season 2 (2018 B) | ||||
| T | 22.2 ± 4.3ab | 39.7 ± 3.9bc | 53.0 ± 8.4ab | 77.5 ± 8.4a |
| M | 22.9 ± 3.2ab | 38.1 ± 3.4b | 56.7 ± 8.2bc | 81.7 ± 9.6a |
| S | 22.5 ± 4.3ab | 38.7 ± 3.5bc | 53.0 ± 8.3ab | 82.1 ± 14.6a |
| V | 25.6 ± 3.5b | 40.0 ± 3.7bc | 58.0 ± 8.1bc | 81.8 ± 10.2a |
| D | 25.8 ± 3.7b | 42.8 ± 5.0c | 61.2 ± 5.7c | 99.5 ± 10.6b |
| C | 19.3 ± 4.8a | 31.3 ± 5.2a | 46.0 ± 7.1a | 73.3 ± 6.0a |
| Grand mean | 23.1 | 38.4 | 54.6 | 82.2 |
| s.e.d. | 1.42 | 1.47 | 2.72 | 3.66 |
| Treatment | Number of Leaves | |||
|---|---|---|---|---|
| Season 1 (2018 A) | ||||
| 2WAP | 4WAP | 6WAP | 8WAP | |
| T | 4.13 ± 0.96 | 5.56 ± 0.81 | 8.25 ± 0.68 | 11.88 ± 1.50 |
| P | 3.94 ± 0.77 | 6.06 ± 0.68 | 8.38 ± 0.62 | 11.56 ± 1.63 |
| S | 4.00 ± 0.89 | 5.75 ± 0.86 | 8.19 ± 0.66 | 11.12 ± 1.71 |
| V | 4.13 ± 0.96 | 5.81 ± 0.91 | 8.50 ± 0.63 | 11.56 ± 0.81 |
| D | 4.00 ± 0.89 | 5.56 ± 0.96 | 8.50 ± 0.63 | 11.13 ± 1.63 |
| C | 3.94 ± 0.85 | 4.81 ± 1.05 | 8.31 ± 0.79 | 10.75 ± 1.44 |
| Grand Mean | 4.21 | 5.59 | 8.35 | 11.33 |
| s.e.d. | 0.32 ns | 0.32 ns | 0.24 ns | 0.49 ns |
| Season 2 (2018 B) | ||||
| T | 4.00 ± 0.89 | 5.25 ± 0.68 | 7.25 ± 0.86 | 9.50 ± 1.03 |
| P | 3.94 ± 0.85 | 5.06 ± 0.77 | 7.34 ± 0.62 | 9.00 ± 0.89 |
| S | 3.99 ± 0.80 | 5.06 ± 0.77 | 7.25 ± 0.86 | 9.38 ± 1.41 |
| V | 4.13 ± 0.89 | 5.55 ± 0.89 | 7.25 ± 0.68 | 9.56 ± 0.73 |
| D | 4.13 ± 1.03 | 5.56 ± 0.81 | 7.18 ± 0.66 | 9.63 ± 0.89 |
| C | 3.88 ± 0.89 | 5.19 ± 0.65 | 7.19 ± 0.83 | 9.25 ± 0.93 |
| Grand Mean | 4.00 | 5.27 | 7.25 | 9.39 |
| s.e.d. | 0.32 ns | 0.27 ns | 0.26 ns | 0.33 ns |
| Treatment | Costs (USD) | |||
|---|---|---|---|---|
| Initial Investment | Annual Costs | Annual Benefits | Annual Net Cash Flow | |
| T | 25 | 1008 | 1637 | 629 |
| M | 114 | 1008 | 1563 | 556 |
| S | 1905 | 1017 | 1875 | 858 |
| V | 150 | 1211 | 3516 | 2305 |
| D | 186 | 989 | 1854 | 865 |
| C | 0 | 989 | 1162 | 174 |
| PW of Benefits (USD) | ||||
| 5% | 10% | 13.9% | 24% | |
| T | 7839 | 5355 | 4190 | 2585 |
| M | 6929 | 4734 | 3704 | 2285 |
| S | 10,693 | 7305 | 5716 | 3527 |
| V | 28,725 | 19,624 | 15,355 | 9474 |
| D | 10,780 | 7364 | 5762 | 3555 |
| C | 2168 | 1481 | 1159 | 715 |
| B/C Ratio | ||||
| T | 314 | 214 | 168 | 103 |
| M | 61 | 42 | 32 | 20 |
| S | 6 | 4 | 3 | 2 |
| V | 192 | 131 | 102 | 63 |
| D | 58 | 40 | 31 | 19 |
| C | - | - | - | - |
| NPV (USD) | ||||
| T | 7817 | 5332 | 4167 | 2562 |
| M | 6810 | 4616 | 3587 | 2170 |
| S | 8792 | 5403 | 3813 | 1623 |
| V | 28,572 | 19,472 | 15,203 | 9323 |
| D | 10,597 | 7180 | 5578 | 3371 |
| C | 2163 | 1477 | 1156 | 713 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jjagwe, J.; Chelimo, K.; Karungi, J.; Komakech, A.J.; Lederer, J. Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results. Agronomy 2020, 10, 69. https://doi.org/10.3390/agronomy10010069
Jjagwe J, Chelimo K, Karungi J, Komakech AJ, Lederer J. Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results. Agronomy. 2020; 10(1):69. https://doi.org/10.3390/agronomy10010069
Chicago/Turabian StyleJjagwe, Joseph, Keneth Chelimo, Jeninah Karungi, Allan John Komakech, and Jakob Lederer. 2020. "Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results" Agronomy 10, no. 1: 69. https://doi.org/10.3390/agronomy10010069
APA StyleJjagwe, J., Chelimo, K., Karungi, J., Komakech, A. J., & Lederer, J. (2020). Comparative Performance of Organic Fertilizers in Maize (Zea mays L.) Growth, Yield, and Economic Results. Agronomy, 10(1), 69. https://doi.org/10.3390/agronomy10010069





